Abstract
Few long-term studies have assessed whether changes in both diet and exercise can improve the health and quality of life of hemodialysis patients. Here we examined whether 12 months of intradialytic protein supplementation and endurance exercise improves physical function, risk of cardiovascular disease, and quality of life in hemodialysis patients in a randomized controlled trial. A total of 138 hemodialysis patients (average age 58 years) were assigned for 12 months to control, intradialytic protein, or protein plus exercise groups. The protein and protein plus exercise groups consumed an oral protein supplement (30 grams of whey) three days/week during dialysis. The protein plus exercise group cycled for 30–45 minutes during dialysis treatment. The primary outcome was change in physical function at 12 months, assessed by a shuttle walk test. Secondary outcomes included arterial stiffness, blood pressure, body composition, muscle strength, markers of nutritional status, and quality of life. Assessments were conducted at baseline, 6 and 12 months. In total, 101 patients completed the intervention. There were no significant differences between groups in shuttle walk test performance from baseline to 12 months. There were trends for improvements in some secondary measures of physical function and strength in the protein and protein plus exercise groups at six or 12 months, but these did not reach statistical significance. Thus, our trial did not demonstrate significant improvements in markers of physical function, risk of cardiovascular disease or quality of life after one year of intradialytic oral protein supplementation and aerobic exercise training. More comprehensive lifestyle management may be needed to uncover robust improvements in the health and quality of life of hemodialysis patients.
Keywords: Nutrition, Exercise, Physical Function, Arterial Stiffness, Hemodialysis
Graphical Abstract
INTRODUCTION
Patients with end-stage kidney disease undergoing chronic hemodialysis (HD) often suffer from multiple comorbidities, including protein-energy wasting (PEW) and cardiovascular disease (CVD), that contribute to a poor quality of life (QOL) and excessive mortality rates.1 The etiology of these comorbidities are multifactorial and complex, but both nutritional factors and a sedentary lifestyle may contribute to their development and progression.
Low intake of dietary protein and energy are associated with poor outcomes in HD patients2, 3. Oral nutrition supplements (ONS) improve nutritional status,4–7 and observational studies suggest reduced mortality from ONS in HD patients.8–10 Among ONS, intradialytic oral protein supplementation (OPS) is a particularly efficient and cost-effective intervention,10–12 However, the efficacy of intradialytic OPS has been explored in only a few short-term RCTs,11–16 and even fewer have been conducted in patients with normal nutritional status (albumin > 3.5 g/L).12
Numerous studies also have demonstrated benefits of exercise training in HD patients, including improvements in physical function and markers of CVD risk.17–20 However, most of these studies had small sample sizes, short intervention periods, or lacked adequate control groups.19 They also differ in many regards, including the frequency, intensity, type (resistance or aerobic exercise), and timing of exercise (intradialytic vs out-of-center). Recently, several longer-term RCTs with larger sample sizes have been published.21–27 While some of the results continue to support the position that exercise training of any variety improves physical function and CVD risk, the benefits have generally been modest or inconsistent. The lack of robust benefits in these trials may be contributing to the low implementation of exercise programs in HD clinics.
Due to the complex etiology of comorbidities, combinations of therapies are likely needed to improve the health and QOL of HD patients. Recently, several studies demonstrated that intradialytic nutritional supplementation acutely increased skeletal muscle amino acid uptake, and this effect was potentiated by concomitant exercise.28, 29 Unfortunately, data from longer-term interventions have largely failed to demonstrate additive benefits of exercise and nutrition on a variety of health-related metrics.30–33 However, these also have generally been uncontrolled studies with short intervention periods and small sample sizes.
The purpose of IHOPE was to determine if the combination of two common interventions, namely intradialytic OPS and intradialytic endurance exercise, improves the health and QOL of HD patients. We hypothesized that 12 months of OPS supplementation alone would improve physical function, measured by a shuttle walk test (SWT), and that concomitant intradialytic endurance exercise training would have additive benefits. We also hypothesized there would be additive benefits of our interventions on secondary outcomes of muscle strength, function, body composition, nutritional status, markers of CVD risk, and QOL.
RESULTS
Patient Characteristics
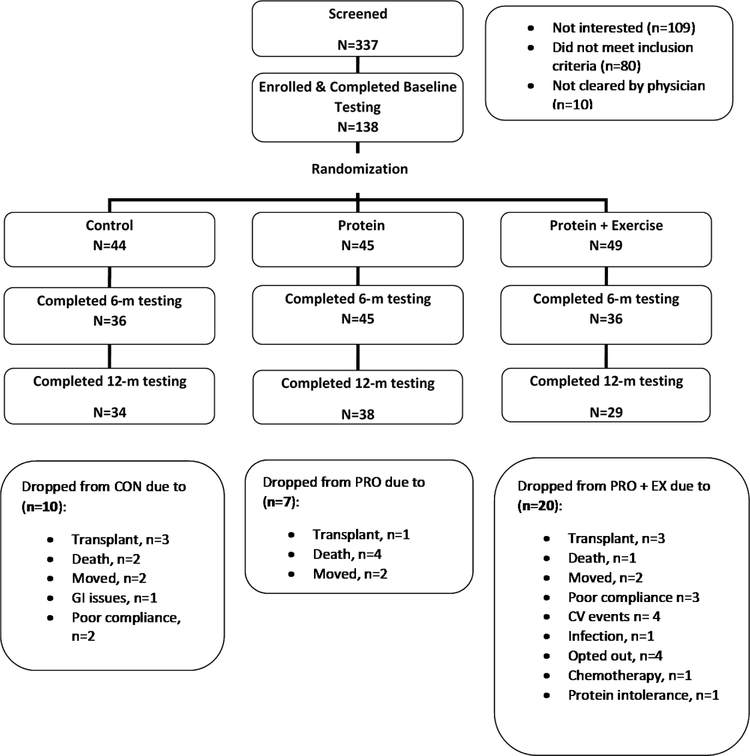
Of 337 patients recruited, 138 met the inclusion criteria, agreed to participate, and were randomized (n=44 CON, n=45 PRO, n=49 PRO+EX). Of these, 117 and 101 patients completed testing at 6 and 12 months, respectively (Figure 1). There were no differences between groups in demographic or clinical characteristics at baseline except smoking status, which was significantly higher in PRO compared to other groups (Table 1).
Figure 1.
Patient Consort Diagram
Table 1.
Demographics and Baseline Characteristics of Study Population
| Characteristics | CON | PRO | PRO+EX | Total | P-value |
|---|---|---|---|---|---|
| Age (yr) | 54.4 ± 12.3 | 56.6 ± 13.0 | 53.7 ± 11.4 | 54.8 ± 12.2 | 0.49 |
| Dialysis Vintage (months) | 47.9 ± 37.5 | 45.6 ± 38.7 | 34.3 ± 34.8 | 42.0 ± 37.1 | 0.19 |
| BMI (kg/m2) | 31.5 ± 7.6 | 30.6 ± 7.1 | 31.9 ± 8.3 | 31.4 ± 7.7 | 0.71 |
| Hip Circumference (cm) | 108.6 ± 15.8 | 107.5 ± 15.6 | 111.6 ± 18.2 | 109.3 ± 16.7 | 0.47 |
| Waist Circumference (cm) | 104.3 ± 17.8 | 101.8 ± 19.5 | 105.7 ± 19.1 | 32.2 ± 5.6 | 0.61 |
| Gender (male, %) | 28 (63.6) | 23 (51.1) | 29 (59.2) | 80 (58.0) | 0.67 |
| Ethnicity (n, %) | |||||
| Hispanic or Latino | 6 (4.3) | 1 (0.7) | 2 (1.4) | 9 (6.5) | 0.08 |
| Not Hispanic | 38 (28.5) | 44 (31.9) | 47 (34.1) | 129 (93.5) | 0.33 |
| Race (n, %) | |||||
| Black | 31 (70.5) | 42 (93.3) | 43 (87.8) | 116 (84.1) | 0.06 |
| White | 12 (27.3) | 3 (6.7) | 5 (10.2) | 20 (14.5) | 0.13 |
| Other | 1 (2.3) | 0 | 1 (2.0) | 2 (0.1) | |
| Diabetes (n, %) | 19 (43) | 22 (52.4) | 24 (49.0) | 66 (47.8) | 0.68 |
| Smoking (n, %) | 6 (13.6) | 14 (31.1) | 7 (14.3) | 28 (20.2) | 0.03 |
| Cause of ESRD (n, %) | 0.26 | ||||
| Diabetes | 13 (29.5) | 12 (26.7) | 18 (36.7) | 43 (31.2) | |
| Hypertension | 20 (45.4) | 24 (54.5) | 22 (44.9) | 66 (47.8) | |
| Nephritis | 5 (11.3) | 0 | 3 (6.1) | 8 (5.8) | |
| PKD | 1 (2.3) | 1 (2.2) | 2 (4.1) | 4 (2.9) | |
| Other/unknown | 5 (11.4) | 8 (17.8) | 4 (8.1) | 17 (12.3) | |
| Beverage Compliance (%) | 91.2 ± 8.0 | 93.5 ± 4.0 | 93.1 ± 4.7 | 90.4 ± 8.8 | 0.34 |
| Exercise Compliance (%) | 78.8 ± 9.7 |
BMI: body mass index; ESRD: end stage renal disease. PKD: Polycystic Kidney Disease P-values indicate the level of difference between groups by ANOVA or χ2 tests.
Intervention Compliance
Compliance with the study beverage (>90%) and exercise sessions (80%) were both high (Table 1), though the dropout rate was greater in PRO+EX compared to CON and PRO (41%, 23% and 16%, respectively, p=0.02). Patients in PRO+EX completing the trial cycled for an average of 40±6 minutes/session. The only reported adverse events attributed to the intervention were 3 complaints of GI distress from the study beverage.
Physical Function, Muscle Strength, and Body Composition
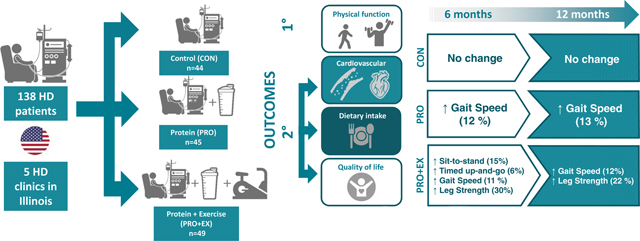
Outcomes related to physical function, muscle strength, and body composition are shown in Table 2. Contrary to our hypotheses, there was no significant group-by-time interactions, indicating our interventions had no significant effect on our primary (SWT), secondary measures of physical function, muscle strength and body composition. However, there was a significant main effect of time for many of these variables, indicating a general improvement across the groups during the study. Within group comparisons assessing the change over time revealed that the magnitude of improvements in the overall study population was largely driven by changes in the intervention groups. For example, at 6 months, performance on the sit-to-stand (STS) and timed up-and-go (TUG) improved by 15% (p=0.018, d=0.4) and 6% (p=0.003, d=0.4) in PRO+EX, but did not change in CON or PRO, and these changes were not sustained at 12 months. Likewise, gait speed improved at 6 months by 11–12% in both PRO (d=0.3) and PRO+EX (d=0.7), but only 7% in CON (d=0.3) (p<0.05 for all). Moreover, these improvements were maintained at 12 months in PRO and PRO+EX, but not in CON. Muscle strength also tended to increase more in PRO+ EX than the other groups. For example, leg maximal flexion force increased by 30% at 6 months (p=0.003, d=0.5) and 21% at 12 months (p=0.05, d=0.3) in PRO+EX group, with no changes in CON or PRO.
Table 2.
Effect of the 12-month Protein Supplementation with and without Exercise Intervention on Physical Function, Muscle Strength, and Body Composition.
| Baseline | 6 months | 12 months | Between Group Comparison by GEE |
||||
|---|---|---|---|---|---|---|---|
| P (T*G) |
P (Time) |
P (Group) |
|||||
| Physical Function | |||||||
| Shuttle Walk (sec) | CON | 231.8 ± 110.2 | 222.2 ± 121.0 | 214.7 ± 124.1 | 0.44 | 0.23 | 0.19 |
| PRO | 221.6 ± 106.9 | 235.1 ± 102.0 | 218.7 ± 113.7 | ||||
| PRO+EX | 263.4 ± 114.4 | 287.7 ± 98.8 | 271.1 ± 121.4 | ||||
| Gait Speed (m/sec) | CON | 0.87 ± 0.24 | 0.93 ± 0.26* | 0.88 ± 0.28 | 0.31 | <.001 | 0.06 |
| PRO | 0.86 ± 0.23 | 0.93 ± 0.21* | 0.96 ± 0.20** | ||||
| PRO+EX | 0.93 ± 0.24 | 1.1 ± 0.22* | 1.05 ± 0.18** | ||||
| STS (rep) | CON | 10.1 ± 3.8 | 10.0 ± 4.5 | 9.8 ± 4.1 | 0.25 | 0.08 | 0.45 |
| PRO | 9.6 ± 4.0 | 9.9 ± 4.6 | 10.7 ± 5.6 | ||||
| PRO+EX | 10.1 ± 3.5 | 11.6 ± 3.4* | 11.7 ± 4.0 | ||||
| TUG (sec) | CON | 8.0 ± 5.4 | 8.0 ± 4.1 | 8.2 ± 5.1 | 0.08 | 0.28 | 0.20 |
| PRO | 7.6 ± 3.5 | 7.3 ± 2.9 | 8.0 ± 3.6** | ||||
| PRO+EX | 7.1 ± 4.1 | 5.9 ± 1.6* | 6.2 ± 1.7 | ||||
| Muscle Strength | |||||||
| Leg Extension (ft•lb) | CON | 85.0 ± 44.5 | 89.5 ± 43.1 | 87.6 ± 45.3 | 0.75 | 0.12 | 0.44 |
| PRO | 73.9 ± 40.2 | 80.3 ± 39.7 | 77.4 ± 38.7** | ||||
| PRO+EX | 80.9 ± 32.8 | 87.1 ± 35.9* | 85.7 ± 36.6** | ||||
| Leg Flexion (f•lb) | CON | 41.6 ± 24.4 | 45.7 ± 28.6 | 45.1 ± 28.5 | 0.24 | 0.03 | 0.45 |
| PRO | 37.8 ± 20.4 | 40.2 ± 21.8 | 38.9 ± 22.1 | ||||
| PRO+EX | 36.5 ± 18.9 | 47.4 ± 26.5* | 45.6 ± 34.8** | ||||
| Body Composition | |||||||
| BMI (kg/m2) | CON | 31.5 ± 7.6 | 32.4 ± 7.9 | 32.3 ± 8.5 | 0.64 | 0.19 | 0.62 |
| PRO | 30.6 ± 7.1 | 31.1 ± 7.0 | 31.5 ± 7.4 | ||||
| PRO+EX | 31.9 ± 8.3 | 32.7 ± 10.0 | 33.9 ± 10.9 | ||||
| WB lean mass (kg) | CON | 58.6 ± 11.7 | 59.3 ± 12.6 | 60.4 ± 124.1 | 0.62 | 0.19 | 0.76 |
| PRO | 56.9 ± 14.5 | 57.7 ± 15.0 | 58.6 ± 15.9 | ||||
| PRO+EX | 59.3 ± 12.3 | 60.7 ± 13.1 | 61.5 ± 13.1 | ||||
| WB fat percent (%) | CON | 30.6 ± 10.2 | 32.2 ± 9.8 | 32.0 ± 10.2 | 0.15 | 0.96 | 0.93 |
| PRO | 32.2 ± 9.9 | 31.6 ± 10.4 | 32.4 ± 10.5 | ||||
| PRO+EX | 31.3 ± 10.1 | 30.5 ± 9.9 | 30.9 ± 11.3 | ||||
| Leg Lean Mass (kg) | CON | 9.38 ± 2.13 | 9.51 ± 2.26 | 9.68 ± 2.40 | 0.99 | 0.18 | 0.57 |
| PRO | 9.13 ± 2.74 | 9.20 ± 2.80 | 9.50 ± 2.95 | ||||
| PRO+EX | 9.65 ± 2.21 | 9.90 ± 2.35 | 10.00 ± 2.46 | ||||
| WB BMD (g/cm2) | CON | 1.10 ± 0.15 | 1.09 ± 0.16 | 1.09 ± 0.15** | 0.25 | 0.07 | 0.52 |
| PRO | 1.09 ± 0.14 | 1.08 ± 0.15 | 1.09 ± 0.13 | ||||
| PRO+EX | 1.12 ± 0.15 | 1.14 ± 0.15 | 1.14 ± 0.15 | ||||
| Leg BMD (g/cm2) | CON | 1.13 ± 0.21 | 1.10 ± 0.20 | 1.14 ± 0.21 | 0.33 | 0.29 | 0.51 |
| PRO | 1.10 ± 0.19 | 1.10 ± 0.19 | 1.11 ± 0.18 | ||||
| PRO+EX | 1.15 ± 0.18 | 1.16 ± 0.18 | 1.16 ± 0.17 | ||||
| Hip BMD (g/cm2) | CON | 0.94 ± 0.21 | 0.94 ± 0.21 | 0.93 ± 0.21** | 0.29 | <0.01 | 0.52 |
| PRO | 0.94 ± 0.19 | 0.93 ± 0.19 | 0.94 ± 0.18 | ||||
| PRO+EX | 0.97 ± 0.18 | 0.99 ± 0.19 | 1.02 ± 0.20 | ||||
BMI: body mass index, WB: whole body, BMD: bone mineral density, STS: Sit-to-Stand Test, TUG: Timed Up-and-Go test
: indicates significant within-group difference between baseline vs 6 months in outcome measures by paired t-test
: indicates significant within-group difference between baseline vs 12 months in outcome measures by paired t-test
Cardiovascular Measures
The change in cardiovascular parameters are shown in Table 3. Contrary to our hypothesis, there was no significant group-by-time interactions, indicating our interventions had no significant effect on pulse wave velocity (PWV) or blood pressure (BP) parameters (p>0.05 for all). There was an overall group difference in brachial and aortic SBP levels, with a significant mean difference between PRO and PRO+EX at baseline, 6 months and 12 months (p<0.05 for all).
Table 3.
Effect of the 12-month Protein Supplementation with and without Exercise Intervention on Arterial Function.
| Baseline | 6 months | 12 months | Between Group Comparison by GEE |
||||
|---|---|---|---|---|---|---|---|
| P (T*G) |
P (Time) |
P (Group) |
|||||
| Arterial Function | |||||||
| bSBP (mmHg) | CON | 140.6±26.6 | 130.5±29.4 | 138.1±27.5 | 0.21 | 0.92 | 0.01 |
| PRO | 144.6±23.1 | 149.2±21.6θ | 148.9±23.3 | ||||
| PRO+EX | 132.3±21.3‡ | 136.4±30.4‡ | 132.4±27.9‡ | ||||
| bDBP (mmHg) | CON | 81.4±15.2 | 74.2±13.8* | 74.9±15.2** | 0.22 | 0.16 | 0.88 |
| PRO | 78.2±13.4 | 78.8±15.1 | 78.5±12.5 | ||||
| PRO+EX | 77.6±13.5 | 77.8±14.4 | 76.6±16.6 | ||||
| aSBP (mmHg) | CON | 129.5±13.5 | 118.4±28.2 | 121.8±21.7 | 0.14 | 0.76 | 0.03 |
| PRO | 130.1±23.9 | 136.0±21.2 | 134.3±22.3 | ||||
| PRO+EX | 123.2±21.3 | 126.3±23.9 | 118.0±25.9 | ||||
| aDBP (mmHg) | CON | 83.5±14.1 | 76.3±14.7* | 75.1±13.8** | 0.29 | 0.10 | 0.73 |
| PRO | 81.7±23.1 | 80.4±15.7 | 79.0±12.1 | ||||
| PRO+EX | 78.5±13.1 | 79.5±14.1 | 76.3±18.0 | ||||
| Aix75 (%) | CON | 24.7±11.7 | 19.7±14.4 | 22.7±10.6 | 0.58 | 0.24 | 0.43 |
| PRO | 25.6±10.9 | 25.6±11.4 | 25.7±11.0 | ||||
| PRO+EX | 25.0±13.1 | 21.3±2.7 | 21.8±14.4 | ||||
| PWV (m/s) | CON | 9.4±2.6 | 8.7±2.3* | 10.1±4.0 | 0.08 | 0.30 | 0.16 |
| PRO | 10.8±3.5 | 10.3±2.8 | 9.6±2.7 | ||||
| PRO+EX | 9.3±3.1 | 9.9±4.0 | 8.7±2.2 | ||||
bSBP: brachial systolic blood pressure, bDBP: brachial diastolic blood pressure, aSBP: aortic systolic blood pressure, aDBP: aortic diastolic blood pressure, Aix 75: augmentation index at heart rate 75 beats per minute, PWV: pulse wave velocity
: indicates significant within-group difference between baseline vs 6 months in outcome measures by paired t-test
: indicates significant within-group difference between baseline vs 12 months in outcome measures by paired t-test
: indicates a significant group difference compared to CON group by LSD post-hoc analysis at a time point
: indicates a significant group difference compared to PRO group by LSD post-hoc analysis at a time point
Nutritional Markers
Nutritional intake and related parameters are shown in Table 4. When intakes from dialysis and non-dialysis days were averaged, protein intake increased at 6 and 12 months in PRO and PRO+EX compared to CON (p=0.017), driven by increases in protein intake in PRO and PRO+EX at 6 and 12 months. On non-dialysis days, there was an increase in total energy intake (p=0.017), driven by modest reductions at 6 and 12 months in CON and modest increases in PRO and PRO+EX. Our interventions had no impact on serum albumin or markers of systemic inflammation (serum IL-6 or CRP).
Table 4.
Effect of the 12-month Protein Supplementation with and without Exercise Intervention on Dietary Intake and Blood Markers
| Baseline | 6-month | 12-month | Between Group Comparison by GEE |
|||||
|---|---|---|---|---|---|---|---|---|
| P (T*G) |
P (Time) |
P (Group) |
||||||
| Dietary Intake | ||||||||
| NDD | Energy(Kcal/kg/d) | CON | 20.3±13.1 | 15.9±9.01* | 16.0±8.10 | 0.02 | 0.91 | 0.67 |
| PRO | 17.8±7.55 | 19.6±10.7 | 19.1±9.70 | |||||
| PRO+EX | 16.9±7.6 | 20.1±13.6 | 20.8±12.9 | |||||
| Protein(g/kg/d) | CON | 0.83±0.49 | 0.69±0.37 | 0.72±0.36 | 0.218 | 0.81 | 0.54 | |
| PRO | 0.76±0.36 | 0.84±0.4 | 0.80±0.40 | |||||
| PRO+EX | 0.77±0.35 | 0.90±0.61 | 0.90±0.52 | |||||
| DD | Energy(Kcal/kg/d) | CON | 16.8±10.6 | 14.1±8.9 | 17.7±9.9 | 0.644 | 0.099 | 0.63 |
| PRO | 18.1±9.9 | 17.1±6.7 | 18.7±9.0 | |||||
| PRO+EX | 16.9±8.9 | 18.8±9.8 | 19.4±11.8 | |||||
| Protein(g/kg/d) | CON | 0.75±0.44 | 0.65±0.39 | 0.73±0.44 | 0.01 | 0.01 | 0.01 | |
| PRO | 0.88±0.79 | 1.07±0.38 | 1.01±0.40 | |||||
| PRO+EX | 0.73±0.52 | 1.07±0.44* | 1.10±0.50** | |||||
| AVG | Energy(Kcal/kg/d) | CON | 18.6±11.2 | 15.4±8.7 | 16.8±8.3 | 0.16 | 0.40 | 0.68 |
| PRO | 17.9±7.5 | 18.5±6.9 | 19.03±8.5 | |||||
| PRO+EX | 16.9±7.4 | 19.2±9.8 | 20.04±11.8 | |||||
| Protein(g/kg/d) | CON | 0.79±0.42 | 0.69±0.36 | 0.73±0.41 | 0.02 | 0.04 | 0.07 | |
| PRO | 0.82±0.46 | 0.95±0.31 | 0.90±0.33 | |||||
| PRO+EX | 0.75±0.40 | 0.97±0.46 | 1.00±0.46 | |||||
| Blood Biomarkers | ||||||||
| Albumin(g/dl) | CON | 4.02±0.30 | 4.00±0.35 | 4.00±0.31 | 0.71 | 0.57 | 0.43 | |
| PRO | 4.00±0.35 | 3.95±0.38 | 4.01±0.31 | |||||
| PRO+EX | 3.92±0.37 | 3.95±0.35 | 3.93±0.51 | |||||
| IL-6(pg/ml) | CON (n=9) | 4.53±2.76 | 4.60±2.49 | 5.78±6.00 | 0.92 | 0.38 | 0.73 | |
| PRO (n=10) | 3.98±1.40 | 5.02±3.53 | 4.75±2.58 | |||||
| PRO+EX (n=7) | 5.90±7.02 | 5.97±5.57 | 6.96±6.40 | |||||
| CRP(mg/L) | CON (n=11) | 7.73±6.42 | 7.44±4.46 | 11.0±10.85 | 0.40 | 0.57 | 0.26 | |
| PRO (n=12) | 18.1±20.9 | 12.8±13.2 | 11.36±7.76 | |||||
| PRO+EX (n=11) | 15.2±14.1 | 15.9±9.02 | 13.17±11.8 | |||||
IL-6: interleukin-6, CRP: C-reactive protein, NDD: non-dialysis days, DD: dialysis days, AVG: average of NDD and DD
: indicates significant within-group difference between baseline vs 6-month in outcome measures by paired t-test
: indicates significant within-group difference between baseline vs 12-month in outcome measures by paired t-test
Self-reported QOL and Physical Activity Level
Self-reported QOL metrics and physical activity (PA) levels were similar at baseline, and the changes over time were not different between groups (Supplement 1). Mental health status (MHS) declined over time across all groups (main effect of time, p < 0.05), but there was no change in physical health status (PHS) over time. There was a significant main effect of time for weekly PA levels, indicating a modest increase in PA across groups during the intervention (234±30, 237±21 and 395±361 Kcal/kg/wk) at 0, 6 and 12 months respectively, p<0.001).
DISCUSSION
The primary finding in this study was that 12 months of intradialytic OPS, with or without intradialytic exercise training, did not significantly improve physical function or any of our secondary outcomes related to muscle strength, body composition, markers of CVD risk, or QOL in HD patients. Within-group comparisons demonstrated improvements in some secondary measures of physical function and muscle strength in PRO and PRO+EX groups at 6 or 12 months, compared to CON. However, similar to previous exercise interventions in HD patients,21–27 the magnitude of these changes was modest and must be interpreted with caution due to the likelihood of Type I statistical errors from multiple comparisons. In summary, our data suggest the benefits of chronic OPS alone, or OPS combined with moderate intensity intradialytic exercise, may be modest.
OPS and exercise training are both commonly prescribed strategies for inhibiting the development and progression of comorbidities in HD patients. However, robust data from long-term RCTs with adequate sample sizes are lacking for both interventions. In regards to OPS, observational studies suggest chronic supplementation is associated with reduced mortality,8–10 but observational studies cannot prove causality. To our knowledge, only three RCTs have examined the efficacy of OPS in HD patients,13–15 and the results have been mixed. Bolasco et al. showed that OPS for three months in HD patients with hypoalbuminemia improved serum albumin and reduced CRP.15 Sohrabi et al. also showed that eight weeks of OPS improved markers of nutritional status, but not inflammation. In contrast, our lab previously showed that six months of OPS reduced markers of systemic inflammation and improved physical function, but did not improve serum albumin.14 Other studies using cross-over designs showed modest or no improvement in markers of nutritional status after 12–24 weeks of OPS.11, 16 In general, data from these studies suggest patients with low serum albumin or impaired nutritional status may benefit the most from OPS. However, due to the relatively short time-frame of these interventions, it is less certain if OPS would be effective in preventing declines in nutritional status over a longer time period, or in patients with adequate nutritional status. Furthermore, our group recently demonstrated that HD patients have muscle anabolic resistance to high-protein meal ingestion,34 providing further evidence that feeding alone may be an ineffective strategy in this population and that adjunct therapies are needed to overcome their hypercatabolic state.
The benefits of exercise training in HD patients have been extensively reviewed.17–19 However, much of these data come from uncontrolled pilot studies that reported positive benefits regarding secondary outcomes that were not corrected for multiple comparisons. By contrast, several of the largest and longest exercise interventions in HD patients to date have yielded mixed results in regards to effects of exercise on physical function, strength, body composition, or QOL.21–27 For example, a recent study by Koh et al. found that neither intradialytic nor at-home endurance exercise improved physical function after 6 months, and QOL was actually reduced in the intradialytic exercise group.23 With this in mind, the failure of the PRO+EX intervention to significantly improve these measures in the current study may not be surprising.
Data regarding the efficacy of exercise training in HD patients on CV-related outcomes are also mixed. While most studies generally confirm a beneficial effect of exercise on traditional CVD risk factors,35 the apparent reverse epidemiology between many traditional CVD risk factors and adverse CV events in HD patients makes these findings difficult to interpret.36 In contrast, markers of arterial stiffness have been consistently associated with CVD mortality in HD patients.37, 38 While three small pilot studies noted some modest benefits of exercise training on markers of arterial stiffness following 3–4 months of intradialytic exercise,39–41 a larger RCT23 did not show improvements in PWV after six months of either intradialytic or home-based aerobic exercise. These data are consistent with our study, in which no improvement in PWV in the PRO+EX group was observed. A possible reason for this null effect of exercise on arterial stiffness may be due to the significant vascular remodeling and calcification in HD patients that may limit the vessels ability to respond to the intervention.42, 43
There is some data to suggest that combining ONS and exercise may have additive benefits in HD patients. For example, a single bout of either endurance or resistance exercise acutely improves the anabolic response to nutritional supplementation.28, 29 However, at least four studies have combined various forms of ONS and exercise training for up to 6 months in HD patients, but none demonstrated additive benefits on strength, function, or body composition.30, 32, 33, 44 Our data extends these findings by showing that benefits of combined interventions also may not be realized after 12 months.
One explanation for the lack of robust findings in this and other exercise related RCTs in HD patients is the low-volume and intensity of the exercise prescriptions that are generally used.23, 24, 26 Recent evidence emphasizes the necessity of both high-volume and intensity of exercise in therapeutic programs for promising disease management.45,46 In contrast, few, if any, exercise-related interventions in HD patients, including ours, have provided an exercise prescription that even approaches standard exercise guidelines.47
An important debate in the literature is whether in-center (intradialytic) or out-of-clinic (interdialytic) exercise is more efficacious. A recent study by Manfredini et al showed that a 6 month interdialytic walking program significantly improved performance on a 6 minute walk test.26 While this finding is encouraging, the magnitude of this improvement was modest (~ 12%), and was similar to the 9% increase in SWT we saw after 6 months of intradialytic exercise. Unfortunately, the improvement in SWT was not maintained at 12 months, possibly due in part to the accelerated physical deterioration that may occur in dialysis patients over one year.48 Others have also noted a leveling off of the benefits of intradialytic cycling after just 3 to 6 months.49 We speculate this may be due in part to a reduced enthusiasm for a mundane exercise prescription such as thrice weekly intradialytic cycling over a prolonged period. Another important consideration regarding the best timing for exercise is that dialysis represents a hypercatabolic period3. Thus, intradialytic exercise could hypothetically exacerbate the catabolic effects of dialysis, limiting its utility. However, there is no clear evidence in the literature suggesting a benefit of interdialytic exercise over intradialytic activity, or vice versa. We speculate this may be due to the low volume and intensity of exercise typically prescribed with both types of activities.
A notable concern with our data is the apparently high dropout rate in our exercise group (27% at 6 months, and 41% by 12 months). However, this is similar to dropout rates in comparable studies, including a 29% dropout after 6 months of intradialytic cycling in Koh et al.23 Studies in other populations have shown that patients dropping out of exercise interventions tend to be less healthy than those completing the intervention, which could bias the results.50, 51 However, this does not appear to be the case in this study, as there were no differences in our primary outcome measures (e.g., physical function measures and serum albumin) at baseline between completers and non-completers in the PRO+EX group in this study. Regardless, these high dropout rates suggests that even in studies where robust benefits of exercise are found, the generalizability of the findings may be low. This is especially true given that the sample of dialysis patients that tend to volunteer for exercise interventions is often biased towards healthier subsets of patients.52 This strongly suggests that new approaches to implementing exercise in HD patients should be considered. Historically, one-size-fits-all approaches using mandated exercise prescriptions (e.g., progressive cycling or resistance training protocols) have been the standard in the HD literature. Strong consideration should be given to changing this paradigm. We believe instead that interventions should be designed to allow patients more autonomy to select types of activities in which they choose to engage. A liberalized activity prescription also may result in greater participation and more sustained and robust changes in patient’s lifestyles.
This study has several limitations. First, we did not include a group that exercised without ONS. Given that dialysis promotes acute muscle catabolism,3 there is concern that intradialytic exercise without nutritional support may exacerbate this, and this may be partially responsible for the equivocal effects of intradialytic exercise seen in many studies.21–27 Second, we only subjectively assessed exercise intensity using rating of perceived exertion (RPE). However, the work rate in this study was similar to the modest intensity used in other intradialytic cycling studies,23, 24 and is practical to implement in HD clinics. While it is standard practice in exercise interventions to use a percentage of maximum heart rate to gauge exercise intensity, this is impractical in HD patients for several reasons, including the high rates of autonomic dysfunction and the excessive use of medicines affecting CV hemodynamics. A third limitation of our study was that the average serum albumin level was 4.0 g/L. Some argue that OPS should not be provided to HD patients with serum albumin of > ~3.5g/dl.53 However, albumin is known to be a poor marker of nutritional status.54 Moreover, there is an ongoing RCT (NCT02933151) to determine if there is a survival benefit from providing OPS to HD patients regardless of albumin levels. One aim of our study was to examine whether long term OPS may prevent declines in physical function and nutritional status (as opposed to increasing them) in patients with adequate nutritional status. Lastly, our dietary data indicated that protein and energy intakes in our patients were below established KDOQI guidelines,55 so we believe providing oral supplements to this group was warranted.
Major strengths of this trial included the inclusion of a true “usual care” control group, the length of the intervention (12 months), the larger sample size (n= 101 completing the trial), and the inclusion of comprehensive health outcomes related to physical function, strength, body composition, cardiovascular function, and QOL.
In conclusion, we found that 12 months of intradialytic OPS, with or without intradialytic exercise training, had limited benefits on physical function, body composition, markers of CVD risk, or QOL in HD patients. Despite this, our findings should not be used to discourage HD patients from incorporating physical activity or better nutrition into their lives, as it is clear that some individuals do benefit from these approaches. Our data highlight the notion that a one-size-fits-all approach may not provide robust benefits for many patients. Instead, more intensive, comprehensive, and tailored nutrition and exercise prescriptions may be needed to offset the deleterious consequences of comorbidities in HD patients. To achieve this, a culture change is needed in HD clinics that will better support comprehensive strategies to improve patient’s lifestyles. In the absence of this shift, we may continue to see only modest benefits from nutrition and exercise related interventions, and waste opportunities to optimize the health and QOL of our patients.
METHODS
Study Design and Participants
The Intra-Hemodialytic Oral Protein and Exercise (IHOPE) trial was a multicenter RCT that recruited patients from five HD clinics in Champaign and Chicago, IL between 2011–2016. Inclusion criteria included: receiving HD treatment ≥3 days/week; dialysis vintage ≥3 months; age 30–80 years; and not currently receiving intradialytic ONS or participating in intradialytic exercise. Written informed consent was obtained prior to baseline testing. Subjects were then assigned a coded study ID that was sent via a password protected file to a research member that was not involved in the data collection at that site for the purpose of allocation concealment. This researcher then then randomly assigned the subject to one of three groups using a random number generator: 1) Control (CON); 2) whey protein supplementation (PRO); or 3) whey protein supplementation + exercise training (PRO+EX). The protocol was approved by the Institutional Review Board at the University of Illinois, conducted in accordance with the Declaration of Helsinki, and registered with Clinicaltrials.gov (NCT01234441) on 11/04/2010.
Intervention Groups
Control (CON)
patients received ~150 grams of a non-nutritive beverage (Crystal Light, Kraft Foods Inc.) during each dialysis session.
Protein Supplementation (PRO)
patients received a 30 g whey protein supplement (True Protein Inc., Vista, CA) at each dialysis session mixed in 4–6 ounces of water and consumed at the beginning of each dialysis session.
Protein + Exercise Training (PRO+EX)
patients received the 30 g whey protein supplement and underwent supervised exercise for up to 45 minutes on cycle ergometers (Rehab trainer 881E, Monark Inc.) during each dialysis session. The training started by cycling at a tolerable pace for 5–10 minutes/session, increasing progressively until able to cycle continuously for up to 45 minutes/session at a rating of perceived exertion of 12–14 (“somewhat hard”). All exercise sessions were monitored by the research staff who provided encouragement to the patient to maintain their RPE throughout each exercise session.
Outcome Measures
Patients underwent testing on a non-dialysis day at baseline, 6 and 12 months, 18–24 hours after a previous dialysis session. Our primary outcome was the change in physical function at 12 months, as assessed by an incremental shuttle walk test (SWT). Secondary outcome variables included measures related to muscle strength, body composition, CVD risk, diet, and QOL.
Physical Function and Strength Measures
The SWT involves walking back and forth over a 10-m course to successively faster times until unable to complete the course in the allotted time. The SWT is frequently used to assess function in diseased populations instead of more objective measures of aerobic capacity such as VO2max testing due to functional deficits like muscle weakness and shortness of breath that prevent these individuals from achieving standard criteria during these tests56. This shuttle walk test is well established for assessing fitness in patients with CVD57, CHF58, and COPD59, and is often preferred to the six minute walk test because it is paced, and therefore more objective. Secondary assessments of strength and physical function included: 1) normal gait speed over a 10-m course; 2) 30-second Sit-To-Stand (STS) test;60 3) 8-foot Timed Up-and-Go (TUG) test;61 and 4) bilateral quadriceps femoris and hamstring muscle strength by isokinetic dynamometry (Biodex Medical Systems, Shirley, New York). Knee extension and flexion isokinetic peak muscle torque (Nm) was evaluated at a speed of 60 degrees/second. Participants performed two sets of 6 repetitions, with a 3-minute rest between sets, and the peak torque was recorded for analysis.
Body Composition
Whole-body fat and lean and bone mass were measured by dual emission x-ray absorptiometry (DEXA, Hologic QDR 4500A). Whole body and regional lean mass was calculated by subtracting the bone mineral content from the lean mass quantity of the whole body or region of interest.
Cardiovascular Measures
Central pulse wave velocity (PWV) was calculated from the pressure waveform at the carotid and femoral site. Radial waveforms for augmentation index (Ai) were measured on the non-access side using applanation tonometry (SphygmoCor,Inc).62 Systolic (SBP) and diastolic (DBP) blood pressure were measured in the brachial artery with an automated cuff in triplicate after having patients sit quietly for 5 minutes.
Blood Chemistry
Serum albumin levels were obtained from clinic records, while C-reactive protein (CRP) and IL-6 were measured from pre-dialysis blood draws in a subset of patients (n= 63) using commercially available ELISA kits.
Self-reported measures
Physical health status (PHS) and mental health status (MHS) were measured by the 12-Item Short Form Survey derived from the Medical Outcomes 36-item Short Form Survey.63 Physical activity (PA) was assessed with a structured PA assessment instrument.64 Diet was assessed via interviewer-administered 24-hour diet recalls using the USDA 5-pass method on a midweek dialysis and non-dialysis day, and analyzed using Nutritionist Pro (Axxya Systems, Redmond, WA) software.
Power Analysis
Our power calculation for this study was based on data from two previous studies in HD patients in our lab14, 20 that demonstrated: 1) a 14% improvement in SWT (216±36 to 247±37 seconds, effect size of 0.8) following 6 months of OPS; and 2) a 13% increase in SWT (273±84 to 310±104 seconds, effect size of 0.5) following 4 months of intradialytic cycling. We expected additive benefits in this study in the group receiving both OPS and exercise (PRO +EX), especially at the 12-month timepoint. Based on this assumption, we estimated an effect size of 0.8 for the SWT performance in our combined intervention group at 12 months. After accounting for a 20% drop out rate and an adjusted family α=0.016 for Bonferroni multiple-comparisons, we required 38 patients in each group to have 80% power to detect a difference between groups at α=0.05.
Statistical Analysis
Data are presented as mean ± standard deviation. Baseline variables were tested for difference between groups by one-way analysis of variance (ANOVA) for continuous variables and χ2 tests for categorical data. LSD post-hoc tests were used when significant group differences were noted. To test the effect of 12-month intervention on primary and secondary outcomes, generalized estimating equations (GEE) analysis was used with Group (CON, PRO, and PRO+EX), Time (0-, 6- and 12-month) and the interactions between Group and Time with the exchangeable correlation structure that generated the smallest AIC, QIC criterion. GEE analysis was chosen because of our primary interest on the overall treatment effect by using the population–averaged estimates of variance. This approach is in contrast to linear mixed models that depends on individual subjects’ variance. GEE handles missing values in much the same way as a mixed models analysis, in that it only excludes patients if all observations are missing for that patient. No imputation was performed to replace missing values from the loss to follow-up. This is because the risk of bias with imputation is considerably high when there is significant dropout (e.g., >20%), reducing the accuracy of the estimates for the missing values.65 Moreover, GEE analysis partially overcomes the compromised power from the incomplete data set by incorporating partially collected data (e.g., data collected at 2 time points instead of all 3 time points). Paired t-tests were also conducted to examine within-group change in outcomes between each time point. Effect sizes for the treatment responses are reported by Cohen’s d values to represent small (d=0.2), medium (d=0.5), and large effect sizes (d=0.8). Sensitivity analysis was conducted using a per-protocol analysis that excluded non-compliant patients (n=beverage >90% and exercise >80%, n=34, 35, and 28 in CON, PRO, and PRO+EX respectively) and showed similar results with intent-to-treat analysis that included all subjects agreeing to participate in data collection. Separate analysis was also conducted with adjustment for baseline values and patient demographics. This generated similar data as the unadjusted analysis, so this data is not presented. SAS version 9.4 (SAS Inc.) and SPSS version 22.0 were utilized for data analysis, and p<0.05 was considered statistically significant.
Supplementary Material
Effects of Intradialytic Oral Protein Supplementation and Aerobic Exercise Training on Self-reported Quality of Life and Physical Activity Levels
Acknowledgements:
This study was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK084016)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary information is available at Kidney International’s website
Disclosures: None
References
- 1.Liu J, Huang Z, Gilbertson DT, Foley RN and Collins AJ. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010;77:141–51. [DOI] [PubMed] [Google Scholar]
- 2.Shinaberger CS, Kilpatrick RD, Regidor DL, McAllister CJ, Greenland S, Kopple JD and Kalantar-Zadeh K. Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am J Kidney Dis. 2006;48:37–49. [DOI] [PubMed] [Google Scholar]
- 3.Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, Mitch WE, Price SR, Wanner C, Wang AY, ter Wee P and Franch HA. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr 2013;23:77–90. [DOI] [PubMed] [Google Scholar]
- 4.Rhee CM, You AS, Koontz Parsons T, Tortorici AR, Bross R, St-Jules DE, Jing J, Lee ML, Benner D, Kovesdy CP, Mehrotra R, Kopple JD and Kalantar-Zadeh K. Effect of high-protein meals during hemodialysis combined with lanthanum carbonate in hypoalbuminemic dialysis patients: findings from the FrEDI randomized controlled trial. Nephrol Dial Transplant 2017;32:1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calegari A, Barros EG, Veronese FV and Thome FS. Malnourished patients on hemodialysis improve after receiving a nutritional intervention. J Bras Nefrol. 2011;33:394–401. [PubMed] [Google Scholar]
- 6.Veeneman JM, Kingma HA, Boer TS, Stellaard F, De Jong PE, Reijngoud DJ and Huisman RM. Protein intake during hemodialysis maintains a positive whole body protein balance in chronic hemodialysis patients. Am J Physiol Endocrinol Metab. 2003;284:E954–65. [DOI] [PubMed] [Google Scholar]
- 7.Pupim LB, Majchrzak KM, Flakoll PJ and Ikizler TA. Intradialytic oral nutrition improves protein homeostasis in chronic hemodialysis patients with deranged nutritional status. J Am Soc Nephrol. 2006;17:3149–57. [DOI] [PubMed] [Google Scholar]
- 8.Weiner DE, Tighiouart H, Ladik V, Meyer KB, Zager PG and Johnson DS. Oral intradialytic nutritional supplement use and mortality in hemodialysis patients. Am J Kidney Dis. 2014;63:276–85. [DOI] [PubMed] [Google Scholar]
- 9.Lacson E Jr., Wang W, Zebrowski B, Wingard R and Hakim RM. Outcomes associated with intradialytic oral nutritional supplements in patients undergoing maintenance hemodialysis: a quality improvement report. Am J Kidney Dis. 2012;60:591–600. [DOI] [PubMed] [Google Scholar]
- 10.Benner D, Brunelli SM, Brosch B, Wheeler J and Nissenson AR. Effects of Oral Nutritional Supplements on Mortality, Missed Dialysis Treatments, and Nutritional Markers in Hemodialysis Patients. J Ren Nutr 2017. [DOI] [PubMed] [Google Scholar]
- 11.Beddhu S, Filipowicz R, Chen X, Neilson JL, Wei G, Huang Y and Greene T. Supervised oral protein supplementation during dialysis in patients with elevated C-reactive protein levels: a two phase, longitudinal, single center, open labeled study. BMC Nephrol 2015;16:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundell MB, Cavanaugh KL, Wu P, Shintani A, Hakim RM and Ikizler TA. Oral protein supplementation alone improves anabolism in a dose-dependent manner in chronic hemodialysis patients. J Ren Nutr. 2009;19:412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sohrabi Z, Eftekhari MH, Eskandari MH, Rezaianzadeh A and Sagheb MM. Intradialytic Oral Protein Supplementation and Nutritional and Inflammation Outcomes in Hemodialysis: A Randomized Controlled Trial. Am J Kidney Dis. 2016;68:122–30. [DOI] [PubMed] [Google Scholar]
- 14.Tomayko EJ, Kistler BM, Fitschen PJ and Wilund KR. Intradialytic protein supplementation reduces inflammation and improves physical function in maintenance hemodialysis patients. J Ren Nutr. 2015;25:276–83. [DOI] [PubMed] [Google Scholar]
- 15.Bolasco P, Caria S, Cupisti A, Secci R and Saverio Dioguardi F. A novel amino acids oral supplementation in hemodialysis patients: a pilot study. Ren Fail. 2011;33:1–5. [DOI] [PubMed] [Google Scholar]
- 16.Moretti HD, Johnson AM and Keeling-Hathaway TJ. Effects of protein supplementation in chronic hemodialysis and peritoneal dialysis patients. J Ren Nutr. 2009;19:298–303. [DOI] [PubMed] [Google Scholar]
- 17.Chung YC, Yeh ML and Liu YM. Effects of intradialytic exercise on the physical function, depression and quality of life for haemodialysis patients: a systematic review and meta-analysis of randomised controlled trials. J Clin Nurs. 2017;26:1801–1813. [DOI] [PubMed] [Google Scholar]
- 18.Barcellos FC, Santos IS, Umpierre D, Bohlke M and Hallal PC. Effects of exercise in the whole spectrum of chronic kidney disease: a systematic review. Clin Kidney J. 2015;8:753–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young HML, March DS, Graham-Brown MPM, Jones AW, Curtis F, Grantham CS, Churchward DR, Highton P, Smith AC, Singh SJ, Bridle C and Burton JO. Effects of intradialytic cycling exercise on exercise capacity, quality of life, physical function and cardiovascular measures in adult haemodialysis patients: a systematic review and meta-analysis. Nephrol Dial Transplant 2018. [DOI] [PubMed] [Google Scholar]
- 20.Wilund KR, Tomayko EJ, Wu PT, Ryong Chung H, Vallurupalli S, Lakshminarayanan B and Fernhall B. Intradialytic exercise training reduces oxidative stress and epicardial fat: a pilot study. Nephrol Dial Transplant. 2010;25:2695–701. [DOI] [PubMed] [Google Scholar]
- 21.Cheema B, Abas H, Smith B, O’Sullivan A, Chan M, Patwardhan A, Kelly J, Gillin A, Pang G, Lloyd B and Singh MF. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18:1594–601. [DOI] [PubMed] [Google Scholar]
- 22.Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J and Shubert T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: A randomized, controlled trial. J Am Soc Nephrol. 2006;17:2307–14. [DOI] [PubMed] [Google Scholar]
- 23.Koh KP, Fassett RG, Sharman JE, Coombes JS and Williams AD. Effect of intradialytic versus home-based aerobic exercise training on physical function and vascular parameters in hemodialysis patients: a randomized pilot study. Am J Kidney Dis. 2010;55:88–99. [DOI] [PubMed] [Google Scholar]
- 24.Kopple JD, Wang H, Casaburi R, Fournier M, Lewis MI, Taylor W and Storer TW. Exercise in maintenance hemodialysis patients induces transcriptional changes in genes favoring anabolic muscle. J Am Soc Nephrol. 2007;18:2975–86. [DOI] [PubMed] [Google Scholar]
- 25.Chen JL, Godfrey S, Ng TT, Moorthi R, Liangos O, Ruthazer R, Jaber BL, Levey AS and Castaneda-Sceppa C. Effect of intra-dialytic, low-intensity strength training on functional capacity in adult haemodialysis patients: a randomized pilot trial. Nephrol Dial Transplant. 2010;25:1936–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manfredini F, Mallamaci F, D’Arrigo G, Baggetta R, Bolignano D, Torino C, Lamberti N, Bertoli S, Ciurlino D, Rocca-Rey L, Barilla A, Battaglia Y, Rapana RM, Zuccala A, Bonanno G, Fatuzzo P, Rapisarda F, Rastelli S, Fabrizi F, Messa P, De Paola L, Lombardi L, Cupisti A, Fuiano G, Lucisano G, Summaria C, Felisatti M, Pozzato E, Malagoni AM, Castellino P, Aucella F, Abd ElHafeez S, Provenzano PF, Tripepi G, Catizone L and Zoccali C. Exercise in Patients on Dialysis: A Multicenter, Randomized Clinical Trial. J Am Soc Nephrol. 2017;28:1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkman DL, Mullins P, Junglee NA, Kumwenda M, Jibani MM and Macdonald JH. Anabolic exercise in haemodialysis patients: a randomised controlled pilot study. J Cachexia Sarcopenia Muscle. 2014;5:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pupim LB, Flakoll PJ, Levenhagen DK and Ikizler TA. Exercise augments the acute anabolic effects of intradialytic parenteral nutrition in chronic hemodialysis patients. Am J Physiol Endocrinol Metab. 2004;286:E589–97. [DOI] [PubMed] [Google Scholar]
- 29.Majchrzak KM, Pupim LB, Flakoll PJ and Ikizler TA. Resistance exercise augments the acute anabolic effects of intradialytic oral nutritional supplementation. Nephrol Dial Transplant. 2008;23:1362–9. [DOI] [PubMed] [Google Scholar]
- 30.Molsted S, Harrison AP, Eidemak I and Andersen JL. The effects of high-load strength training with protein- or nonprotein-containing nutritional supplementation in patients undergoing dialysis. J Ren Nutr. 2013;23:132–40. [DOI] [PubMed] [Google Scholar]
- 31.Dong J, Sundell MB, Pupim LB, Wu P, Shintani A and Ikizler TA. The effect of resistance exercise to augment long-term benefits of intradialytic oral nutritional supplementation in chronic hemodialysis patients. J Ren Nutr. 2011;21:149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hristea D, Deschamps T, Paris A, Lefrancois G, Collet V, Savoiu C, Ozenne S, Coupel S, Testa A and Magnard J. Combining intra-dialytic exercise and nutritional supplementation in malnourished older haemodialysis patients: Towards better quality of life and autonomy. Nephrology (Carlton). 2016;21:785–90. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Alemany G, Valdez-Ortiz R, Olvera-Soto G, Gomez-Guerrero I, Aguire-Esquivel G, Cantu-Quintanilla G, Lopez-Alvarenga JC, Miranda-Alatriste P and Espinosa-Cuevas A. The effects of resistance exercise and oral nutritional supplementation during hemodialysis on indicators of nutritional status and quality of life. Nephrol Dial Transplant. 2016;31:1712–20. [DOI] [PubMed] [Google Scholar]
- 34.van Vliet S, Skinner SK, Beals JW, Pagni BA, Fang HY, Ulanov AV, Li Z, Paluska SA, Mazzulla M, West DWD, Moore DR, Wilund KR and Burd NA. Dysregulated Handling of Dietary Protein and Muscle Protein Synthesis After Mixed-Meal Ingestion in Maintenance Hemodialysis Patients. Kidney Int Rep. 2018;3:1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheema BS and Singh MA. Exercise training in patients receiving maintenance hemodialysis: a systematic review of clinical trials. Am J Nephrol. 2005;25:352–64. [DOI] [PubMed] [Google Scholar]
- 36.Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, Gillen D, Kovesdy CP and Kalantar-Zadeh K. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis. 2014;56:415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME and London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–9. [DOI] [PubMed] [Google Scholar]
- 38.Sato M, Ogawa T, Otsuka K, Ando Y and Nitta K. Stiffness parameter beta as a predictor of the 4-year all-cause mortality of chronic hemodialysis patients. Clin Exp Nephrol. 2013;17:268–74. [DOI] [PubMed] [Google Scholar]
- 39.Mustata S, Chan C, Lai V and Miller JA. Impact of an exercise program on arterial stiffness and insulin resistance in hemodialysis patients. J Am Soc Nephrol. 2004;15:2713–8. [DOI] [PubMed] [Google Scholar]
- 40.Toussaint ND, Polkinghorne KR and Kerr PG. Impact of intradialytic exercise on arterial compliance and B-type natriuretic peptide levels in hemodialysis patients. Hemodial Int 2008;12:254–63. [DOI] [PubMed] [Google Scholar]
- 41.Cooke AB, Ta V, Iqbal S, Gomez YH, Mavrakanas T, Barre P, Vasilevsky M, Rahme E and Daskalopoulou SS. The Impact of Intradialytic Pedaling Exercise on Arterial Stiffness: A Pilot Randomized Controlled Trial in a Hemodialysis Population. Am J Hypertens. 2017. [DOI] [PubMed] [Google Scholar]
- 42.Briet M, Bozec E, Laurent S, Fassot C, London GM, Jacquot C, Froissart M, Houillier P and Boutouyrie P. Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int. 2006;69:350–7. [DOI] [PubMed] [Google Scholar]
- 43.Garnier AS and Briet M. Arterial Stiffness and Chronic Kidney Disease. Pulse (Basel). 2016;3:229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong J, Sundell MB, Pupim LB, Wu P, Shintani A and Ikizler TA. The Effect of Resistance Exercise to Augment Long-term Benefits of Intradialytic Oral Nutritional Supplementation in Chronic Hemodialysis Patients. J Ren Nutr 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ashor AW, Lara J, Siervo M, Celis-Morales C and Mathers JC. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PloS one. 2014;9:e110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Autier P and Pizot C. Meaningless METS: studying the link between physical activity and health. BMJ. 2016;354:i4200. [DOI] [PubMed] [Google Scholar]
- 47.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA and Castaneda-Sceppa C. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1435–45. [DOI] [PubMed] [Google Scholar]
- 48.Pupim LB, Heimburger O, Qureshi AR, Ikizler TA and Stenvinkel P. Accelerated lean body mass loss in incident chronic dialysis patients with diabetes mellitus. Kidney Int. 2005;68:2368–74. [DOI] [PubMed] [Google Scholar]
- 49.Anding K, Bar T, Trojniak-Hennig J, Kuchinke S, Krause R, Rost JM and Halle M. A structured exercise programme during haemodialysis for patients with chronic kidney disease: clinical benefit and long-term adherence. BMJ Open. 2015;5:e008709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ormel HL, van der Schoot GGF, Sluiter WJ, Jalving M, Gietema JA and Walenkamp AME. Predictors of adherence to exercise interventions during and after cancer treatment: A systematic review. Psychooncology. 2018;27:713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nam S, Dobrosielski DA and Stewart KJ. Predictors of exercise intervention dropout in sedentary individuals with type 2 diabetes. J Cardiopulm Rehabil Prev. 2012;32:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shalom R, Blumenthal JA, Williams RS, McMurray RG and Dennis VW. Feasibility and benefits of exercise training in patients on maintenance dialysis. Kidney Int. 1984;25:958–63. [DOI] [PubMed] [Google Scholar]
- 53.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, McAllister CJ, Alcorn H Jr., Kopple JD and Greenland S. Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant 2005;20:1880–8. [DOI] [PubMed] [Google Scholar]
- 54.Mukai H, Villafuerte H, Qureshi AR, Lindholm B and Stenvinkel P. Serum albumin, inflammation, and nutrition in end-stage renal disease: C-reactive protein is needed for optimal assessment. Semin Dial 2018. [DOI] [PubMed] [Google Scholar]
- 55.Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis. 2000;35:S1–140. [DOI] [PubMed] [Google Scholar]
- 56.Painter P Physical functioning in end-stage renal disease patients: update 2005. Hemodial Int. 2005;9:218–35. [DOI] [PubMed] [Google Scholar]
- 57.Cardoso FM, Almodhy M, Pepera G, Stasinopoulos DM and Sandercock GR. Reference values for the incremental shuttle walk test in patients with cardiovascular disease entering exercise-based cardiac rehabilitation. J Sports Sci. 2017;35:1–6. [DOI] [PubMed] [Google Scholar]
- 58.Lewis ME, Newall C, Townend JN, Hill SL and Bonser RS. Incremental shuttle walk test in the assessment of patients for heart transplantation. Heart. 2001;86:183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh SJ, Morgan MD, Hardman AE, Rowe C and Bardsley PA. Comparison of oxygen uptake during a conventional treadmill test and the shuttle walking test in chronic airflow limitation. Eur Respir J. 1994;7:2016–20. [PubMed] [Google Scholar]
- 60.Mong Y, Teo TW and Ng SS. 5-repetition sit-to-stand test in subjects with chronic stroke: reliability and validity. Arch Phys Med Rehabil. 2010;91:407–13. [DOI] [PubMed] [Google Scholar]
- 61.Podsiadlo D and Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. [DOI] [PubMed] [Google Scholar]
- 62.Van Bortel LM, Duprez D, Starmans-Kool MJ, Safar ME, Giannattasio C, Cockcroft J, Kaiser DR and Thuillez C. Clinical applications of arterial stiffness, Task Force III: recommendations for user procedures. Am J Hypertens. 2002;15:445–52. [DOI] [PubMed] [Google Scholar]
- 63.Ware JE, Keller SK SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston: The Health Institute; 1994. [Google Scholar]
- 64.Matthews CE, Ainsworth BE, Hanby C, Pate RR, Addy C, Freedson PS, Jones DA and Macera CA. Development and testing of a short physical activity recall questionnaire. Medicine and science in sports and exercise. 2005;37:986–94. [PubMed] [Google Scholar]
- 65.Schulz KF and Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet. 2002;359:781–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of Intradialytic Oral Protein Supplementation and Aerobic Exercise Training on Self-reported Quality of Life and Physical Activity Levels