Abstract
Automated synthesis of a radiopharmaceutical 3-((2-fluoro-4-(5-(2′-methyl-2-(trifluoromethyl)-[1,1′-biphenyl]-4-yl)-1,2,4-oxadiazol-3-yl)benzyl)(methyl-11C)amino)propanoic acid ([11C]CS1P1) for PET imaging sphingosine-1 phosphate receptor 1 (S1P1) was accomplished by a two-step-one-pot procedure in a Siemens CTI methylation automated module using TR-19 cyclotron. The synthesis of [11C]CS1P1 was successfully validated under current Good Manufacturing Practices (cGMP) conditions, resulting in a consistent average radiochemical yield of ~ 15%, molar activity of ~ 3129 GBq/μmol (decay corrected to end of bombardment, EOB), and radiochemical purity > 95%. The radiopharmaceutical product meets all quality control criteria for human use for an Investigational New Drug (IND) application to permit human studies.
Keywords: Positron emission tomography, Sphingosine-1 phosphate receptor 1, Automation, Radiopharmaceutical, [11C]CS1P1
1. Introduction
Sphingosine-1-phosphate receptor 1 (S1P1), originally named as endothelial differentiation gene 1 (EDG1) is a protein encoded by the S1P1 gene in humans. It belongs to a sphingosine-1-phosphate receptor family, which has five subtypes (S1P1-5). S1P1 plays an important role in regulating vascular and lymphatic systems, immune system, central nervous system (CNS), and oncogenesis by binding with a bioactive signaling molecule sphingosine 1-phosphate (S1P) (Allende et al., 2003; Garris et al., 2013; Lee et al., 1999; Matloubian et al., 2004; Pham et al., 2008; Rutherford et al., 2013). Interest in S1P1 has increased with the recent approval by the USA Food and Drug Administration (FDA) of the first orally administered S1P receptor-targeted drug for multiple sclerosis (MS). This drug, FTY720 (Fingolimod), a sphingosine analogue, acts as an S1P1 modulator after phosphorylation by sphingosine kinase in vivo, in which it engages the S1P1, leading to internalization and subsequent degradation of the S1P1, thereby leading to lymphocyte sequestration in lymph nodes. This prevents them from moving into the CNS and causing a relapse of MS (Brinkmann, 2009; Cohen and Chun, 2011). The success of treating MS using FTY720 has triggered abundant research efforts to investigate the role of S1P1 in the pathogenesis and progression of MS and other inflammatory diseases (Camp et al., 2015; Choi et al., 2011; Gonzalez-Cabrera et al., 2012; Li et al., 2016; Liu et al., 2018).
Positron emission tomography (PET) provides a unique non-invasive medical imaging technique to visualize, characterize, and measure the biological processes in humans and other living subjects. It has become an indispensable tool for many diseases in oncology, cardiology, and neurology. Using suitable radiotracers, PET is used widely in preclinical and clinical investigations to directly quantify the expression of target proteins in vivo for guiding the therapeutic drug development (Das, 2015). In our preclinical research, we developed and evaluated a promising C-11 radiolabeled radiotracer, 3-((2-fluoro-4-(5-(2′-methyl-2-(trifluoromethyl)-[1,1′-biphenyl]-4-yl)-1,2,4-oxadiazol-3-yl)benzyl)(methyl-11C)amino)propanoic acid ([11C]CS1P1) targeting S1P1. In vivo animal studies demonstrated that [11C]CS1P1 has the capability of imaging S1P1 expression that reflects the inflammatory response in three different models of inflammatory diseases including MS, neointimal hyperplasia, and vascular inflammation (Jin et al., 2017; Liu et al., 2016; 2017; 2018). To explore the clinical utilization of [11C]CS1P1 for human use, [11C]CS1P1 needs to be prepared under current Good Manufacturing Practices (cGMP) using an automated synthesis module to meet FDA regulation of radiopharmaceutical. Herein, we report our efforts on the automated production of [11C]CS1P1 in our institutional cGMP facilities.
2. Materials and methods
2.1. General
Unless otherwise indicated, all the chemicals and solvents were purchased from Sigma-Aldrich (St. Louis, MO). The reference standard and precursor for [11C]CS1P1 were prepared according to our published procedure (Jin et al., 2017). Sep-Pak® C18 plus light cartridge (WAT023501) and plus short cartridge (WAT020515) were purchased from Waters (Milford, MA). 0.9% Sodium chloride USP and sterile water USP were purchased from Hospira (Lake Forest, IL). All the chemicals and consumables were received and meet the requirement of FDA regulations for preparing [11C]CS1P1 used in human studies.
No-carrier added [11C]CO2 was produced through Washington University, Mallinckrodt Institute of Radiology TR19 cyclotron (Advanced Cyclotron Systems, Richmond, BC, Canada) from the 14N(p,α)11C nuclear reaction with proton bombardment of an aluminum target filled with 0.5% O2 in nitrogen gas. The irradiation was achieved with a beam of 18.0 MeV at current of 40 μA for 25 min. After the irradiation was finished, the [11C]CO2 was shunted to the GE TracerLab FX Mel module to generate reactive [11C]methyl triflate ([11C]CH3OTf) for tracer production. Briefly, the [11C]CO2 was trapped in molecular sieves and further reduced to [11C]CH4 in the presence of Ni catalyst and hydrogen at 350 °C. After removal of unreacted CO2 and H2O through passing a double ascarite/sicapent trap, the [11C]CH4 was concentrated and purified by trapping in a carbosphere column. Once the [11C]CH4 was released, it reacted with iodine at 740 °C to produce [11C]CH3I in a recirculation loop. The [11C]CH3I was trapped in a Porapak-Q column and released by heating to 190 °C in a stream of helium (20 mL/min). The released [11C]CH3I was further passed through a heated tube (190 °C) containing graphitised carbon impregnated with silver triflate to generate the [11C]CH3OTf.
2.2. Automated radiosynthesis of [11C]CS1P1
The radiosynthesis route of [11C]CS1P1 was shown in Scheme 1 and the automated production carried out in a Siemens CTI methylation automated module (Figure 2). Before starting the production, each vial was loaded with reagents as follows. The reactor: 1.5 mg of precursor, tert-butyl 3-((2-fluoro-4-(5-(2′-methyl-2-(trifluoromethyl)-[1,1′-biphenyl]-4-yl)-1,2,4-oxadiazol-3-yl)benzyl)amino)propanoate in anhydrous acetonitrile (300 μL); vial #1: trifluoroacetic acid (250 μL); vial #2: 51% acetonitrile in 0.1 M ammonium formate buffer, pH 4.5 (650 μL) and 5 N NaOH (200 μL). The [11C]CH3OTf was delivered to the reaction module with a helium flow rate of 20 mL/min. Upon completion of delivery, the reaction vessel was heated to 70 °C for 5 min. Then, trifluoroacetic acid (250 μL) in vial #1 was added to the reaction vessel by N2 flow for 20 seconds, followed by heating to 70 °C again for another 5 min. At last, the quench reagent in vial #2 was added to the reaction vessel and the reaction mixture was loaded onto a C-18 semi-preparative column (Phenomnenex Luna® 5 μm C18(2) 100 Å, 250 × 10 mm) for HPLC purification. The HPLC system consisted of a 5 mL injection loop, 51% acetonitrile in 0.1 M ammonium formate buffer (pH 4.5) as a mobile phase, a flow rate of 4.0 mL/min; and UV wavelength of 254 nm. The radioactive product peak fraction was collected from 19-21 min into a collection vial containing 60 mL of sterile water. Then the collection vial was pressurized by activating V07 to push the product solution out of the collection vial to pass through a Sep-Pak C18 plus light cartridge (WAT023501). [11C]CS1P1 was trapped on the C18 cartridge and the water containing the mobile phase was passed through to the waste bottle in the hot cell product drawer. Once all the water with the product passed through the C18 cartridge and into the waste bottle, V07 was deactivated.
Scheme 1.
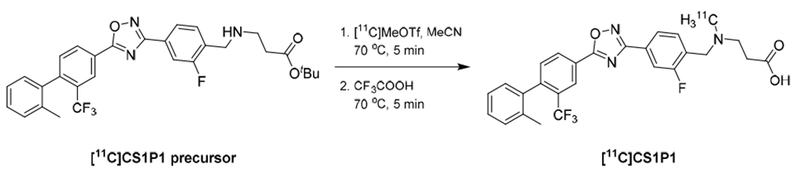
The radiosynthesis of [11C]CS1P1 using the two-step-one-pot procedure.
Figure 2.
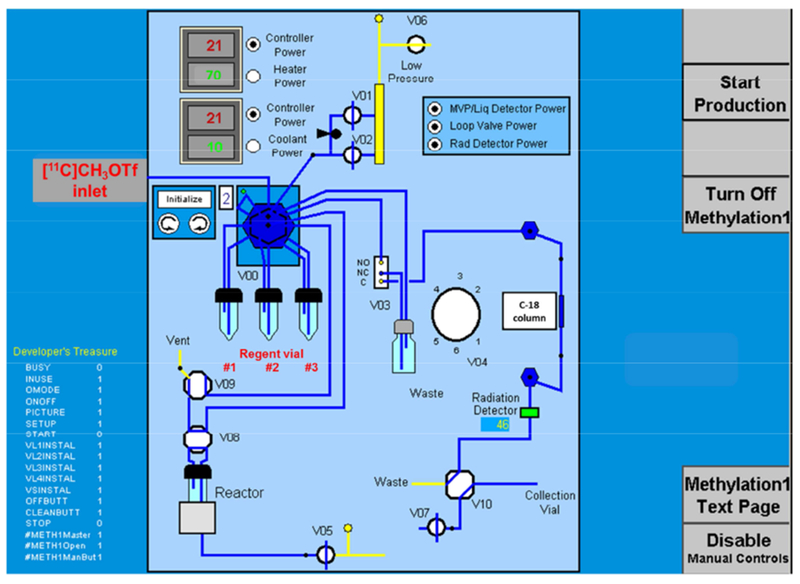
Scheme of the CTI methylation tubings and valves configuration for the synthesis of [11C]CS1P1
2.3. Dose formulation of [11C]CS1P1
The dose formulation of [11C]CS1P1 was accomplished as shown in Figure 3. From the rinse line, the C18 cartridge with the product was flushed with 20 mL of sterile water by manually switching 3-way valve #1 attached to the C18 cartridge to get rid of the residual acetonitrile and ammonium formate. Additional air was pushed through to remove the water residue. Then, 3-way valve #2 attached to the output of the C-18 cartridge was manually switched from waste to the pre-formulation vial. The product [11C]CS1P1 was eluted from C-18 cartridge to the pre-formulation vial using 1.0 mL of absolute ethanol followed with another 1.0 mL of 0.9% saline. Additional air was push through to remove the liquid residue. Finally, the pre-formulation vial was shaken to mix the solution and a spinal needle was inserted down to the bottom of the vial. The content in the pre-formulation vial was pressurized through the sterilizing filter (Millipore GV Filter, 0.22 μm, 13 mm diameter) into the final product vial (FPV) preloaded with aseptic 9.0 mL of 0.9% saline to give the final dose.
Figure 3.
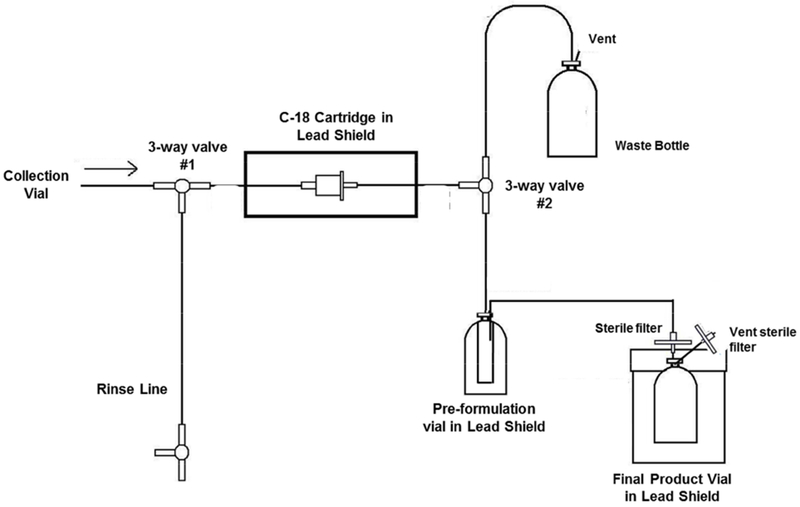
C-18 trap and release cartridge setup diagram
2.4. Quality control of radiopharmaceutical [11C]CS1P1
Conventional methodology and requirement for radiopharmaceutical as described in the United States Pharmacopoeia was employed for [11C]CS1P1 to ensure that the [11C]CS1P1 met acceptance specifications for clinical use.
2.4.1. Visual inspection and pH value
Doses of [11C]CS1P1 were examined visually and needed to be clear, colorless, and free of particulate matter. The pH value was confirmed by applying a small amount of the formulated product onto MACHEREY-NAGEL pH test strips (pH-Fix 3.1-8.3) and determined by comparison with the scale provided.
2.4.2. Analytic HPLC analysis
Radiochemical and chemical purities were determined using an analytic HPLC on Dionex U3000 system equipped with a Bioscan radioactivity-HPLC-flow detector under the following conditions: Agilent Eclipse XDB-C18, 5 μm, 250 × 4.6 mm, 60% acetonitrile in ammonium formate buffer (0.1 M, pH = 4.5) as mobile phase, flow rate at 1.5 mL/min, UV wavelength at 254 nm, and 20 μL of injection volume.
2.4.3. Residual solvent analysis
Analysis for residual organic solvents including acetonitrile and ethanol was carried out using an Agilent 7890B GC (Agilent Technologies Inc., USA) equipped with a headspace auto-injector, split/splitless inlet, a flame ionization detector (FID), and a capillary column (length 30 m, ID 0.520 mm, DB-WAX 1.0 μm, Agilent Technologies lnc.,USA).
2.4.4. Endotoxin and sterility testing
Apyrogenicity (Charles River Laboratories Inc., USA) tests were performed to ensure that doses of [11C]CS1P1 contained < 175 endotoxin units (EU) per maximum total dose. The sterility testing was performed via direct inoculation into growth media.
2.4.5. Filter integrity testing
Final filter membrane integrity test was performed by a standard bubble-point method (Hayashi et al., 2014). After completing the synthesis of [11C]CS1P1, the 0.22 μm membrane filter (Millipore GV Filter) was removed from C-18 trap and release cartridge setup diagram and attached a needle, which was pierced into a sealed vial filling with water. Adjust the nitrogen gas flow rate through a pressure gauge from another end of the filter. As pressure is applied, record the pressure reading when a continuous stream of bubbles is formed in water.
2.4.6. Radionuclidic identity
Radionuclidic purity was confirmed by half-life determination of the [11C]CS1P1 using a Capintec CRC-15R Radioisotope Dose Calibrator. The half-life of the sample was calculated from the averaged slope and compared with the known theoretical half-life of carbon-11 (t1/2 = 20.3 min).
2.4.7. Stability testing
The 1 h stability testing of the [11C]CS1P1 was performed to check the radiochemical purity and pH value using the above-described methods.
3. Results and discussion
3.1. Automated radiosynthesis of [11C]CS1P1
A two-step-one-pot procedure was followed for the automated production of GMP quality [11C]CS1P1. The N-[11C]methylation of the tert-butyl protected precursor was accomplished using [11C]CH3OTf followed by in situ deprotection of tert-butyl group with trifluoroacetic acid (TFA). The originally reported procedure (Jin et al., 2017) was optimized accordingly after transferring to the automated methylation module. In the optimized procedure, the amount of TFA was increased to 250 μL because using 100 μL of TFA in the automated module led to a low yield due to the unhydrolyzed [11C]intermediate in the reaction mixture, whereas 250 μL of TFA was able to completely hydrolyze the [11C]intermediate. This difference was due to the greater dead volume in the module than the previously reported manually-controlled procedure. The crude product was purified using a semipreparative C-18 column, and the product peak was eluted between 19-21 min (Figure 4). A saturated UV peak at 13-17 min was confirmed as 3-((2-fluoro-4-(5-(2′-methyl-2-(trifluoromethyl)-[1,1′-biphenyl]-4-yl)-1,2,4-oxadiazol-3-yl)benzyl)-amino)propanoic acid, the deprotection of tert-butyl group of precursor having a same retention time. The total synthesis time was ~ 50 min, including the trapping of [11C]CH3OTf, the reaction, HPLC purification, and dose formulation. When no-carrier added [11C]CO2 was produced from TR19 cyclotron, the average decay-corrected (EOB) radioactivity of [11C]CS1P1 product was ~ 13.9 GBq with molar activity of ~ 3129 GBq/μmol (Table 1, validations 1-3). Additionally, a Siemens cyclotron, RDS-111 (CTI, Knoxville, TN, now Siemens Medical Solutions) is a compact, dual beam, 11 MeV, proton-only accelerator which has lower energy extraction than the TR-19. Generally, when no-carrier added [11C]CO2 was produced by RDS-111 cyclotron with 60 μA beam current and 45 min bombard, it was able to produce 14.6 GBq of [11C]CS1P1 at 1172 GBq/μmol of molar activity (EOB). Although the molar activity is slightly lower from the RDS-111 cyclotron than that from the TR-19 cyclotron, the quality control of the product still meets our release specifications (Table 1, validation 4) for human use. The capability of Siemens cyclotron, RDS-111 producing [11C]CS1P1 provides flexibility within our cGMP facilities for delivering radioactive [11C]CS1P1 doses for clinical use.
Figure 4.
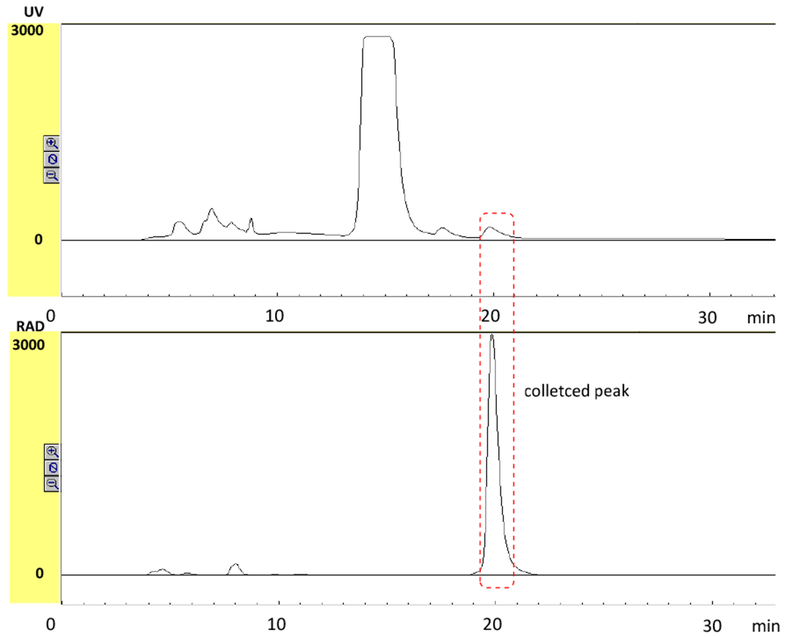
Representative HPLC chromatogram for separating [11C]CS1P1. Top: UV absorbance at 254 nm; Bottom: radioactivity detection. The retention time of the product was 19-21 min.
Table 1.
[11C]CS1P1 specifications and validation data
| Quality control test | Release criteria | Validation 1 | Validation 2 | Validation 3 | Validation 4d |
|---|---|---|---|---|---|
| Product activity | |||||
| (GBq, decay corrected to EOB) | > 5.0 | 16.2 | 12.5 | 12.9 | 14.6 |
| Appearancea | Clear, particle-free and colorless | Pass | Pass | Pass | Pass |
| pH | 4.5 – 7.5 | 5.0 | 5.0 | 5.0 | 5.0 |
| Filter integrity | Meet manufacturer’s requirement | Pass | Pass | Pass | Pass |
| Radionuclidic identity | 19.32 – 21.35 min | 20.35 min | 20.36 min | 20.36 min | 20.35 min |
| Radiochemical identityb | Retention time of [11C]CS1P1 and injected CS1P1 must be within ± 10% | Pass | Pass | Pass | Pass |
| Radiochemical purityb | ≥ 90% | 96.6% | 100% | 100% | 98.2% |
| Radiochemical stability | Purity > 90% at 1.0 h | Pass | Pass | Pass | Pass |
| Molar activity (GBq/μmol, decay corrected to EOB) | > 500 | 2289 | 1818 | 5281 | 1172 |
| Chemical purity: Mass of CS1P1 | ≤ 10.0 μg/injected dose | 3.63 μg/total volume | 3.53 μg/total volume | 1.25 μg/total volume | 6.40 μg/total volume |
| Total unidentified mass impurities | All to be ≤ 1.0 μg/injected dose | 0.50 μg/total volume | 0.41 μg/total volume | 0.65 μg/total volume | 0.21 μg/total volume |
| Residual solvent: acetonitrilec | ≤ 0.41 μg/mL (4.1 μg/day max) | < 0.050 μg/mL | < 0.050 μg/mL | < 0.050 μg/mL | < 0.050 μg/mL |
| Residual solvent: ethanolc | ≤ 100 μg/mL | 85.8 μg/mL | 72.4 μg/mL | 67.5 μg/mL | 72.1 μg/mL |
| Bacterial endotoxin | ≤ 175 EU/total dose | < 46.5 EU | < 53.5 EU | < 52.0 EU | < 48.0 EU |
| Sterility test | No visible microorganism growth | Pass | Pass | Pass | Pass |
Visual inspection for color and particulates;
determined by HPLC;
determined by GC;
[11C]CS1P1 was synthesized using the [11C]CO2 generated from the RDS-111 cyclotron with bombardment of 45 min at beam of 60 μA.
3.2. Dose formulation of [11C]CS1P1
To maximize radioactive product recovery from the Sep-Pak® C-18 cartridge, different C-18 cartridges including Sep-Pak® plus short (360 mg of sorbent) and Sep-Pak® plus light (130 mg of sorbent) were used and compared. Both cartridges have similar trapping efficiencies that collecting ~ 99% of radioactive product collected from HPLC collections. However, after eluting the radioactive product from the C-18 cartridge into a pre-formulation vial using 1.0 mL of ethanol, and followed by 1.0 mL of 0.9% saline, the recovery yield was ~ 97% from the Sep-Pak® plus light cartridge, and ~ 90% from the Sep-Pak® plus short cartridge. The Sep-Pak® plus light cartridge offered higher recovery yield for [11C]CS1P1 and a shorter elution time. Therefore, for our standard protocol, the Sep-Pak® plus light cartridge was selected for solid phase extraction (SPE) for [11C]CS1P1. The ~ 2.0 mL of pre-formulated solution was passed through a sterile filter to the FPV preloading with 9.0 mL of sterile 0.9% saline to afford the final dose.
To select a suitable sterile filter to reduce the activity lost in the filter, we tested the recovery efficiency of different sterile filters including Supor® Membrane 0.2 μm (B. Braun Medical Inc.), Millex®-GS 0.22 μm (Millipore Ltd.), Millex®-GV 0.22 μm, 33 mm diameter (Millipore Ltd.), and Millex®-GV 0.22 μm, 13 mm diameter (Millipore Ltd.). It was observed that most of the filters showed very low filtration efficiency (< 5%) except the Millex®-GV filters. The filtration efficiency showed ~60% from Millex-GV® filter (33 mm diameter) and ~90% from Millex-GV® filter (13 mm diameter). Therefore, Millex-GV® filter (13 mm diameter) was selected for the production of [11C]CS1P1.
3.3. Quality control of radiopharmaceutical [11C]CS1P1
Quality control protocols for radiopharmaceutical [11C]CS1P1 were established according to the USP guidelines. For an exploratory Investigational New Drug application, three successful consecutive syntheses (Validation 1-3) were completed to validate the synthetic procedure to produce [11C]CS1P1 in our cGMP facility. The results are summarized in Table 1. Quality control of [11C]CS1P1 demonstrated that [11C]CS1P1 could be consistently manufactured in the CTI methylation module, and the product [11C]CS1P1 was able to meet the specifications for clinical utility. The product was clear, colorless, and free of particulate matter by visual inspection. The pH test strips indicated the pH value was 5.0, and the test result of filter membrane integrity met the manufacturer’s pressure requirement. The radionuclidic identity was confirmed by half-life measurement of t1/2 = 20.36 min and the radioactive [11C]CS1P1 was identified by injection of cold standard CS1P1 onto analytical HPLC with retention time within ± 10% (Figure 5). Analytical HPLC result showed that the radiochemical purity was > 95% for all batches at EOS (Figure 5), and no change on chemical and radiochemical purity was observed after 1 h, suggesting [11C]CS1P1 is stable for at least 1 h after synthesis. The total average mass of three batches (validations 1-3) of non-radioactive CS1P1 was 2.80 ± 1.35 μg per synthesized dose and the mass of unknown impurities was 0.52 ± 0.12 μg. Together, the total mass of each batch production was far less than 10 μg, the maximum mass allowed to inject into the patient. The residual solvent analysis was determined by gas chromatography, indicated that the acetonitrile concentration was less than 0.050 mg/mL and for the ethanol was 75.2 ± 9.5 mg/mL; both were below the limit of USP recommendations. Endotoxin testing results indicated that the bacterial endotoxins were all below the regulatory maximum of 175 endotoxin units/total dose. The sterility testing results indicated that the radiopharmaceutical [11C]CS1P1 was sterile post-filtration.
Figure 5.
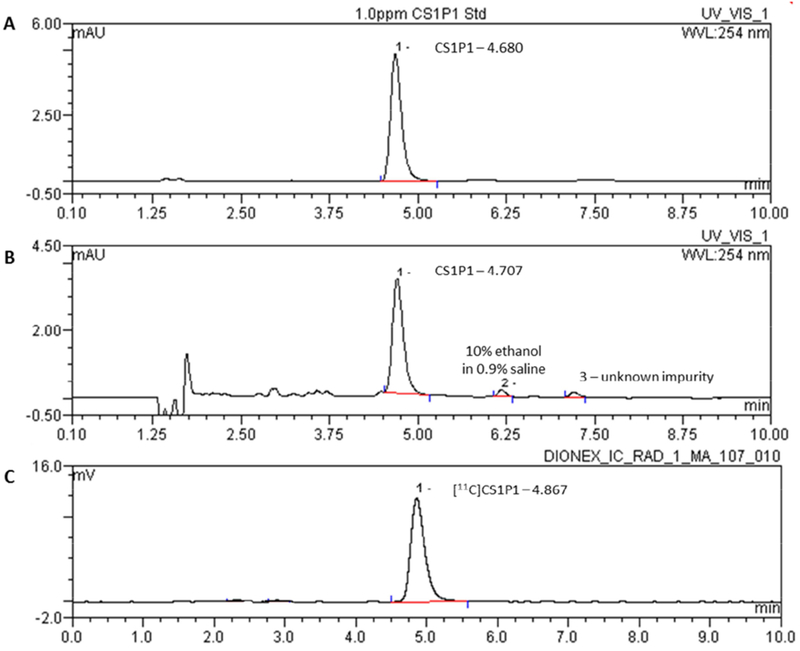
Representative analytical HPLC chromatogram of CS1P1 and formulated [11C]CS1P1. A: Reference standard CS1P1 at the concentration of 1.0 ppm; B: UV trace of formulated [11C]CS1P1 at 254 nm; C: radioactive trace of formulated [11C]CS1P1.
4. Conclusion
We have successfully developed a dependable procedure in our cGMP facilities for automated production of [11C]CS1P1, a radiotracer targeting S1P1. The production of [11C]CS1P1 was accomplished following a two-step-one-pot procedure in approximately 60 min with high radiochemical purity (> 95%) and molar activity ~ 3129 GBq/μmol, EOB). The total mass of the CS1P1 per batch was found to be far less than 10 μg. The chemical and radiochemical purities of [11C]CS1P1 don’t change after 1 h, indicating the dose of [11C]CS1P1 is stable up to 1 h post-production. The quality control of three consecutive validation tests was accomplished, and the final product doses met all release criteria of [11C]CS1P1 for human use. These methods can be used to submit for the necessary regulatory approvals to produce [11C]CS1P1 for use in human subjects.
Figure 1.
Structure of FTY720 and [11C]CS1P1
Automated production of [11C]CS1P1 was performed by a two-step-one-pot procedure under cGMP conditions.
[11C]CS1P1 was achieved with high molar activity and radiochemical purity.
The procedure can be applied for a routine production of [11C]CS1P1.
The radiopharmaceutical [11C]CS1P1 meets all quality control criteria for human use.
Acknowledgements
This work was supported by the National Institutes of Health, United States [NS103988 and EB025815]. We thank Nerissa Brame-Torrey and Reiko Oyama for their assistance with the quality control of this tracer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allende ML, Yamashita T, Proia RL, 2003. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 102, 3665–3667. 10.1182/blood-2003-02-0460. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, 2009. FTY720 (fingolimod) in Multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br. J. Pharmacol. 158, 1173–1182. 10.1111/j.1476-5381.2009.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp SM, Chiang ET, Sun C, Usatyuk PV, Bittman R, Natarajan V, Garcia JGN, Dudek SM, 2015. Pulmonary endothelial cell barrier enhancement by novel FTY720 analogs: methoxy-FTY720, fluoro-FTY720, and β-glucuronide-FTY720. Chem. Phys. Lipids 191, 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, Chun J, 2011. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc. Natl. Acad. Sci. U.S.A. 108, 751–756. 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Chun J, 2011. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann. Neurol. 69, 759–777. 10.1002/ana.22426. [DOI] [PubMed] [Google Scholar]
- Das BK, 2015. Development of positron emission tomography (PET): a historical perspective, in: Das BK (Ed.), Positron Emission Tomography: A Guide for Clinicians. Springer; India, New Delhi, pp. 7–11. [Google Scholar]
- Garris CS, Wu L, Acharya S, Arac A, Blaho VA, Huang Y, Moon BS, Axtell RC, Ho PP, Steinberg GK, Lewis DB, Sobel RA, Han DK, Steinman L, Snyder MP, Hla T, Han MH, 2013. Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat. Immunol. 14, 1166–1172. 10.1038/ni.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cabrera PJ, Cahalan SM, Nguyen N, Sarkisyan G, Leaf NB, Cameron MD, Kago T, Rosen H, 2012. S1P(1) receptor modulation with cyclical recovery from lymphopenia ameliorates mouse model of multiple sclerosis. Mol. Pharmacol. 81, 166–174. 10.1124/mol.111.076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Douhara K, Kashino G, 2014. Evaluation of the bubble point test of a 0.22-μm membrane filter used for the sterilizing filtration of PET radiopharmaceuticals. Ann. Nucl. Med. 28, 586–592. 10.1007/s12149-014-0830-0. [DOI] [PubMed] [Google Scholar]
- Jin H, Yang H, Liu H, Zhang Y, Zhang X, Rosenberg AJ, Liu Y, Lapi SE, Tu Z, 2017. A promising carbon-11-labeled sphingosine-1-phosphate receptor 1-specific PET tracer for imaging vascular injury. J. Nucl. Cardiol. 24, 558–570. 10.1007/s12350-015-0391-1. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha’afi RI, Hla T, 1999. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99, 301–312. 10.1016/S0092-8674(00)81661-X. [DOI] [PubMed] [Google Scholar]
- Li Y, Xie P, Sun M, Xiang B, Kang Y, Gao P, Zhu W, Ning Z, Ren T, 2016. S1PR1 expression correlates with inflammatory responses to Newcastle disease virus infection. Infect., Genet. Evol. 37, 37–42. [DOI] [PubMed] [Google Scholar]
- Liu H, Jin H, Han J, Yue X, Yang H, Zayed MA, Gropler RJ, Tu Z, 2018. Upregulated sphingosine 1-phosphate receptor 1 expression in human and murine atherosclerotic plaques. Mol. Imaging Biol. 20, 448–456. 10.1007/s11307-017-1141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Jin H, Yue X, Han J, Baum P, Abendschein DR, Tu Z, 2017. PET study of sphingosine-1-phosphate receptor 1 expression in response to vascular inflammation in a rat model of carotid injury. Mol. Imaging 16, 1–7. 10.1177/1536012116689770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Jin H, Yue X, Luo Z, Liu C, Rosenberg AJ, Tu Z, 2016. PET imaging study of S1PR1 expression in a rat model of multiple sclerosis. Mol. Imaging Biol. 18, 724–732. 10.1007/s11307-016-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG, 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360. 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG, 2008. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity 28, 122–133. 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford C, Childs S, Ohotski J, McGlynn L, Riddick M, MacFarlane S, Tasker D, Pyne S, Pyne NJ, Edwards J, Palmer TM, 2013. Regulation of cell survival by sphingosine-1-phosphate receptor S1P1 via reciprocal ERK-dependent suppression of Bim and PI-3-kinase/protein kinase C-mediated upregulation of Mcl-1. Cell Death Dis. 4, e927 10.1038/cddis.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]


