Abstract
Neuroimaging in the context of examining atypical parkinsonian tauopathies is an evolving matter. Positron emission tomography and single photon emission computed tomography (SPECT) bring tools, which may be reasonable in supplementary examination, however, cannot be interpreted as a criterion standard for correct diagnosis. The aim of this observational study was to assess the differentiating potential of perfusion SPECT in 3 types of atypical parkinsonisms: multiple system atrophy parkinsonian type (MSA-P), corticobasal syndrome (CBS), and progressive supranuclear palsy (PSP). The study was carried out using the comparison of standard deviations of perfusion in patients from these 3 groups. Data obtained from 10 patients with clinical diagnosis MSA-P, 14 patients with CBS and 21 patients with PSP, which were analyzed using Tukey honest significant difference post-hoc test, revealed significant differences of perfusion P < .05 between MSA-P and PSP within the cerebellum and thalamus. No significant differences between CBS and PSP were observed.
Keywords: corticobasal degeneration, corticobasal syndrome, multiple system atrophy parkinsonian type, perfusion single photon emission computed tomography, progressive supranuclear palsy
1. Introduction
Atypical parkinsonisms during more than 50 years history have not been associated with any definitive method of examination except histopathological post-mortem analysis. Clinical assessment of the diseases based on recent criteria of the diseases bring often doubtful diagnoses. Neuroimaging is highlighted in recent criteria of progressive supranuclear palsy (PSP), corticobasal syndrome (CBS), and multiple system atrophy-parkinsonian type (MSA-P).[1–3] PSP criteria present neuroimaging as an additional tool. Among possibilities authors present factors demonstrating predominant midbrain atrophy or hypometabolism, which can be observed using magnetic resonance imaging (MRI) and [18]-fluorodeoxyglucose positron emission tomography (PET). Another factor indicated in the criteria is associated with postsynaptic striatal dopaminergic degeneration, which can be observed using iodobenzamide single photon emission computed tomography (SPECT) or [18]-fluoro desmethoxyfallypride PET. CBS criteria show atrophy patterns, which are associated with different pathologies, for example, Alzheimer disease (AD), PSP, and corticobasal degeneration (CBD). Neuroimaging is interpreted as an additional tool possibly differentiating other pathological bases of CBS such as Creutzfeldt-Jakob disease (CJD). MSA criteria show the role of presentation of nigrostriatal denervation using SPECT or PET. Authors indicated differentiation of PD and MSA using sympathetic postganglionic imaging with SPECT. Another aspect of imaging in MSA shows the role of MRI in differentiation using analysis of increased putaminal and middle cerebellar peduncle diffusivity examination.
Recently growing interest was related to new radiotracers in PET. Radiotracers such as fluorodopa and AV-1451 highlighted possible examination of deteriorations within the dopaminergic system or presence of tau protein within the central nervous system. Nevertheless, proper and efficient examination remains unknown. The aim of this research was to assess the role of SPECT with hexamethylpropyleneamine oxime (HMPAO). Only very few articles highlighting the role of perfusion SPECT in the examination PSP, CBS, and MSA-P were published.[1–17]
All of the articles showed the role of perfusion SPECT using of 99mTc-HMPAO as a radiotracer, however, in a different context. 99mTc-HMPAO SPECT/CT was showed in those works as a possible method of examination, which may be interpreted as an additional tool. The aim of this study was to verify whether similar clinical manifestations of the diseases are associated with potential differences in perfusion.
2. Materials and methods
The project was approved by the local Ethical Committee—AKBE243/2016. The mentioned patients gave written informed consent before the study. Written informed consent was obtained from the participants for the publication in a publicly accessible journal.
2.1. Subjects
All patients were recruited in the Department of Neurology between January 2017 and November 2018. Forty-five patients, men and women, were included in the study. Age of the patients varied from 45 to 85 years. Twenty-one patients with clinical diagnosis of PSP, 14 with CBS, and 10 with MSA-P were examined in this study. Criteria of inclusion to indicated groups were based on clinical examination and correlated with recent criteria of diagnosis.[1–3] Patients with relevant coexisting ailments possibly hindering appropriate assessment of the disease were excluded. Among comorbidities excluding patients from examined groups were history of stroke, vascular dementia, and lack of capability of undergoing SPECT examination.
The patients fulfilling the inclusion criteria were subsequently divided into 3 groups:
-
1.
Patients with PSP with clinical manifestations of Richardson-Steele-Olszewski syndrome and PSP resembling idiopathic Parkinson disease (PD). Age of the patients (21 patients: 9 women, 12 men) varied from 57 to 77. Duration of the disease was between 2 and 6 years. The diagnosis of PSP was made according to recent published criteria[1] (Fig. 1, Table 1).
-
2.
Patients with clinical diagnosis of CBS. Age of the patients (14 patients: 12 women, 2 men) varied from 53 to 82 years. Duration of the disease was between 1, 5, and 4 years. Patients were diagnosed according to recent published criteria[2] (Fig. 2, Table 1).
-
3.
Patients with clinical diagnosis of MSA-P. Age of the patients (10 patients: 3 women, 7 men) varied from 45 to 70 years. Duration of the disease was between 3 and 6 years. Patients were diagnosed according to published criteria of diagnosis[3] (Fig. 3, Table 1).
Figure 1.
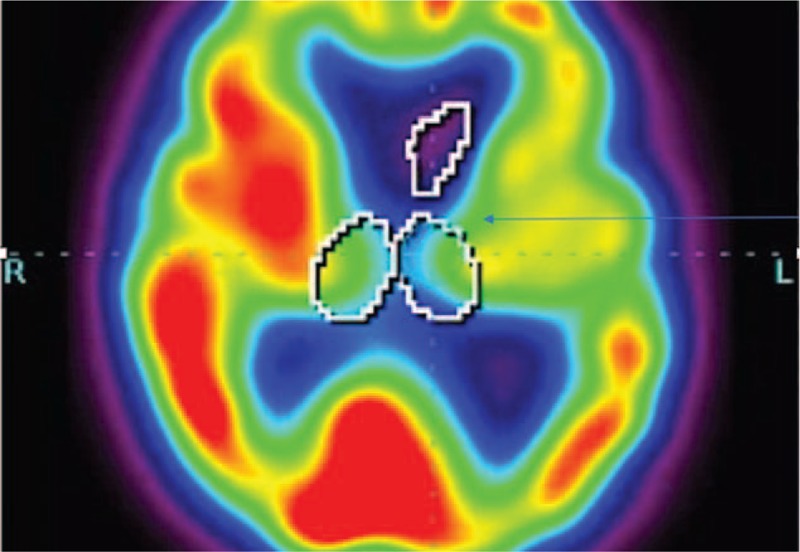
A patient with clinical diagnosis of progressive supranuclear palsy (PSP). Hypoperfusion in the thalami bilaterally.
Table 1.
General information considering patients examined in the study.
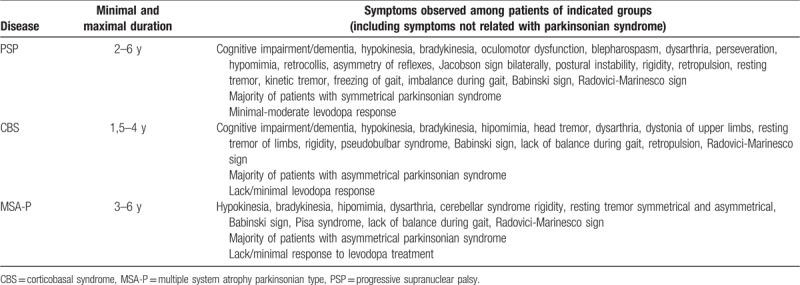
Figure 2.
A patient with clinical diagnosis of corticobasal syndrome (CBS). Hypoperfusion within the left thalamus.
Figure 3.
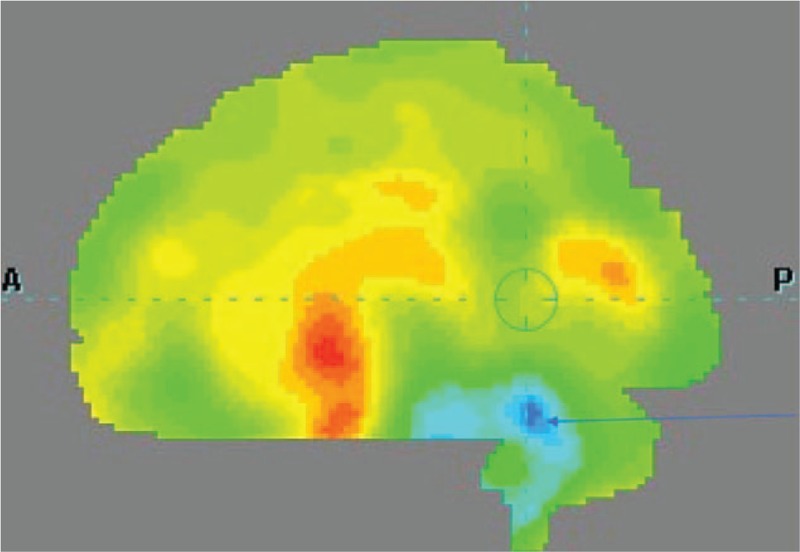
A patient with clinical diagnosis of multiple system atrophy (MSA). Hypoperfusion in the cerebellum.
2.2. Single photon emission computed tomography
All of the mentioned patients were examined using SPECT with Technetium-99m HMPAO (99mTc-HMPAO) as a radiotracer.
All patients were placed in a quiet, dimly lit room in supine position. 740 MBq (0,02 Ci) of technetium-99m hexamethyl propylene amine oxime (99mTc-HMPAO) was administered. All patients underwent brain SPECT/CT scan (Symbia T6, Siemens) on dual-head gamma camera with low energy high-resolution parallel-hole collimator. Step and shoot acquisition mode was used and sequences of 128 frames on a 128 × 128 matrix were obtained (64 projections per head, 30 seconds per projection). The photopeak was set at 140 keV with 10% window on either site of the photopeak. All images were reconstructed with filtered back projection and smoothed with a Butterworth filter. The reconstructed images were corrected for gamma-ray attenuation with measured correction matrix obtained from CT scan.
2.3. Image analysis
Postprocessing analysis was performed with Scenium software (Siemens Medical Solutions USA, Inc. Hoffman Estates, IL). The SPECT images of investigated patients were normalized. The reference database comprised 99mTc-HMPAO brain scans of 20 healthy volunteers with an age range of 64 to 86 years (mixed population of female and male). The number of standard deviations of perfusion from the mean for each voxel was computed. The mean was taken from the corresponding voxel in the normal brain. Statistics were displayed on a voxel-by-voxel basis. The regions of interest (ROIs) were predefined on a high-resolution T1 magnetic resonance imaging volume scan.[18] Mean standard deviation of perfusion was calculated for each ROI.
2.4. Statistical analysis
Statistical analysis was performed with Statistica v. 13. Statistical significance of differences between subgroups of patients in hemispheres of cerebella and thalami perfusion was examined using multivariate analysis of variance (if the data had a normal distribution and homogenous variance) or analysis of variance Kruskal-Wallis test (in the data had not met previous conditions). After that a post-hoc analysis was performed using Tukey honest significant difference test. In all analyses statistical significance was defined as P value <.05.
3. Results
3.1. Statistical results
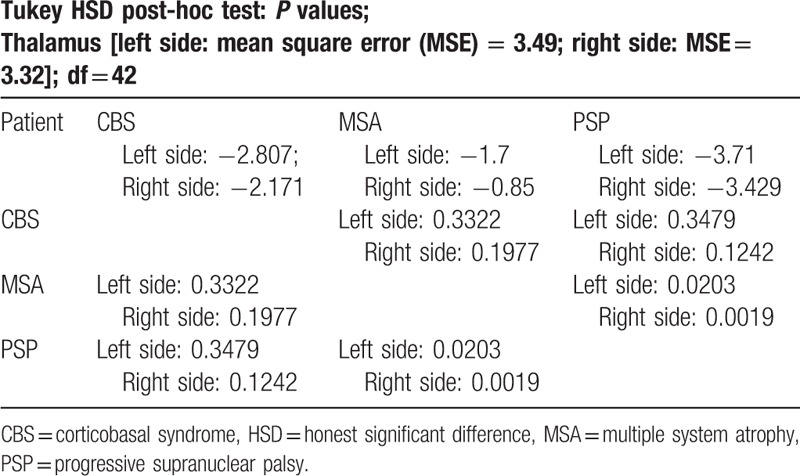
Final results revealed significant differences (P < .05) of perfusion between MSA-P and PSP in the thalami and left hemisphere of cerebellum. A decrease of perfusion compared to healthy volunteers was observed in the cerebellum in MSA-P and in the thalami in PSP (Tables 2–4). No significant differences between PSP and CBS were observed.
Table 2.
MANOVA analysis.
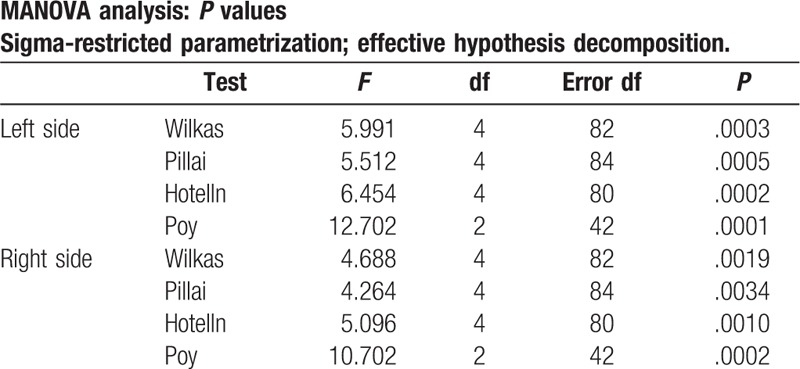
Table 4.
Post-hoc analysis—cerebellum.
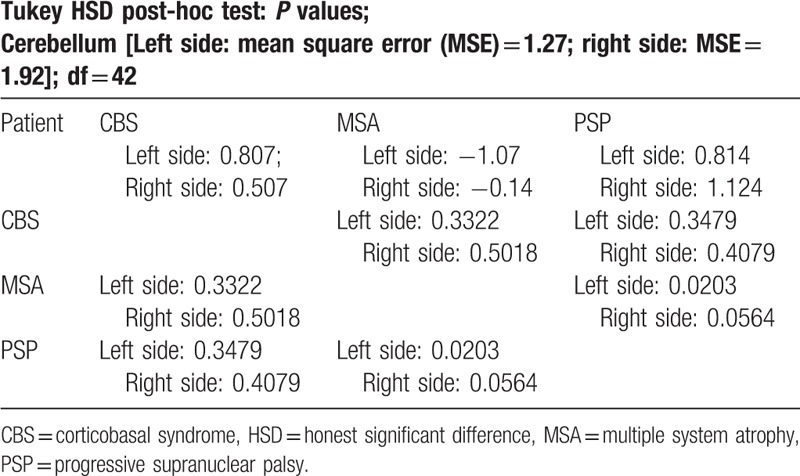
Table 3.
Post-hoc analysis—thalamus.
The differences of perfusion between PSP and MSA-P within the right hemisphere of cerebellum was above the borderline of significance (P > .05), however, relatively close to it, which may suggest that examination based on a larger group of patients may reveal significance in this location.
3.2. Clinical significance of SPECT in the examination of atypical parkinsonism
Our study based on a relatively small group of patients, showed that 99mTc-HMPAO SPECT/CT and the analysis of regions of hypoperfusion using this method, may be interpreted as a possible method of differentiation of atypical parkinsonisms. Significant differences of perfusion were observed only between MSA-P and PSP in the thalami and between MSA-P and PSP. Results of the study suggest that the assessment of the decrease of perfusion within the thalami and cerebellum may be interpreted as a possible additional tool, especially when examining patients with unclear clinical manifestation of parkinsonian syndrome. The outcome of this study showed that potential differentiation of diseases using SPECT should be proceeded by extensive clinical examination. The study did not show any beneficial role of 99mTc-HMPAO SPECT/CT in the differentiation of tauopathic parkinsonism plus. Additional research in the field based on a larger group of patients is required; however, results of this study may be interpreted as an argument in favor of 99mTc-HMPAO SPECT/CT in differentiation of certain atypical parkinsonism.
4. Discussion
This study presented 99mTc-HMPAO SPECT/CT as a possible method of differentiation of parkinsonian plus syndromes. Obtained results showed that analysis of perfusion in the thalami may be beneficial in the assessment of atypical parkinsonism in the context of differentiation between MSA-P and PSP. In addition hypoperfusion in the left hemisphere of cerebellum differentiated MSA-P and PSP. Significantly decreased perfusion of the cerebellum in MSA-P may suggest possible overlapping of MSA-P and cerebellar subtype of MSA.
99mTc-HMPAO SPECT/CT as a method of examination of atypical parkinsonian syndrome was mentioned in a few works. One of the articles evaluated the role of SPECT in the examination of clinical manifestations of frontotemporal dementia.[4] CBS and PSP were some of the manifestations. Authors interpreted SPECT as a possibly useful tool in examination of the diseases.[4] Another study done in 2001 analyzed whether SPECT 99mTc-HMPAO could be helpful in examination of PSP and CBS.[5] Another study presented by Rollin-Slllaire et al[6] showed 48 patients with neurodegenerative or vascular dementia. It revealed sensitivity at the level of 83%, specificity of 87, and accuracy of 85% of 99mTc-HMPAO SPECT/CT in the context of clinical diagnosis of CBS and PSP. The diagnoses were confirmed in neuropathological examination. Another study assessed the role of 99mTc-HMPAO SPECT/CT in the examination of different types of dementia.[7] This work showed a mild decrease in the perfusion of the superior frontal cortex of patients with PSP.[7] A different work showed symmetrical CBS associated with a novel c.314 dup progranulin maturation.[8] In the article authors described a case report of a patient with CBS who underwent 99mTc-HMPAO SPECT/CT examination. 99mTc-HMPAO SPECT/CT revealed symmetric parietal hypoperfusion.[8] Another study showed comparison of patients with CBS. The aim of this work was to compare CBS with underlying AD pathology and CBD.[9] This work showed parietal hypoperfusion in AD patients and frontotemporal hypoperfusion in CBD.[9] MSA in the context of 99mTc-HMPAO SPECT/CT was presented in a work evaluating differentiation of mildly symptomatic idiopathic PD and MSA-P. Authors highlighted hypoperfusion in frontal cortex in PD and MSA-P and decrease of hypoperfusion in occipital lobe in PD with maintained normal perfusion in MSA-P.[10]
To the best of our knowledge, this is the first study examining differences of perfusion in PSP, CBS, and MSA-P at the same time. Authors of the manuscript verified the number of patients with PSP, CBS, or MSA-P examined using perfusion SPECT in other studies. Results of the search revealed 10 with PSP and 8 with CBS in Takaya et al[11] study, 31 patients with either PSP or CBS in Valotassiou et al[4] study, 9 patients with PSP in Harada et al,[12] 21 patients with PSP in Chiu et al[13] study, 24 patients with MSA and 12 patients with PSP in Van Laere et al[14] study, 2 with PSP and 2 with CBS in Sławek et al,[5] 12 with PSP and 12 with CBS in Zhang et al,[15] 5 with PSP and 6 with CBS in Okuda et al,[16] 11 patients with PSP in Johnson et al.[17] This analysis shows that only one of the studies indicated 21 patients with PSP and none of them examined the number of patients with CBS as in this manuscript. Works assessing MSA-P analyzed up to 20 to 30 patients (e.g., Song et al -21)[10]; however, generally the number of patients was much lower (e.g., Takaya et al -15).[11] In conclusion this manuscript analyzes the largest group of patients with the diagnosis of either PSP or CBS assessing them with SPECT and is the first bringing the issue of differentiation of PSP, CBS, and MSA using this method on a total number of 45 patients.
5. Limitations
Authors of the study had to address certain limitations, among which can be mentioned the rarity of the diseases combined with the fact that certain patients were not analyzed in the study due to their lack of consent. Nevertheless the number of examined patients with PSP and CBS was higher than in most papers analyzing the role of SPECT. It should also be stressed that all of the examined patients were alive during the study, which overruled possible neuropathological examination, which is vital in the analysis of atypical parkinsonisms. Nevertheless the main objective which was to verify whether SPECT may be interpreted as a notable tool was partly achieved.
Author contributions
Conceptualization: Piotr Alster.
Data curation: Piotr Alster.
Formal analysis: Piotr Alster, Michał Nieciecki, Dariusz M. Koziorowski.
Investigation: Piotr Alster.
Methodology: Piotr Alster, Andrzej Cacko.
Project administration: Piotr Alster, Ingeborga Charzyńska.
Supervision: Piotr Alster, Michał Nieciecki, Dariusz M. Koziorowski, Leszek Królicki, Andrzej Friedman.
Validation: Piotr Alster.
Visualization: Piotr Alster.
Writing – original draft: Piotr Alster, Michał Nieciecki, Dariusz M. Koziorowski, Leszek Królicki, Andrzej Friedman.
Writing – review and editing: Piotr Alster, Michał Nieciecki, Dariusz Mariusz Koziorowski, Ingeborga Charzyńska, Leszek Królicki, Andrzej Friedman.
Dariusz Mariusz Koziorowski orcid: 0000-0001-8920-8024.
Footnotes
Abbreviations: AD = Alzheimer disease, CBD = corticobasal degeneration, CBS = corticobasal syndrome, CT = computed tomography, HMPAO = hexamethylpropyleneamine oxime, MRI = magnetic resonance imaging, MSA-P = multiple system atrophy parkinsonian type, PET = positron emission tomography, PSP = progressive supranuclear palsy, ROI = region of interest, SPECT = single photon emission computed tomography.
The study was funded using internal funds of the Department of Neurology.
The authors have no conflicts of interest to disclose.
References
- [1].Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 2017;32:853–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Valotassiou V, Papatriantafyllou J, Sifakis N, et al. Brain perfusion SPECT with Brodmann areas analysis in differentiating frontotemporal dementia subtypes. Curr Alzheimer Res 2014;11:941–54. [DOI] [PubMed] [Google Scholar]
- [5].Sławek J, Lass P, Derejko M, et al. Cerebral blood flow SPECT may be helpful in establishing the diagnosis of progressive supranuclear palsy and corticobasal degeneration. Nucl Med Rev Cent East Eur 2001;4:73–6. [PubMed] [Google Scholar]
- [6].Rollin-Sillaire A, Bombois S, Deramecourt V, et al. Contribution of single photon emission computed tomography to the differential diagnosis of dementia in a memory clinic. J Alzheimers Dis 2012;30:833–45. [DOI] [PubMed] [Google Scholar]
- [7].Habert MO, Spampinato U, Mas JL, et al. A comparative technetium 99m hexamethylpropylene amine oxime SPET study in different types of dementia. Eur J Nucl Med 1991;18:3–11. [DOI] [PubMed] [Google Scholar]
- [8].Dopper EG, Seelaar H, Chiu WZ, et al. Symmetrical corticobasal syndrome caused by a novel C.314dup progranulin mutation. J Mol Neurosci 2011;45:354–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hu WT, Rippon GW, Boeve BF, et al. Alzheimer's disease and corticobasal degeneration presenting as corticobasal syndrome. Mov Disord 2009;24:1375–9. [DOI] [PubMed] [Google Scholar]
- [10].Song IU, Yoo I, Chung YA, et al. The value of brain perfusion SPECT for differentiation between mildly symptomatic idiopathic Parkinson's disease and the Parkinson variant of multiple system atrophy. Nucl Med Commun 2015;36:1049–54. [DOI] [PubMed] [Google Scholar]
- [11].Takaya S, Sawamoto N, Okada T, et al. Differential diagnosis of parkinsonian syndromes using dopamine transporter and perfusion SPECT. Parkinsonism Relat Disord 2018;47:15–21. [DOI] [PubMed] [Google Scholar]
- [12].Harada K, Saeki H, Matsuya E, et al. Detection of cerebral hypoperfusion using single photon emission computed tomography image analysis and statistical parametric mapping in patients with Parkinson's disease or progressive supranuclear palsy [in Japanese]. Nihon Hoshasen Gijutsu Gakkai Zasshi 2013;69:1281–4. [DOI] [PubMed] [Google Scholar]
- [13].Chiu WZ, Papma JM, De Koning I, et al. Midcingulate involvement in progressive supranuclear palsy and tau positive frontotemporal dementia. J Neurol Neurosurg Psychiatry 2012;83:910–5. [DOI] [PubMed] [Google Scholar]
- [14].Van Laere K, Casteels C, De Ceuninck L, et al. Dual-tracer dopamine transporter and perfusion SPECT in differential diagnosis of parkinsonism using template-based discriminant analysis. J Nucl Med 2006;47:384–92. [PubMed] [Google Scholar]
- [15].Zhang L, Murata Y, Ishida R, et al. Differentiating between progressive supranuclear palsy and corticobasal degeneration by brain perfusion SPET. Nucl Med Commun 2001;22:767–72. [DOI] [PubMed] [Google Scholar]
- [16].Okuda B, Tachibana H, Kawabata K, et al. Cerebral blood flow in corticobasal degeneration and progressive supranuclear palsy. Alzheimer Dis Assoc Disord 2000;14:46–52. [DOI] [PubMed] [Google Scholar]
- [17].Johnson KA, Sperling RA, Holman BL, et al. Cerebral perfusion in progressive supranuclear palsy. J Nucl Med 1992;33:704–9. [PubMed] [Google Scholar]
- [18].Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single- subject brain. Neuroimage 2002;15:273–89. [DOI] [PubMed] [Google Scholar]


