Abstract
Background:
The aim of the study was to evaluate the diagnostic accuracy of cervical elastography in predicting preterm delivery (PTD).
Methods:
We searched the PubMed, EMBASE, and Cochrane databases to identify relevant studies that applied ultrasound (US) elastography to assess cervical stiffness and predict PTD. All the studies were published before December 11, 2018, and only studies published in English were collected. The cervical length (CL) was considered a comparator, and the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool was applied to assess the quality of the included studies. Summary receiver operating characteristic (SROC) modeling was performed to evaluate the diagnostic performance of cervical elastography in predicting PTD. Subgroup analyses were also performed.
Results:
Seven studies, including 1488 pregnant women, were included in this meta-analysis. Cervical elastography showed a summary sensitivity of 0.84 [95% confidence interval (CI): 0.68, 0.93], a specificity of 0.82 (95% CI: 0.63, 0.93), a diagnostic odds ratio of 25 (95% CI: 7, 93), and an area under the curve (AUC) of SROC of 0.90 (95% CI: 0.87–0.93). CL measurement showed that the AUC of SROC was 0.60 (95% CI: 0.56–0.64). The results of subgroup analysis showed that the summary sensitivity and specificity were different in the QUADAS-2 score subgroups.
Conclusion:
Cervical elastography is a promising and reliable method to predict PTD. Cervical elastography showed better diagnostic performance to predict PTD than CL measurement.
Keywords: cervical elastography, meta-analysis, pregnant women, preterm delivery
1. Introduction
Preterm delivery (PTD) is defined as birth occurring fewer than 37 complete weeks of gestational age and is responsible for 75% of all neonate deaths.[1] Premature babies are at a higher risk for cerebral palsy, delayed development, and hearing and sight problems. Despite numerous efforts to decrease the rate of PTD, the World Health Organization has estimated that 11% of all births are preterm.[2] Obstetricians must identify patients at high risk for PTD. New approaches to detect and treat PTD could decrease 35% of neonatal deaths[3,4] and severe complications such as long-term neurodevelopmental disorders, respiratory distress syndrome and sepsis.[5,6] Existing methods to evaluate the risk of PTD include clinical examination, modified Bishop score calculation, and the cervical length (CL) measured by ultrasound (US). The CL evaluated by US is the most effective way to identify women at high risk for PTD[7,8] because US is an objective, more reliable, and easier method than clinical examination and modified Bishop score calculation.[9]
Extracellular matrix changes in collagen organization, the water content, and proteoglycan concentration are the modifications in biomechanical properties that make a cervix soft or hard.[10] As pregnancy progresses, collagenolytic activity increases in cervical tissue, and this activity is more significant in patients with cervical insufficiency.[11] Moreover, such changes in the cervix may be identified before the cervix begins to shorten.[12] However, conventional US cannot detect the changes in cervical stiffness.
US elastography is an imaging technique that can visualize the change in the stiffness of an examined region. Traditionally, elastography is mainly used to observe the size, location, region, and stiffness of breast, thyroid, prostate tumors. Recently, some physicians have used this new tool to assess cervical lesions.[13] Stanziano et al[14] found that cervical ultrasound elastography, by identifying cervical tissue inhomogeneity, may be helpful to predict embryo transfer ease in infertile women candidates to embryo transfer or intracytoplasmic sperm injection. Many radiologists or obstetricians have applied this technique to predict labor induction success.[15,16] A recent meta-analysis reported that, in women treated by medical induction of labor, cervical elastography seemed to be a promising approach to predict the successfulness of labor induction and vaginal delivery.[9] In addition, many clinical studies have applied this method to predict PTD and have reported preliminary results.[17–23] Therefore, searching and collecting currently available studies must be performed to assess the diagnostic performance of cervical elastography in predicting PTD. Currently, no systematic review and meta-analysis have assessed the diagnostic accuracy of cervical elastography in predicting PTD. Thus, we investigated whether cervical elastography is useful in predicting PTD.
2. Methods
2.1. Literature search strategy
We systematically searched the PubMed (National Institutes of Health's National Library of Medicine, Bethesda, MD), EMBASE (Elsevier BV, Amsterdam, The Netherlands), Cochrane (The Cochrane Collaboration, John Wiley & Sons, Inc, Hoboken, New Jersey) databases to identify relevant studies that applied US elastography to predict PTD. The following keywords were used: “cervix uteri,” “cervix,” “elasticity,” “elasticity imaging techniques,” “elastography,” “preterm birth,” and “premature birth.” Our search only included English language published papers. The starting date of the search was not specified and was continually updated until December 11, 2018. All the searched results were exported to a bibliography manager.
2.2. Inclusion criteria
All the studies, including subsets of studies, investigated the diagnostic accuracy of cervical elastography in PTD and satisfied all the following inclusion criteria:
-
1.
The number of patients who underwent cervical US elastography should not exceed 20. No age constriction for the study participants was considered.
-
2.
The diagnosis of PTD was based on birth occurring fewer than 37 complete weeks of gestational age.
-
3.
The studies were prospective or retrospective.
-
4.
The study results showed sufficient detail to evaluate the diagnostic accuracy of cervical US elastography.
In this meta-analysis, the number of true positives (TPs), false positives (FPs), false negatives (FNs), and true negatives (TNs) was collected when analyzing the diagnostic performance of cervical elastography in PTD. US elastography can be used alone or combined with other diagnostic approaches (e.g., CL and modified Bishop score).
2.3. Exclusion criteria
The studies satisfied all the following exclusion criteria:
-
1.
Studies with a sample size fewer than 20 patients.
-
2.
Literature including editorials, letters, review papers, comments, and conference proceedings.
-
3.
Studies about no human subjects.
-
4.
Articles that were not written in English.
-
5.
Studies that could not extracted the number of TPs, FPs, FNs, and TNs.
-
6.
Studies with overlapping patients and data.
Two independent reviewers (XX and SC) selected the published studies. Consensus agreements were reached after consultation when 2 reviewers had different opinions.
2.4. Index and comparator tests
In this meta-analysis, cervical US elastography was considered as the index test. Although different elastography methods or commercial ultrasound scanners were adopted by physicians from different centers, we considered all eligible elastography approaches because they are all ultrasound-based methods to assess cervical tissue characterization. The primary methods that physicians use to assess cervical tissue can be broadly divided into 2 categories: strain elastography (SE) and shear wave elastography (SWE).
SE needs mechanically induced or passive internal physiologically induced or active external displacement of the tissue surface to generate elastograms. The main SE methods used in this meta-analysis were the following: tissue Doppler imaging (TDI) (Toshiba Medical Systems, Tokyo, Japan), which was originally used for cardiac imaging, has been largely applied in liver, breast and cervix disease.[21] When pressure is applied on the anterior lip of the cervix, tissue movement can be tracked by TDI, where TDI-Q (Q-Quantification) software (Toshiba Medical Systems) can estimate tissue stiffness. Elastoscan (Samsung Medison), a cervical elastography method in which tissue movements could be represented on a color map, allowing visual evaluation of stiffness.[18,19] Another method is real-time SE (Hitachi Medical Systems, Tokyo, Japan) that evaluates tissue stiffness by calculating the tissue displacement in the axial direction of the ultrasound beam.[17,24]
SWE, including acoustic radiation force impulse (ARFI) imaging and supersonic shear imaging (SSI), needs acoustic radiation force impulses to generate elastograms. SWE is a quantitative method to assess tissue stiffness using shear wave speed (SWS) or Young modulus. ARFI (Siemens Healthcare, Erlangen, Germany) is a technique that uses short-duration pushing pulses to cause tissue displacement and ARFI-based (eSie Touch EI and Virtual Touch tissue quantification) elasticity models to generate SWS to assess tissue stiffness.[20] SSI (SuperSonic Imagine, Aix-en-Provence, France), which refreshes at up to several times per second, also uses SWS to assess tissue stiffness.[22]
CL measurement is considered a comparator test in several literature sources; however, in this meta-analysis, no comparator test was considered for eligibility.
2.5. Quality assessment
Two viewers (JW and MY) used the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) to assess the quality of published studies.[25] Consensus agreements were reached after consultation when the 2 reviewers had different opinions. To assess publication bias, Deeks funnel plot was applied.[26]
2.6. Statistical analysis and data synthesis
Data were analyzed by STATA 14 (StataCorp LP, College Station, TX). TPs, TNs, FPs, and FNs for the diagnostic accuracy of cervical elastography were extracted or calculated from the source literature directly. We also obtained the sensitivity, specificity, positive and negative likelihood ratios (LRs), diagnostic odds ratio (DOR), and the 95% confidence intervals (CIs) of each study. The I2 index was used to determine the heterogeneity among the studies. An I2 index value >50% and a P value <.10 were considered statistically significant signs for heterogeneity.[27] When heterogeneity was noted, the Spearman correlation coefficient between the sensitivity and FP rate was calculated. A Spearman correlation coefficient >0.6 indicated a considerable threshold effect.[25] Summary receiver operating characteristic (SROC) was plotted to obtain area under the curve (AUC). Subgroup analysis was performed to identify the cause of heterogeneity. The following subgroups were considered: elastography mode (SE vs SWE), ultrasound examination approach (transvaginal US vs transabdominal US), QUADAS-2 score (5 vs 6), and pregnancy trimester (only second trimester vs others). Results were considered statistically significant at P < .05 for subgroup analysis
3. Results
3.1. Search results
Figure 1 describes the selection process. The literature search of EMBASE, PubMed, and Cochrane database initially identified 128 articles, of which 42 were removed due to duplicates. Next, 67 articles were excluded after reviewing the titles and abstracts. After viewing the full-text of the selected 19 articles, 12 studies were excluded because we could not extract the number of TPs, FPs, FNs, and TNs. Finally, we included 7 studies in our meta-analysis.[17–23]
Figure 1.

Flow chart of the literature search and selection.
3.2. Characteristics of the included studies
Table 1 summarizes the general characteristics of 7 included studies. In this meta-analysis, besides cervical elastography, we also extracted CL data to compare the diagnostic accuracy of these 2 methods. Table 2 summarizes the cervical elastography and CL data. These 7 included studies, published between 2014 and 2018, enrolled 1488 pregnant women for cervical ultrasound examination: 2 studies were conducted in the USA,[17,22] 2 studies were conducted in India,[20,23] and the remaining 3 studies were conducted in Europe.[18,19,21] The median age of pregnant women in 7 included studies range from 23 to 33. All the studies were prospective, and PTD and at-term pregnancies were included. In those included studies, physicians applied different ultrasound devices to assess cervix stiffness. Two studies used the Samsung Medison V20 Prestige imaging system (Samsung Medison, Seoul, South Korea).[18,19] Two studies used the Acuson S2000 (Siemens Medical Solution, Erlangen, Germany).[20] One study used the SuperSonic Imagine imaging system (SuperSonic Imagine).[22] The other studies used the Hitachi HI Vision 900 imaging system (Hitachi Medical Cooperation, Tokyo, Japan)[17] and Toshiba Aplio XG imaging system (Toshiba Medical Systems Europe, Zoetermeer, The Netherlands).[21] All the included studies provided cutoff values. Other detailed information is presented in Tables 1 and 2.
Table 1.
Main characteristics of the included studies.
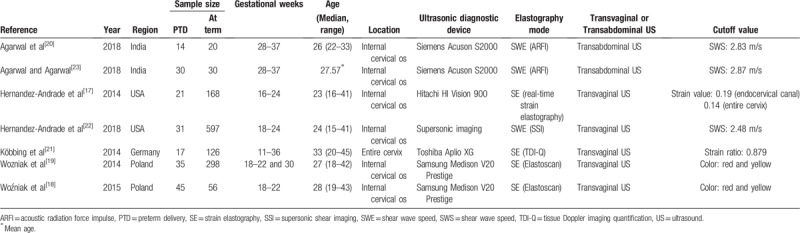
Table 2.
Data of the included studies.

3.3. Quality assessment of the included studies
Figure 2 shows the quality assessment of the included studies using QUADAS-2. The same reference standard was used in all the included studies. The index test results were blinded in all cases. The 7 included articles were prospective studies with appropriate exclusion criteria such as cervical surgery (cerclage) and chromosome abnormalities. One study only enrolled pregnant women with PTD syndrome.[20] Agarwal and Agarwal's[23] design was an observational case control study. Woźniak et al[18] studied pregnant women with a short cervix (CL < 25 mm) and those with a low risk for PTD (CL > 25 mm).[19] The remaining studies included pregnant women regardless of PTD syndrome or a short cervix. Because no cervix elastography standard has been published to date to predict PTD, no threshold value of the included 7 studies was prespecified. The quality of all the included studies was moderate, and all satisfied at least 5 of the 7 items.
Figure 2.
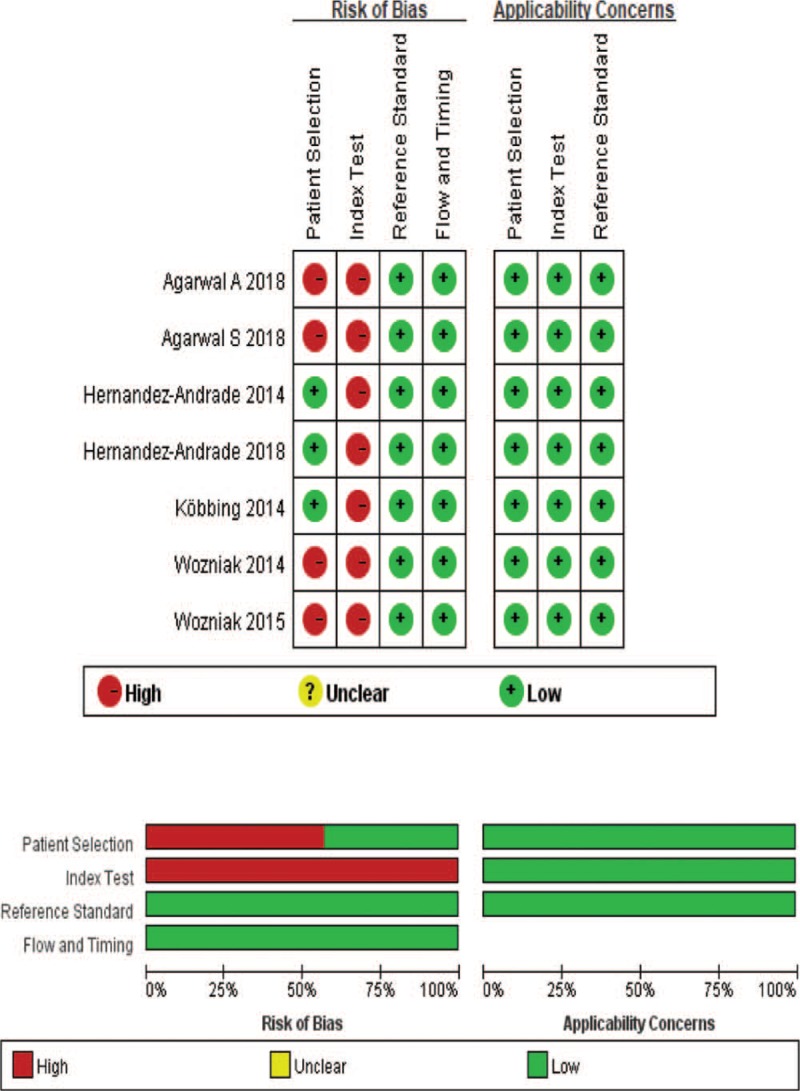
Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) criteria for the included studies.
3.4. Bias risk assessment
Studies included in this meta-analysis adopted different US systems and elastography methods to assess cervical stiffness, which may cause heterogeneity. However, the authors of the included 7 studies believed that cervical elastography could predict PTD and agreed to pursue this technique in obstetrics. The P value of Deeks asymmetry test was .73, indicating that no significant publication bias exists among the included 7 studies (Fig. 3).
Figure 3.
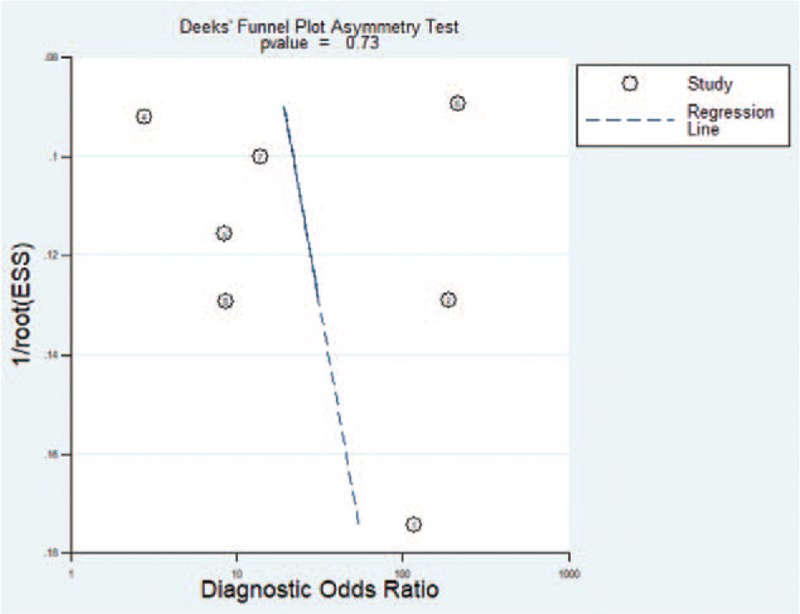
Deeks funnel plot to evaluate potential publication bias.
3.5. Main analysis
In this meta-analysis, the sensitivity and specificity of each included study ranged from 48% to 97% and 30% to 97%, respectively. Higgins I2 statistics showed considerable heterogeneity in this meta-analysis (I2 = 96, 95%). The Spearman correlation coefficient of sensitivity and 1-specificity was −0.571 (P = .18). The forest plot of sensitivity and specificity also showed no threshold effect (Fig. 4). The proportion of heterogeneity likely due to the threshold effect was 0.01. Cervical elastography showed a summary sensitivity of 0.84 (95% CI: 0.68, 0.93), a specificity of 0.82 (95% CI: 0.63, 0.93), a positive likelihood ratio (+LR) of 4.7 (95% CI: 2.1, 10.7), a negative likelihood ratio (−LR) of 0.19 (95% CI: 0.09, 0.42), and a DOR of 25 (95% CI: 7, 93). In sensitivity analysis, 1 study caused considerable heterogeneity.[17] After excluding that study, I2 was reduced from 96, 95% to 55, 95%, and the recalculated sensitivity and specificity were 0.81 (95% CI: 0.64, 0.92) and 0.88 (95% CI: 0.78, 0.94). Subgroup analysis revealed that the QUADAS-2 score was a significant factor affecting study heterogeneity (Table 3). The diagnostic accuracy of CL measurement in predicting PTD was also analyzed to compare cervical elastography. The CL showed a summary sensitivity of 0.36 (95% CI: 0.20, 0.55), a specificity of 0.86 (95% CI: 0.65, 0.95), a +LR of 2.5 (95% CI: 1.3, 4.7), a −LR of 0.75 (95% CI: 0.63, 0.89), and a DOR of 3 (95% CI: 2, 6). The AUC values of SROC of elastography and CL were 0.90 (95% CI: 0.87–0.93) and 0.60 (95% CI: 0.56–0.64) (Fig. 5).
Figure 4.
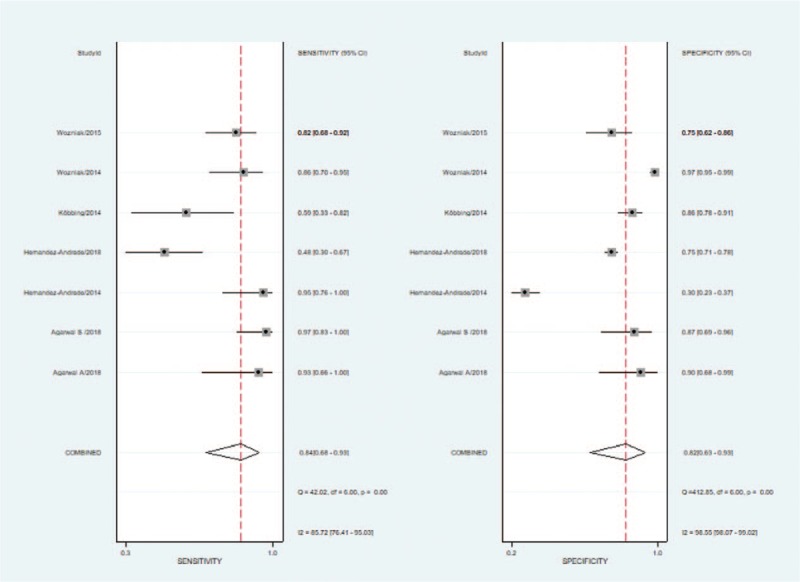
Couple forest plot of sensitivity and specificity of cervical elastography to predict preterm delivery.
Table 3.
Subgroup analysis.
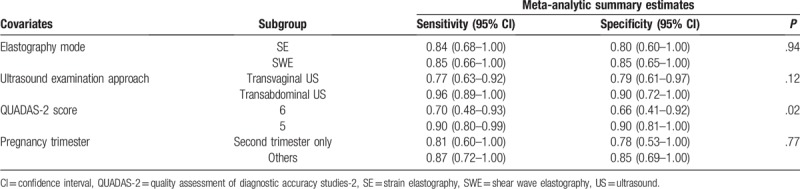
Figure 5.
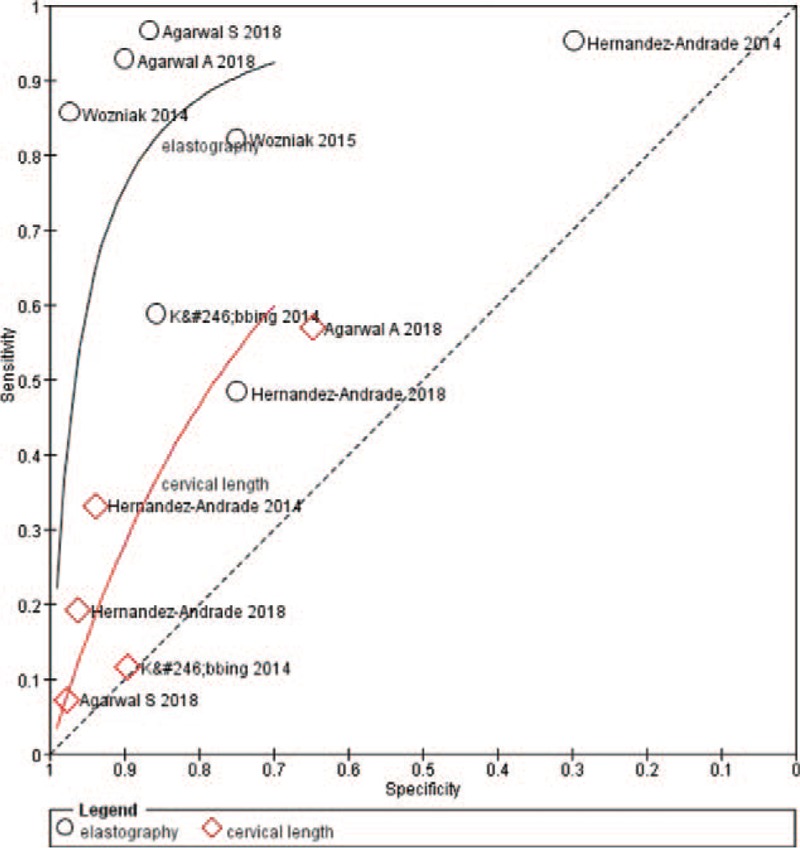
Summary receiver operating characteristic (SROC) curve of the diagnostic performance of cervical elastography and cervical length to predict preterm delivery.
4. Discussion
This meta-analysis found that cervical elastography showed a summary sensitivity of 0.84 (95% CI: 0.68, 0.93) and specificity of 0.82 (95% CI: 0.63, 0.93), as well as a recalculated sensitivity of 0.81 (95% CI: 0.64, 0.92) and specificity of 0.88 (95% CI: 0.78, 0.94), to predict PTD. The DOR and AUC were 25 (95% CI: 7, 93) and 0.90 (95% CI: 0.87–0.93), respectively, indicating that cervical elastography is useful to predict PTD. Compared with CL measurement, which showed an AUC of 0.60 (95% CI: 0.56–0.64), cervical elastography is likely a better choice to predict PTD.
How to predict PTD with high sensitivity and specificity is a major challenge in the current field of obstetrics. Many modalities are used to predict PTD, but they are inaccurate in more than half of the cases.[28] Many overlapping pathophysiology mechanisms cause PTD that are more associated with the remodeling of the uterus cervix. During pregnancy and labor, the cervix changes dynamically and can be roughly described as cervical softening, dilatation and effacement. Cervical dilatation and effacement can be observed directly on ultrasound imaging. Existing studies and current obstetricians use 25 mm as a criterion for premature risk that may cause underestimation in approximately 63% of PTD patients whose CL is >25 mm.[19,29] Thus, many studies have observed cervical softening through cervical elastography with promising results.
Both SE and SWE produce satisfactory reproducibility and repeatability elasticity measurements.[30,31] However, when assessed by SE, the compression is not standardized, making quantification of tissue stiffness impossible.[32] In addition, when assessed by SWE, placing a transducer next to the cervix likely causes a change in cervical stiffness.[32] SWE has overcome the limitation of SE by automatically generating acoustic forces to create shear waves that can be measured directly and used as an estimation of elastography by Young's modulus.[33] However, SWE and SE use different mechanisms to generate elastograms. Some studies have found that, in the differentiation of benign and malignant breast lesions, the diagnostic accuracy for SWE is similar to that of SE.[34,35] In our study, subgroup analysis also showed no significant difference between cervical SE and SWE to predict PTD (P = .94). However, due to the limited number of published studies, biases can affect the interpretation of the results.
The meta-analysis results proved that the cervical elastography approaches are promising. In our meta-analysis, many elastography methods, including ARFI, SSI, strain ratio calculation, and TDI-Q, have been used, likely leading to the loss of data reliability. We cannot perform metaregression due to the limited number of published studies. However, many physicians have performed studies comparing different elastography systems for clinical applications, and their results showed high intraobserver and interobserver correlation.[31] Anesa et al[36] found out that different systems had a coefficient of variation in the range 0.00 to 0.21 for all 4 phantoms, equivalent to low variance and high repeatability when using shear wave elastography assessing liver fibrosis.
When observing the cervix through abdominal US, a full bladder is needed for better imaging of cervix. A full bladder or maternal body habitus may affect the transabdominal visualization of the cervix.[37,38] Bladder fullness also can impact transabdominal CL.[39] In some studies, when measuring CL, transabdominal measurement does not reflect the transvaginal measurement accurately.[40,41] Thus, when assessing cervical stiffness, the cervix condition may be affected by the compression of bladder fullness. However, subgroup analysis showed no significant difference in transvaginal US and transabdominal US when assessing the elastography of the cervix (P = .12). The compression caused by a full bladder may not sufficiently impact cervix stiffness. Nevertheless, future studies are needed to determine whether transvaginal US and transabdominal US produce different results in assessing cervical stiffness.
The gestational weeks of pregnant women included in our study varied. Most studies comprised second-trimester pregnant women.[17–19,21,22] In this meta-analysis, we could not identify which trimester showed the best diagnostic accuracy when assessing cervical stiffness. Although Köbbing et al's[21] study selected 182 pregnant women between 11 and 36 gestational weeks, and found out that, when assessing cervical stiffness to predict PTD, the third-trimester group had a better diagnostic performance (sensitivity: 0.88; specificity: 0.70) than the second-trimester group (sensitivity: 0.57; specificity: 0.50). Which gestational week is optimal to identify pregnant women with a high risk of PTD remains uncertain. In addition, during the evaluation, which intervention would have a better effect on the PTD outcome should be considered.
Our meta-analysis showed considerable heterogeneity in the diagnostic performance of cervical elastography in predicting PTD, which affected the interpretation of our results. Thus, sensitivity analysis and subgroup analysis were performed. The sensitivity analysis showed that heterogeneity was reduced (I2: from 96, 95% to 55, 95%), and the recalculated sensitivity and specificity were 0.81 (95% CI: 0.64, 0.92) and 0.88 (95% CI: 0.78, 0.94). Hernandez-Andrade et al's study,[17] which was excluded in our sensitivity analysis, used dichotomous variables to identify a hard cervix with a lower risk of spontaneous PTD, whereas other studies identified the soft cervix with a higher risk of PTD.[18–22] After sensitivity analysis, the recalculated sensitivity and specificity showed that cervical elastography is a good method to predict PTD.
The subgroup analysis results showed that the QUADAS-2 score was a significant factor affecting study heterogeneity. The difference between 2 QUADAS-2 score groups is likely due to patient sampling. One study only enrolled pregnant women with clinical signs of PTD.[20] Agarwal and Agarwal's[23] design was an observational case control study with a group of pregnant women with clinical signs of PTD and another group of pregnant women with no PTD symptom. Woźniak et al[18] studied pregnant women with a short cervix (CL < 25 mm) or a low risk for PTD (CL > 25 mm).[19] The remaining studies, which were in the higher QUADAS-2 score group, included pregnant women regardless of PTD symptoms and CL. That pregnant women with PTD symptoms or with short CL are more likely to have a prior PTD may have caused heterogeneity in our study.[20,22] Sensitivity and subgroup analysis may partly explained the heterogeneity; however, some of the heterogeneity was unexpected.
5. Limitation
Our study had several limitations. First, only 7 studies were included in our study; thus, the sample size was relatively small and insufficient. The incongruence discussed above should be considered and evaluated in a future study. Second, considerable heterogeneity was found in our study, and interpretation should be cautiously made. Patient sampling (pregnant women with clinical signs and a short CL) may be the main cause for heterogeneity. Third, due to limited studies, other factors (i.e., ethnic group, PTD history, age, and ultrasonic diagnostic device) that may affect the diagnostic performance of cervical elastography were not discussed in this study. Given the limited number of studies included in the analysis, the findings from our meta-analysis should be used with caution and those finding should be confirmed in future research.
Despite these limitations, in this meta-analysis, we aimed to evaluate whether cervical elastography could predict PTD. In addition to the currently available studies and evidence, our study is crucial to help obstetricians advance their daily clinical practice.
6. Conclusion
Cervical elastography is a promising method to predict PTD. Cervical elastography showed better diagnostic performance to predict PTD compared with CL measurement. Future multicenter studies with a large sample size are required to confirm these findings.
Author contributions
Conceptualization: Bo Wang, Baiyun He.
Data curation: Bo Wang, Shuangshuang Chen.
Formal analysis: Bo Wang, Yong Zhang, Shuangshuang Chen.
Methodology: Bo Wang, Baiyun He, Bing Hu.
Project administration: Bing Hu.
Resources: Yong Zhang, Bing Hu.
Software: Yong Zhang, Xiaowei Xiang, Juan Wen, Mei Yi.
Supervision: Baiyun He, Bing Hu.
Validation: Xiaowei Xiang.
Visualization: Shuangshuang Chen.
Writing – original draft: Bo Wang.
Writing – review and editing: Bo Wang, Shuangshuang Chen.
Bo Wang orcid: 0000-0002-7283-6611.
Footnotes
Abbreviations: ARFI = acoustic radiation force impulse, AUC = area under the curve, CI = confidence interval, CL = cervical length, DOR = diagnostic odds ratio, FN = false negative, FP = false positive, LR = likelihood ratio, PTD = preterm delivery, QUADAS-2 = Quality Assessment of Diagnostic Accuracy Studies-2, SE = strain elastography, SROC = summary receiver operating characteristic, SSI = supersonic shear imaging, SWE = shear wave elastography, SWS = shear wave speed, TDI = tissue Doppler imaging, TN = true negative, TP = true positive, US = ultrasound.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hannah B, Simon C, Mikkel ZO, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2162–72. [DOI] [PubMed] [Google Scholar]
- [3].Belizán JM, Mcclure EM, Goudar SS, et al. Neonatal death in low-middle income countries: a global network study. Am J Perinatol 2012;29:649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012;379:2151–61. [DOI] [PubMed] [Google Scholar]
- [5].Escobar GJ, Mccormick MC, Zupancic JAF, et al. Unstudied infants: outcomes of moderately premature infants in the neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed 2006;91:F238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Goldenberg RL, Hoffman HJ, Cliver SP. Neurodevelopmental outcome of small-for-gestational-age infants. Eur J Clin Nutr 1998;52suppl:S54–8. [PubMed] [Google Scholar]
- [7].Andersen HF, Nugent CE, Wanty SD, et al. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol 1990;163:859–67. [DOI] [PubMed] [Google Scholar]
- [8].Vincenzo B, Michele B. Cervical length assessment by ultrasound. Acta Obstet Gynecol Scand 2005;84:543–4. [DOI] [PubMed] [Google Scholar]
- [9].Londero AP, Schmitz R, Bertozzi S, et al. Diagnostic accuracy of cervical elastography in predicting labor induction success: a systematic review and meta-analysis. J Perinat Med 2016;44:167–78. [DOI] [PubMed] [Google Scholar]
- [10].Edgar HA, Roberto R, Korzeniewski SJ, et al. Cervical strain determined by ultrasound elastography and its association with spontaneous preterm delivery. J Perinat Med 2014;42:159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rechberger T, Uldbjerg N, Oxlund H. Connective tissue changes in the cervix during normal pregnancy and pregnancy complicated by cervical incompetence. Obstet Gynecol 1988;71:563–7. [PubMed] [Google Scholar]
- [12].Fuchs T, Woytoń R, Pomorski M, et al. Sonoelastography of the uterine cervix as a new diagnostic tool of cervical assessment in pregnant women—preliminary report. Ginekol Pol 2013;84:12–6. [DOI] [PubMed] [Google Scholar]
- [13].Lu R, Xiao Y, Liu M, et al. Ultrasound elastography in the differential diagnosis of benign and malignant cervical lesions. J Ultrasound Med 2014;33:667–71. [DOI] [PubMed] [Google Scholar]
- [14].Stanziano A, Caringella AM, Cantatore C, et al. Evaluation of the cervix tissue homogeneity by ultrasound elastography in infertile women for the prediction of embryo transfer ease: a diagnostic accuracy study. Reprod Biol Endocrinol 2017;15:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hwang HS, Sohn IS, Kwon HS. Imaging analysis of cervical elastography for prediction of successful induction of labor at term. J Ultrasound Med 2013;32:937–46. [DOI] [PubMed] [Google Scholar]
- [16].Pereira S, Frick AP, Poon LC, et al. Successful induction of labor: prediction by preinduction cervical length, angle of progression and cervical elastography. Ultrasound Obstet Gynecol 2015;44:468–75. [DOI] [PubMed] [Google Scholar]
- [17].Hernandez-Andrade E, Romero R, Korzeniewski SJ, et al. Cervical strain determined by ultrasound elastography and its association with spontaneous preterm delivery. J Perinat Med 2014;42:159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Woźniak S, Czuczwar P, Szkodziak P, et al. Elastography for predicting preterm delivery in patients with short cervical length at 18-22 weeks of gestation: a prospective observational study. Ginekol Pol 2015;86:442–7. [DOI] [PubMed] [Google Scholar]
- [19].Wozniak S, Czuczwar P, Szkodziak P, et al. Elastography in predicting preterm delivery in asymptomatic, low-risk women: a prospective observational study. BMC Pregnancy Childbirth 2014;14:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Agarwal A, Agarwal S, Chandak S. Role of acoustic radiation force impulse and shear wave velocity in prediction of preterm birth: a prospective study. Ultrasound (Leeds, England) 2018;59:755–62. [DOI] [PubMed] [Google Scholar]
- [21].Köbbing K, Fruscalzo A, Hammer K, et al. Quantitative Elastography of the uterine cervix as a predictor of preterm delivery. J Perinatol 2014;34:774–80. [DOI] [PubMed] [Google Scholar]
- [22].Hernandez-Andrade E, Maymon E, Luewan S, et al. A soft cervix, categorized by shear-wave elastography, in women with short or with normal cervical length at 18-24 weeks is associated with a higher prevalence of spontaneous preterm delivery. J Perinat Med 2018;46:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Agarwal S, Agarwal A. Fetal adrenal gland biometry and cervical elastography as predictors of preterm birth: a comparative study. Ultrasound 2018;26:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Von Schoning D, Fischer T, Von Tucher E, et al. Cervical sonoelastography for improving prediction of preterm birth compared with cervical length measurement and fetal fibronectin test. J Perinat Med 2015;43:531–6. [DOI] [PubMed] [Google Scholar]
- [25].Devillé WL, Buntinx F, Bouter LM, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 2002;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kim KW, Lee J, Choi SH, et al. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part I. General guidance and tips. Korean J Radiol 2015;16:1175–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim H, Hwang HS. Elastographic measurement of the cervix during pregnancy: current status and future challenges. Obstet Gynecol Sci 2017;60:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Celik E, To M, Gajewska K, et al. Cervical length and obstetric history predict spontaneous preterm birth: development and validation of a model to provide individualized risk assessment. Ultrasound Obstet Gynecol 2010;31:549–54. [DOI] [PubMed] [Google Scholar]
- [30].Swiatkowska-Freund M, Pankrac Z, Preis K. Intra- and inter-observer variability of evaluation of uterine cervix elastography images during pregnancy. Ginekol Pol 2014;85:360–4. [DOI] [PubMed] [Google Scholar]
- [31].Mulabecirovic A, Vesterhus M, Gilja OH, et al. In vitro comparison of five different elastography systems for clinical applications, using strain and shear wave technology. Ultrasound Med Biol 2016;42:2572–88. [DOI] [PubMed] [Google Scholar]
- [32].Fruscalzo A, Mazza E, Feltovich H, et al. Cervical elastography during pregnancy: a critical review of current approaches with a focus on controversies and limitations. J Med Ultrason 2016;43:493–504. [DOI] [PubMed] [Google Scholar]
- [33].Tsuyoshi S, Nightingale KR, Palmeri ML, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 1: basic principles and terminology. Ultrasound Med Biol 2015;41:1126–47. [DOI] [PubMed] [Google Scholar]
- [34].Chang JM, Won JK, Lee KB, et al. Comparison of shear-wave and strain ultrasound elastography in the differentiation of benign and malignant breast lesions. AJR Am J Roentgenol 2013;201:W347–56. [DOI] [PubMed] [Google Scholar]
- [35].Youk JH, Son EJ, Gweon HM, et al. Comparison of strain and shear wave elastography for the differentiation of benign from malignant breast lesions, combined with B-mode ultrasonography: qualitative and quantitative assessments. Ultrasound Med Biol 2014;40:2336–44. [DOI] [PubMed] [Google Scholar]
- [36].Anesa M, Batman MA, Helge GO, et al. Repeatability of shear wave elastography in liver fibrosis phantoms—Evaluation of five different systems [J]. PLoS One 2018;13:e0189671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].To MS, Skentou C, Cicero S, et al. Cervical assessment at the routine 23-weeks’ scan: problems with transabdominal sonography. Ultrasound Obstet Gynecol 2000;15:292–6. [DOI] [PubMed] [Google Scholar]
- [38].Andersen HF. Transvaginal and transabdominal ultrasonography of the uterine cervix during pregnancy. J Clin Ultrasound 2010;19:77–83. [DOI] [PubMed] [Google Scholar]
- [39].Chaudhury K, Ghosh M, Halder A, et al. Is transabdominal ultrasound scanning of cervical measurement in mid-trimester pregnancy a useful alternative to transvaginal ultrasound scan? J Turk Ger Gynecol Assoc 2013;14:225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Westerway SC, Pedersen LH, Hyett J. Cervical length measurement: Comparison of transabdominal and transvaginal approach. Australas J Ultrasound Med 2016;18:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cho HJ, Roh HJ. Correlation between cervical lengths measured by transabdominal and transvaginal sonography for predicting preterm birth. J Ultrasound Med 2016;35:537–44. [DOI] [PubMed] [Google Scholar]


