Abstract
Background:
To identify the clinical correlations between mechanical power and transforming growth factor-β1 (TGF-β1) and connective tissue growth factor (CTGF) in acute respiratory distress syndrome (ARDS) patients, their clinical significance in pulmonary structural remodeling in ARDS patients was investigated.
Methods:
Ninety-five patients with moderate or severe ARDS, who required mechanical ventilation therapy, were randomly selected among hospitalized patients from January 2017 to February 2019. Their mechanical power was monitored and recorded, the TGF-β1 and CTGF levels were detected by enzyme-linked immunosorbent assay (ELISA), their relevance was analyzed, and the relationship between mechanical power and 28-day survival rate was investigated. According to the high-resolution computed tomography (HRCT) examination, the patients were divided into an ARDS group and an ARDS pulmonary fibrosis (ARDS-PF) group. The differences in mechanical power, TGF-β1, and CTGF between the 2 groups were compared, and the significance of TGF-β1 and CTGF in the diagnosis of ARDS pulmonary interstitial fibrosis were evaluated.
Results:
A significant positive correlation between mechanical power and serum TGF-β1 and CTGF in patients with ARDS was found and the correlation coefficients were 0.424 and 0.581, respectively. The difference between mechanical power and 28-day survival rate was statistically significant (P < .05), while the area under the receiver operating characteristic curves of TGF-β1 and CTGF for the diagnosis of ARDS pulmonary fibrosis was 0.838 and 0.884, respectively (P < .05).
Conclusion:
A significant correlation between mechanical power and serum fibrosis biomarkers TGF-β1 and CTGF in ARDS patients was found, and its level was related to the survival prognosis of patients. Mechanical power, TGF-β1, and CTGF were clinically evaluated for the assessment of lung structural remodeling, such as ARDS pulmonary fibrosis. This study has particular significance to the early prevention of ventilator-induced lung injury and pulmonary fibrosis in patients with ARDS receiving mechanical ventilation.
Keywords: acute respiratory distress syndrome, connective tissue growth factor, mechanical power, pulmonary structural remodeling, transforming growth factor-β1
1. Introduction
The acute respiratory distress syndrome (ARDS) is a common illness in critical care medicine, accounting for 10% of intensive care unit (ICU) patients, and the mortality rate is as high as 46%.[1] Mechanical ventilation plays a key role in the treatment of patients with ARDS, however, it may cause ventilator-induced lung injury (VILI), as well as cause or accelerate pulmonary fibrosis.[2] Studies have shown that the severity of pulmonary fibrosis is closely related to mortality, prognosis, and long-term quality of life in patients with ARDS.[3] The study of how to effectively reduce the mortality rate, actively prevent the occurrence and development of pulmonary fibrosis, and improve the prognosis of patients with ARDS, has always been the focus of research in critical care. Therefore, the early identification of post-ARDS pulmonary fibrosis has important clinical implications for patients. With each breath delivered by the mechanical ventilator, a certain amount of energy is transferred to the respiratory system of the patient. This energy is called mechanical power (MP) and is mainly used to overcome the resistance of the airways and expand the thorax wall. A fraction of this energy acts directly on the lung skeleton, or extracellular matrix, as such deforming the epithelial and endothelial cells anchored to it.[4] In recent years, it has been found that, during mechanical ventilation of ARDS patients, MP can be used more comprehensively to evaluate and prevent VILI than other mechanical parameters, such as the driving pressure, transpulmonary pressure, respiratory rate, and positive end-expiratory pressure (PEEP).[5] Through MP, pressure injuries and volume injuries, as well as biological injuries caused by mechanical aggravation, can be considered as a whole, and it is very helpful for clinicians to perform lung-protective ventilation.[6,7] At present, there is still a lack of sensitive and highly specific biomarkers able to predict post-ARDS fibrosis. Transforming growth factor-β1 (TGF-β1) and connective tissue growth factor (CTGF) are cytokines involved in pulmonary fibrosis,[8,9] however, their early secretion in patients with ARDS is not completely clear. In this study, the mechanical power during mechanical ventilation was monitored and its correlation with TGF-β1 and CTGF was evaluated. Furthermore, the significance of MP, TGF-β1, and CTGF in the prediction and evaluation of pulmonary fibrosis in patients with ARDS was discussed.
2. Research objects and methods
2.1. Research objects
The study was approved by the Hospital Ethics Committee of the First People's Hospital of Lianyungang. One hundred seven patients with moderate to severe ARDS, who required invasive mechanical ventilation in the ICU ward from January 2017 to February 2019 were screened. Twelve cases were excluded due to death shortly after admission or difficulty in completing the lung HRCT examination. A total of 95 cases (60 men and 35 women) were included in the study. Their basic information, which included sex, age, basic medical history, 24-h acute physiology and chronic health evaluation II (APACHE II) score, and sequential organ failure assessment (SOFA) score, was recorded. All enrolled patients met the diagnostic criteria of the ARDS Berlin definition proposed by the European Society of Critical Care Medicine and the American Thoracic Society in 2012.[10] Patients with previous chronic obstructive pulmonary disease (COPD), chronic kidney disease, autoimmune diseases, tumors, and pulmonary fibrosis were excluded. All patients underwent HRCT examination of the lungs from day 3 to day 7 after admission, and were divided into an ARDS group and an ARDS pulmonary fibrosis (ARDS-PF) group, according to whether pulmonary interstitial fibrosis changes were included.
2.2. Research methods
2.2.1. ARDS standard mechanical ventilation procedure and treatment strategy
Within 24 hours of admission in the ICU, all enrolled patients underwent tracheal intubation and mechanical ventilation, and then a standard procedure was followed to place the manometry cathete. In order to reduce the occurrence of patient-ventilator dyssynchrony, a Richmond Agitation-Sedation Scale score of –3 to –4 had to be reached. Mechanical ventilation was delivered in a volume-controlled mode with constant inspiratory flow and tidal volume targeted to 6 ± 1 mL/kg of predicted body weight. The pleural pressure was indirectly assessed by the esophageal pressure and PEEP was adjusted to obtain an end-expiratory transpulmonary pressure >0 cmH2O, maintaining the alveoli open state at the end of expiration and limiting the end-inspiratory transpulmonary pressure to <25 cmH2O. The inhaled oxygen concentration was adjusted to maintain the patient's SpO2 between 88% and 95% and PaO2 between 55 and 80 mmHg.[11] If the above ventilation goals were not achieved, the patient would be given a pressure control method for lung recruitment maneuver. Prone position ventilation was performed in patients with severe ARDS who were still unable to fulfill the oxygenation goal after performing lung recruitment.[12] Extracorporeal membrane oxygenation (ECMO) was applied only to a few patients for the last treatment of severe ARDS.
2.2.2. Mechanical power monitoring in ARDS patients
The ventilator can monitor the esophageal pressure (Pes) through the esophageal manometer and then calculate the transpulmonary pressure (Ptp) and the driving pressure (▵P). As it has been previously proposed, the MP was calculated using tidal volume (VT), peak pressure (Ppeak), respiratory rate (RR), and driving pressure (▵P) data: MP (J/min) = 0.098 × VT × RR × (Ppeak–1/2 × ▵P).[4] The MP was recorded during the first 24 hours of mechanical ventilation and the mean results were calculated. In a recently published study, it was shown that there was a consistent increase in the risk of death with MP >17.0 J/min.[4] Therefore, all patients were divided into a high mechanical power group (HMP) and a low mechanical power group (LMP) according to whether the MP was higher than 17.0 J/min. Afterwards, the 28-day survival status of the patients was analyzed and recorded.
2.2.3. Pulmonary fibrosis biomarker measurements
Five milliliters of blood samples were collected within 24 hours after admission. After standing for 30 minutes, the serum was centrifuged and stored at –80 °C. The serum TGF-β1 and CTGF levels were determined by enzyme-linked immunosorbent assay (ELISA).
2.3. Ethics statement
The study protocol was approved by the ethics committee of Lianyungang Clinical College of Nanjing Medical University. A written informed consent was obtained from the patients before the start of the study. Patient records/information was anonymized and deidentified prior to analysis.
2.4. Statistical processing
Data processing and mapping were performed using the SPSS 22.0 (SPSS Inc, Chicago, IL) statistical software and GraphPad Prism 6.0 (GraphPad Software, San Diego, California). The measurement data were expressed as mean ± standard deviation (x ± s). Differences between the 2 groups were compared using 2 independent sample t tests. The correlation between mechanical power and cytokines was analyzed by Spearman correlation analysis. The TGF-β1 and CTGF values in the diagnosis of ARDS pulmonary fibrosis were analyzed by receiver operating characteristic (ROC) curve analysis. The 28-day mortality of the patients was recorded and a single-factor analysis was performed to plot the survival curves of ARDS patients.
3. Results
In total, 107 patients were enrolled in this study. Twelve patients were excluded: 2 patients refused to participate in the study, 3 patients died soon after admission, and 7 patients failed to complete the HRCT examination. From the remaining 95 patients, 50 comprised the ARDS group and 45 the ARDS-PF group according to whether pulmonary interstitial fibrosis changed (Fig. 1).
Figure 1.
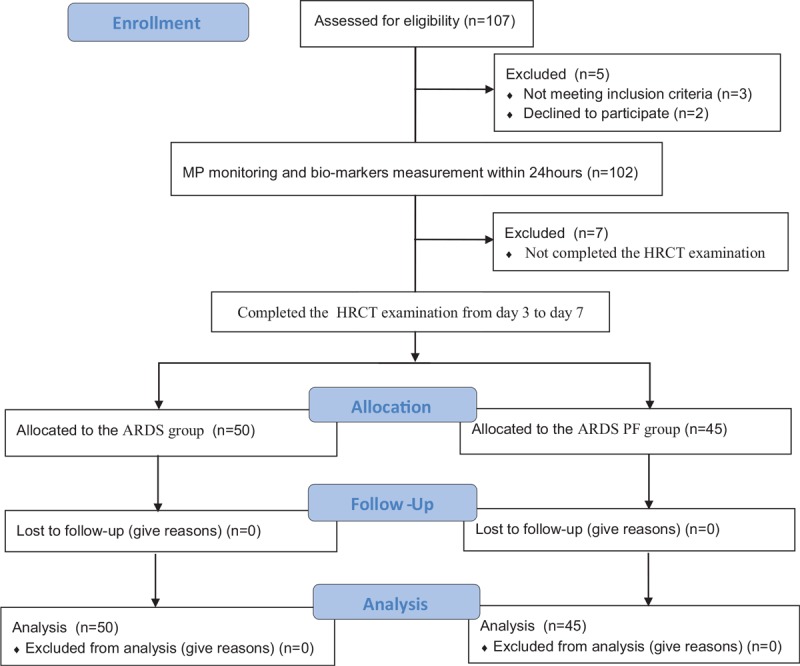
Overview of the included patients.
3.1. Baseline and mechanical ventilation characteristics of the included patients
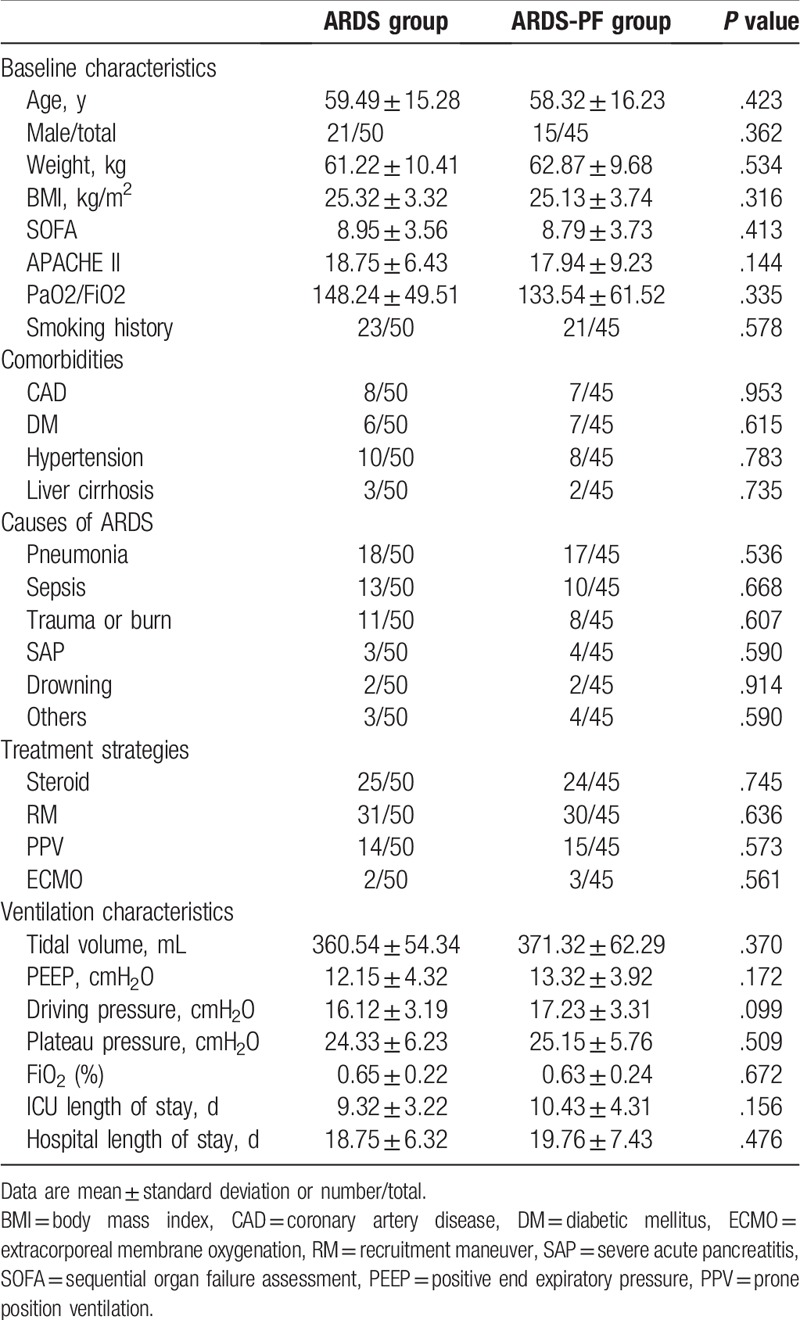
No significant differences (P > .05) were found in the sex, age, body weight, body mass index (BMI), comorbidities, smoking history, oxygenation index, APACHE II, SOFA score, causes of ARDS, treatment strategies, and ventilation characteristics between the ARDS group and the ARDS-PF group. In addition, no significant differences were found in the ICU length of stay and the hospital length of stay between the 2 groups (Table 1).
Table 1.
Comparison of the baseline and mechanical ventilation characteristics between the 2 groups.
3.2. Mechanical power and TGF-β1 and CTGF serum levels in the 2 groups
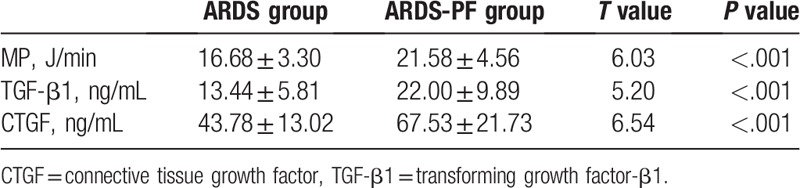
The mechanical power of the ARDS-PF group was significantly higher (P < .001) than that of the ARDS group, and the expression levels of serum TGF-β1 and CTGF were also significantly higher (P < .001) (Table 2).
Table 2.
Comparison of mechanical power and serum TGF-β1 and CTGF expression levels in 2 groups.
3.3. Correlation between mechanical power and serum TGF-β1, CTGF, sequential organ failure assessment, and acute physiology and chronic health evaluation II
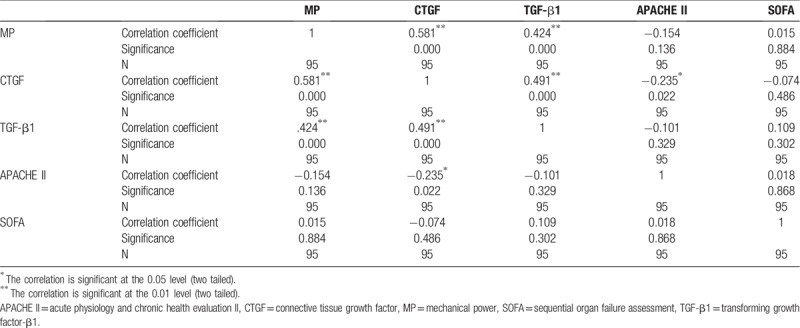
A correlation analysis between the mechanical power and serum TGF-β1, CTGF, and SOFA and APACHE II scores in all ARDS patients was performed and the results are shown in Table 3. According to the results, there was a significant positive correlation between mechanical power and serum TGF-β1 and CTGF, with a correlation coefficient of 0.424 and 0.581, respectively (P < .01). On the other hand, there was no clear correlation between mechanical power and APFCHE II and SOFA score (P > .05).
Table 3.
Correlation between mechanical power and serum TGF-β1, CTGF, APACHE II and SOFA score.
3.4. Predictive diagnostic value of TGF-β1 and CTGF to post-ARDS pulmonary fibrosis
Based on the ROC curves of TGF-β1 and CTGF (Fig. 2) for the diagnosis of ARDS pulmonary fibrosis, the area under the curve of TGF-β1 and CTGF was calculated and found to be 0.838 ± 0.039 and 0.884 ± 0.037, respectively, indicating a high diagnostic predictive value to post-ARDS pulmonary fibrosis (P < .05).
Figure 2.
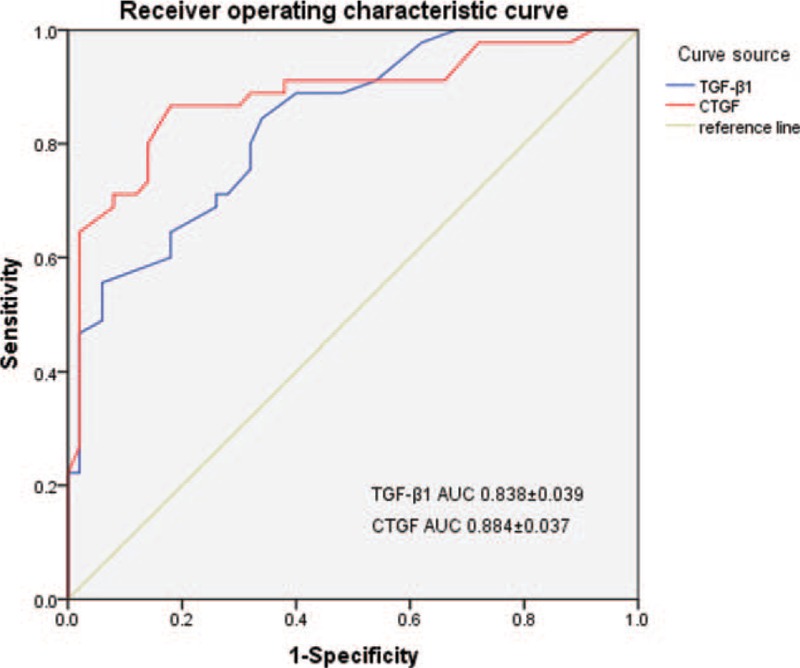
ROC curve of serum TGF-β1 and CTGF in predicting the diagnosis of post-ARDS. ARDS = acute respiratory distress syndrome, CTGF = connective tissue growth factor, ROC = receiver operating characteristic ,TGF-β1 = transforming growth factor-β1.
3.5. Relationship between mechanical power and prognosis in ARDS patients
The survival curve of patients with ARDS indicated that there was a statistically significant difference in the 28-day mortality between the HMP group and the LMP group (P < .05) (Fig. 3).
Figure 3.
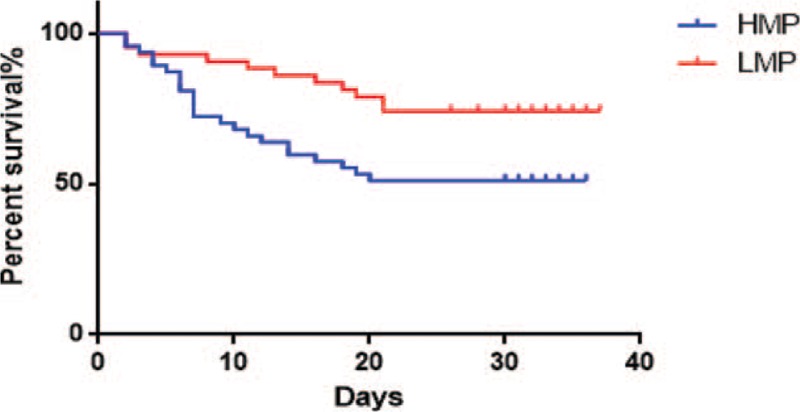
Mechanical power and patient 28-day survival curve.
4. Discussion
This study showed that the mechanical power and the expression levels of serum TGF-β1 and CTGF in the ARDS-PF group were significantly higher than those in the non-fibrotic ARDS group (P < .01), while there was a significant positive correlation between MP and serum TGF-β1 and CTGF, with correlation coefficients of 0.424 and 0.581, respectively (P < .01). However, no significant differences in the oxygenation index, APACHE II and SOFA scores were found between the 2 groups (P > .05). This indicated that higher mechanical power is more likely to cause ventilator-associated lung injury in patients with ARDS, further induce aggravation of biological injury,[13] lead to increased secretion of TGF-β1 and CTGF, and accelerate the progression of lung tissue fibrosis in patients with ARDS. In the present study, a significant difference was also found in the 28-day mortality in patients between the LMP group and the HMP group, which indicated that the level of mechanical power is related to the prognosis of patients and may be related to the severity of ARDS. This can be attributed to the fact that in order to open the collapsed alveoli and maintain them open in early ARDS, the ventilator requires greater transpulmonary pressure and PEEP. Therefore, poorly compliant lungs necessarily require greater mechanical power in the early stages of mechanical ventilation.[14] Consequently, the mechanical power can be related to the severity of the patient's lung disease. This study initially explored the hypothesis that the mechanical power is expected to provide clinical help in assessing the severity of ARDS, similar to the oxygenation index. At the same time, constant high mechanical power may be more predictive of adverse outcome in assessing the prognosis of ARDS.
Tissue damage and fibroplasia are important pathological processes in the progression of ARDS. More and more studies have shown that there are pulmonary fibrosis changes in patients with early stage ARDS. More specifically, a study by Ichikado et al[15] reported that in 47% of patients, the presence of pulmonary fibrosis was confirmed by CT on the first day of the first ARDS diagnosis. In this study, the serum fibrosis biomarkers TGF-β1 and CTGF exhibited significant differences in early-stage ARDS patients, indicating that collagen fibrosis is simultaneously activated at the early stage of ARDS. Studies have shown that cytokines play an important role in the development of pulmonary fibrosis. TGF-β1 regulates the expression of effector genes, such as cellular collagen, through intracellular transduction signals, and is considered to be the most critical fibrotic cytokine .[8,16,17] TGF-β1 is involved in the pathogenesis of growth and tissue repair, it regulates a variety of cellular functions, including inhibiting or stimulating cell growth, cell differentiation, cell death, or apoptosis. It is also an inducer of extracellular matrix and the predominant regulator of collagen in a variety of tissues.[18] In various experimental models and clinical fibrotic diseases, TGF-β1 is continuously up-regulated, accelerating the progression of fibrosis, and thus it is considered to be a key regulator of tissue fibrosis.[19] CTGF is a cysteine-rich secreted growth factor with a relative molecular weight of 38 kDa. It is an efficient downstream response element of TGF-β1, it mediates the partial biological effects of TGF-β1, which stimulate fibroblast proliferation and extracellular matrix synthesis, and it promotes the development of tissue and organ fibrosis.[20] Moreover, CTGF has a mitogenic effect and promotes proliferation of cells, such as vascular smooth muscle cells, it stimulates the synthesis of extracellular matrix, inhibits its degradation, leads to accumulation of extracellular matrix and structural alteration,[17] thereby accelerating tissue fibrosis, and in the development of ARDS, it is closely related to pulmonary fibrosis.[9] The ROC curves of serum TGF-β1 and CTGF presented in this study, indicated that serum TGF-β1 and CTGF have clinical diagnostic significance for lung fiber remodeling in patients with ARDS. It was also demonstrated that cytokines TGF-β1 and CTGF exert active biological effects during the remodeling of ARDS lung tissue structure.
5. Limitations
This study was associated with some limitations. First, the patients were divided into an ARDS group and an ARDS-PF group according to the HRCT results from day 3 to day 7. The course of pulmonary fibrosis among some ARDS patients may appear much later than the 3rd to the 7th day. Additional studies are needed to investigate post-ARDS pulmonary fibrosis. Second, only the SOFA and APACHEII scores were evaluated within 24 hours of the ARDS patient and the severity of the patient's condition was not dynamically assessed. Third, only one time point for the TGF-β1 and CTGF concentration determination was obtained, while in future studies, more time points need to be evaluated.
6. Conclusions
In summary, VILI and pulmonary fibrosis during ARDS mechanical ventilation are gradually attracting the attention of clinicians. Mechanical power can more comprehensively assess and prevent VILI and pulmonary fibrosis changes during mechanical ventilation of ARDS patients, and can be the new standard to guide ARDS lung-protective ventilation. At the same time, the serum biomarkers TGF-β1 and CTGF have a clear correlation with the development of pulmonary fibrosis in ARDS patients. Therefore, they are of great significance in predicting and evaluating ARDS pulmonary fibrosis and have great clinical promotion value.
Author contributions
Formal analysis: Xiaomin Li.
Funding acquisition: Xiaomin Li.
Methodology: Yangli Wang, Xiaomin Li.
Supervision: Kexi Liu, Xiaomin Li.
Writing – original draft: Yongpeng Xie.
Writing – review & editing: Yongpeng Xie.
Footnotes
Abbreviations: ARDS = acute respiratory distress syndrome, CAD = coronary artery disease, COPD = chronic obstructive pulmonary disease, CTGF = connective tissue growth factor, DM = diabetic mellitus, ECMO = extracorporeal membrane oxygenation, HMP = high mechanical power group, HRCT = high-resolution computed tomography, ICU = intensive care unit, LMP = low mechanical power group, MP = mechanical power, PEEP = positive end-expiratory pressure, PPV = prone position ventilation, RM = recruitment maneuver, SAP = severe acute pancreatitis, TGF-β1 = transforming growth factor-β1, VILI = ventilator-induced lung injury.
This work was funded by Lianyungang City Science and Technology Plan Funding Project (SH1601).
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- [1].Maca J, Jor O, Holub M, et al. Past and present ARDS mortality rates: a systematic review. Respir Care 2017;62:113–22. [DOI] [PubMed] [Google Scholar]
- [2].Nieman GF, Satalin J, Andrews P, et al. Personalizing mechanical ventilation according to physiologic parameters to stabilize alveoli and minimize ventilator induced lung injury (VILI). Intensive Care Med Exp 2017;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pais FM, Sinha P, Liu KD, et al. Influence of clinical factors and exclusion criteria on mortality in ards observational studies and randomized controlled trials. Respir Care 2018;63:1060–9. [DOI] [PubMed] [Google Scholar]
- [4].Serpa NA, Deliberato RO, Johnson A, et al. Mechanical power of ventilation is associated with mortality in critically ill patients: an analysis of patients in two observational cohorts. Intensive Care Med 2018;44:1914–22. [DOI] [PubMed] [Google Scholar]
- [5].Tonetti T, Vasques F, Rapetti F, et al. Driving pressure and mechanical power: new targets for VILI prevention. Ann Transl Med 2017;5:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gattinoni L, Marini JJ, Collino F, et al. The future of mechanical ventilation: lessons from the present and the past. Crit Care 2017;21:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McNicholas BA, Rooney GM, Laffey JG. Lessons to learn from epidemiologic studies in ARDS. Curr Opin Crit Care 2018;24:41–8. [DOI] [PubMed] [Google Scholar]
- [8].Souma K, Shichino S, Hashimoto S, et al. Lung fibroblasts express a miR-19a-19b-20a sub-cluster to suppress TGF-beta-associated fibroblast activation in murine pulmonary fibrosis. Sci Rep 2018;8:16642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li X, Yu T, Shan H, et al. lncRNA PFAL promotes lung fibrosis through CTGF by competitively binding miR-18a. FASEB J 2018;32:5285–97. [DOI] [PubMed] [Google Scholar]
- [10].Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526–33. [DOI] [PubMed] [Google Scholar]
- [11].Wang C, Wang X, Chi C, et al. Lung ventilation strategies for acute respiratory distress syndrome: a systematic review and network meta-analysis. Sci Rep 2016;6:22855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Scholten EL, Beitler JR, Prisk GK, et al. Treatment of ARDS with prone positioning. Chest 2017;151:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hoegl S, Burns N, Angulo M, et al. Capturing the multifactorial nature of ARDS - “Two-hit” approach to model murine acute lung injury. Physiol Rep 2018;6:e13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gattinoni L, Tonetti T, Cressoni M, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med 2016;42:1567–75. [DOI] [PubMed] [Google Scholar]
- [15].Ichikado K, Muranaka H, Gushima Y, et al. Fibroproliferative changes on high-resolution CT in the acute respiratory distress syndrome predict mortality and ventilator dependency: a prospective observational cohort study. BMJ Open 2012;2:e000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hu HH, Chen DQ, Wang YN, et al. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem Biol Interact 2018;292:76–83. [DOI] [PubMed] [Google Scholar]
- [17].Chai KX, Chen YQ, Fan PL, et al. STROBE: The correlation of Cyr61, CTGF, and VEGF with polymyositis/dermatomyositis. Medicine (Baltimore) 2018;97:e11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rosenbloom J, Macarak E, Piera-Velazquez S, et al. Human fibrotic diseases: current challenges in fibrosis research. Methods Mol Biol 2017;1627:1–23. [DOI] [PubMed] [Google Scholar]
- [19].Tian Y, Wang Y, Chen W, et al. Role of serum TGF-beta1 level in atrial fibrosis and outcome after catheter ablation for paroxysmal atrial fibrillation. Medicine (Baltimore) 2017;96:e9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Song Y, Yao S, Liu Y, et al. Expression levels of TGF-beta1 and CTGF are associated with the severity of Duchenne muscular dystrophy. Exp Ther Med 2017;13:1209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


