Abstract
We report a case of systemic oxalosis involving the eyes and joints due to long-term use of high-dose vitamin C in a patient receiving maintenance peritoneal dialysis (PD). This 76-year-old female with autosomal dominant polycystic kidney disease underwent living unrelated kidney transplantation ten years earlier. The transplant failed six months prior to presentation, and she initiated hemodialysis before transitioning to PD four months later. Over the month prior to presentation, the patient noted worsening arthralgias and decreased vision. Ophthalmologic exam revealed proliferative retinopathy and calcium oxalate crystals. Plasma oxalate (POx) was markedly elevated at 187 μmol/L (reference range < 1.7 μmol/L), and a urine oxalate-creatinine ratio was high (0.18 mg/mg). The patient reported taking up to four grams of vitamin C per day for several years. Workup for causes of primary and secondary hyperoxaluria was otherwise negative. Vitamin C was discontinued, and the patient transitioned to daily hemodialysis for two weeks; POx prior to the dialysis session decreased but remained higher (30–53 μmol/L) than typical for dialysis patients. Upon discharge, the patient remained on thrice weekly hemodialysis with stabilized vision and improved joint symptoms. This case highlights the risk of high-dose vitamin C in patients with advanced CKD, especially when maintained on PD.
Keywords: Ascorbic acid, oxalosis, peritoneal dialysis (PD), retinopathy, vitamin C, supplements, dialytic clearance, oxalate removal, vision loss, case report
INTRODUCTION
Vitamin C (ascorbic acid) is often self-prescribed by many individuals in the general population for its anti-oxidant properties, but many who use it are unaware of potential harmful side-effects. Vitamin C is under investigation as a treatment for advanced cancer1 and sepsis.2 Vitamin C deficiency has been noted in the dialysis population and has been associated with poor response to erythrocyte-stimulating agents (ESA).3,4
Systemic oxalosis has been well-described in patients with genetic causes of hyperoxaluria who lose native kidney function and are maintained on dialysis5,6. Vitamin C can convert to oxalate, and there are reports of oxalate disorders attributed to vitamin C administration.7,8 Here we describe a case of systemic oxalosis with eye and joint involvement due to long-term use of high-dose vitamin C in a patient receiving maintenance peritoneal dialysis (PD). This case highlights the potential risk of vitamin C in this patient population.
CASE REPORT
A 76-year-old female presented to our emergency department for evaluation of arthralgias and decreased vision. She had a prior history of end-stage kidney disease secondary to autosomal dominant polycystic kidney disease and received a living unrelated kidney transplant 10 years prior to presentation. Nine years later, kidney biopsy performed for declining graft function revealed severe arteriosclerosis, arteriolar hyalinosis, glomerulomegaly with focal segmental glomerulosclerosis, early transplant glomerulopathy and no calcium oxalate crystals. Due to allograft failure, the patient initiated hemodialysis (HD) six months prior to presentation. All immunosuppressants were discontinued at the time of dialysis initiation. Four months later due to patient preference, she transitioned to nightly PD on a cycler. Her residual kidney function was minimal (Kt/V of 0.07), but she achieved adequate clearance on PD with a weekly Kt/V of at least 2.05 until presentation.
About one month prior to admission, the patient noted decreasing vision and arthralgias affecting her fingers, knees, and shoulders. An abnormal ophthalmologic exam at an outside facility suggested crystal deposition (images previously published9). Thus she was referred to our facility and admitted to the hospital for further management. Ophthalmologic examination revealed retinal hemorrhages and crystalline deposits consistent with calcium oxalate deposition (Figure 1A and B). Plasma oxalate (POx) upon admission was markedly elevated (187 μmol/L; reference range < 1.7 μmol/L). A random urine oxalate-creatinine ratio was high (225 mmol/mol, reference range <40 mmol/mol) while urine glycolate, glycerate, and 4-hydroxy-2-oxoglutarate (HOG) excretions were normal. There was no history of diarrhea, gastrointestinal symptoms, or prior gastrointestinal surgeries. A random stool fat analysis did not suggest fat malabsorption. The patient self-prescribed high-dose vitamin C for at least five years (4000 mg/d in the winter and 2000 to 3000 mg/d in the summer) due to personal belief of its homeopathic properties. No history of excessive intake of high-oxalate foods was identified. Knee synovial fluid analysis was negative for crystals or inflammation, but the significant synovitis led to the presumptive diagnosis of oxalate arthritis. Echocardiogram revealed only mild left ventricular enlargement, and an electrocardiogram was unremarkable. The patient was severely anemic (hemoglobin 6.6 g/dL), but a bone marrow biopsy was normocellular without calcium oxalate crystals.
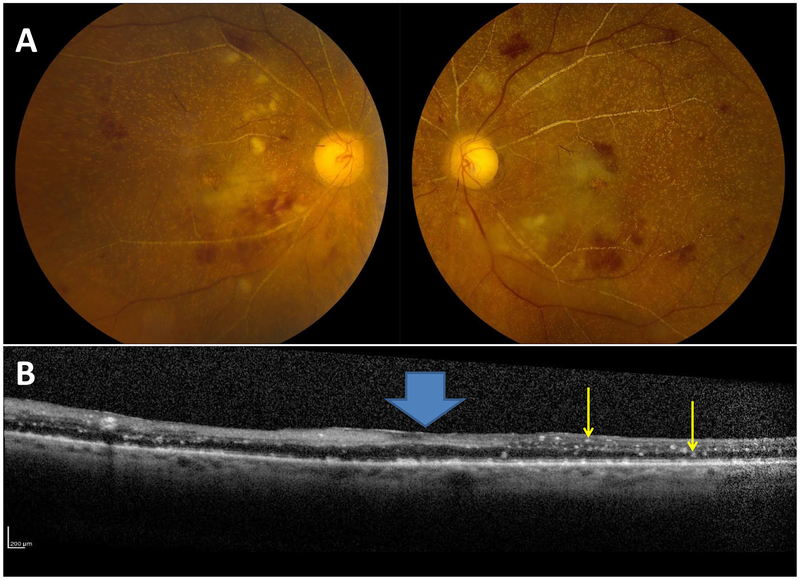
Figure 1. Ophthalmic examination in retinal oxalosis.
Funduscopic examination revealed typical crystalline deposits due to oxalate deposition with secondary retinal hemorrhages (Panel A) and optical coherence tomography showed hyperreflective deposits (yellow arrows) throughout the inner and outer retina with loss of normal, concave foveal contour (blue block arrow, Panel B).
Exogenous vitamin C was discontinued, and she received nearly daily hemodialysis for two weeks. Pre-dialysis POx fell to between 30 to – 53 μmol/L (Figure 2A). The dialysis frequency was decreased to four times per week for an additional two weeks. Repeated ophthalmologic evaluations confirmed the presence of retinal calcium oxalate crystals with associated ischemic change, and subsequently development of retinal neovascularization. The patient’s vision stabilized with the intensive dialysis, while her joint symptoms improved markedly with empiric steroids. Pre-dialysis POx remained stable at 33.0 μmol/L four weeks later. At that point, an oxalate removal study (Figure 2B) documented 1.03 mmol of oxalate in the dialysate over a 4-hour hemodialysis treatment, similar to patients with PH.10 Genetic testing did not reveal any pathogenic polymorphisms in AGXT, GRHPR or HOGA1.5 She was discharged on three days per week hemodialysis. Follow-up pre-dialysis POx level two months later remained elevated at 35.2 μmol/L. Ten months later pre-dialysis POx was improved to 20.3 μmol/L, a value more typical of patients on maintenance hemodialysis without a specific disorder of oxalate metabolism.
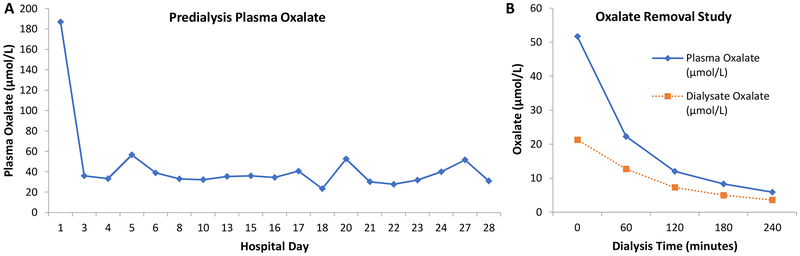
Figure 2: Plasma oxalate concentrations over time.
POx concentrations (Panel A) were markedly increased on presentation (187 μmol/L), fell with frequent dialysis, but were persistently higher (30–52 μmol/L) than typically expected for a hemodialysis patient. On hospital day 27, an oxalate removal study was performed and a total of 1.03 mmol oxalate was removed after four hours of hemodialysis (Panel B). The first dialysate oxalate level was measured ten minutes after starting hemodialysis. The efficiency of oxalate removal progressively declined over the 4-hour session as the POx concentration fell, but POx increased to 31.0 μmol/L pre-dialysis the next morning on the day of hospital discharge (Panel A). Conversion factor for oxalate in mg/L to μmol/L, x11.1.
DISCUSSION
This case demonstrates the potential danger of high-dose vitamin C ingestion, especially in patients with chronic kidney disease (CKD). Vitamin C is an important exogenous antioxidant integral for collagen formation and catecholamine synthesis. The recommended daily dose is 70–90 mg/d which can easily be met when one’s diet is rich in fruits and vegetables. The absorption of vitamin C occurs via sodium dependent (SVCT [sodium-ascorbate cotransporter]) and hexose (GLUT) transporters in the gut. Vitamin C can also be intravenously administered and is increasingly utilized as an alternative or adjunct treatment for malignancy and other critically ill patients, including for septic shock. Vitamin C is water soluble and primarily cleared by the kidneys.11 Vitamin C can be found in the urine when intake is more than 50 mg/d.12 Patients with CKD are instructed to limit intake of potassium and are thus at risk of vitamin C deficiency since many food sources of vitamin C are also rich in potassium.3 Given these considerations, vitamin C was often administered to improve ESA responsiveness.4 However, routine supplementation with vitamin C (500 mg by mouth daily over six months) in a hemodialysis population was found to be associated with elevated POx concentrations.13 Therefore, the most recent KDIGO guideline suggests that vitamin C not be used to enhance ESA responsiveness.14
Once vitamin C is absorbed, the vast majority resides in the intracellular compartment throughout the body. This intracellular vitamin C is then converted to dehydroascorbate, which can then form 2,3-diketogluconic acid, an unstable molecule that subsequently breaks down into oxalic acid.15 Thus, higher vitamin C intake is associated with increased urinary oxalate excretion.16 In humans, oxalate is an end-product of metabolism that is primarily cleared by the kidneys.17 Therefore, as GFR falls, overproduction or over absorption results in elevated blood concentrations and retention throughout body tissues.18 For example, patients with PH produce excessive amounts of oxalate in their liver due to mutations in one of the three known causative genes AGXT, GRHPR or HOGA1 for PH 1, 2 and 3, respectively. When a patient with PH develops advanced CKD, oxalate is retained in the body, and systemic oxalosis can ensue. Oxalate nephropathy, nephrocalcinosis, retinal, joint, and cardiac lesions have all been described.5,6,19 Similar presentations can occur with exogenous intake and/or over absorption of oxalate in the setting of reduced GFR.8 The diagnosis is suspected when the 24 hour urine oxalate excretion or a random oxalate-creatinine ratio is elevated, although either may be falsely low in the setting of a markedly reduced GFR.18 Normal urine glycolate, glycerate, and HOG excretions, which are elevated in PH type 1, 2, and 3 respectively, make genetic causes of hyperoxaluria less likely.5,20,21 PH is definitively ruled out when genetic testing reveals no pathogenic variants of AGXT, GRHPR or HOGA1..
Management of oxalosis from exogenous oxalate and/or precursors begins with discontinuing all supplemental forms of vitamin C and eating low oxalate diet. The subsequent management aims at oxalate removal. We previously reported that hemodialysis can be used to clear oxalate from the blood. In a cohort of 13 PH patients, POx was reduced by an average of 78.4% +/−7.7% (SD) after each HD session; approximately half of the oxalate was removed during the first hour of HD, similar to our patient (Figure 2B), with diminishing amounts removed in subsequent hours. Thus, although HD effectively clears oxalate from the blood, oxalate has a large volume of distribution and, in PH, a limited amount of total body oxalate is removed during a single HD treatment and POx rebounds quickly after each session.10 Accordingly, among PH patients with ESRD, frequent HD sessions are needed to keep up with the ongoing liver production, often 5 or 6 days per week.10 In our case, since the source of oxalate production was removed when vitamin C was discontinued, the total body oxalate burden was gradually reduced and the frequency of dialysis could be safely decreased. While peritoneal transfer of oxalate can occur, due to the relatively low volumes in each exchange, PD does not adequately remove oxalate when oxalosis is a concern.22
Although oxalosis has been largely described as a complication of PH, there are reports of oxalosis associated with high dose vitamin C on the background of decreased kidney function.7,8 Thus in our patient, the combination of high dose vitamin C and the switch to PD both contributed to her oxalosis. Furthermore, despite effective HD, it took many months to decrease her total body oxalate burden, as evidenced by the persistently high pre-dialysis POx values. Although there was evidence of decreased retinal crystallization, the patient’s visual symptoms only marginally improved, similar to another published case of retinal oxalosis in which long-term progressive macular changes associated with atrophy and fibrosis from neovascularization around sites of crystal deposition and minimal improvement of visual symptoms were reported.19
In conclusion, this case provides a cautionary tale regarding the risk of high-dose vitamin C in patients with CKD, especially those on PD.
Support:
Investigators on this study were partially supported by the Mayo Foundation as well as the Rare Kidney Stone Consortium (U54KD083908), a member of the NIH Rare Diseases Clinical Research Network (RDCRN), funded by the NIDDK and the National Center for Advancing Translational Sciences (NCATS); Dawn S. Milliner, MD (President) and John C. Lieske, MD (Vice President) are members of the Rare Kidney Stone Consortium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Peer Review: Received September 3, 2018. Evaluated by 2 external peer reviewers, with direct editorial input from an Associate Editor and a Deputy Editor. Accepted in revised form January 18, 2019.
REFERENCES
- 1.Fritz H, Flower G, Weeks L, et al. Intravenous Vitamin C and Cancer: A Systematic Review. Integrative cancer therapies 2014;13:280–300. [DOI] [PubMed] [Google Scholar]
- 2.Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017;151:1229–38. [DOI] [PubMed] [Google Scholar]
- 3.Clase CM, Ki V, Holden RM. Water-soluble vitamins in people with low glomerular filtration rate or on dialysis: a review. Seminars in dialysis 2013;26:546–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deicher R, Ziai F, Habicht A, Bieglmayer C, Schillinger M, Horl WH. Vitamin C plasma level and response to erythropoietin in patients on maintenance haemodialysis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2004;19:2319–24. [DOI] [PubMed] [Google Scholar]
- 5.Hoppe B An update on primary hyperoxaluria. Nature reviews Nephrology 2012;8:467–75. [DOI] [PubMed] [Google Scholar]
- 6.Lorenz EC, Michet CJ, Milliner DS, Lieske JC. Update on oxalate crystal disease. Current rheumatology reports 2013;15:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cossey LN, Rahim F, Larsen CP. Oxalate nephropathy and intravenous vitamin C. American journal of kidney diseases : the official journal of the National Kidney Foundation 2013;61:1032–5. [DOI] [PubMed] [Google Scholar]
- 8.Wells CG, Johnson RJ, Qingli L, Bunt-Milam AH, Kalina RE. Retinal oxalosis. A clinicopathologic report. Archives of ophthalmology (Chicago, Ill : 1960) 1989;107:1638–43. [DOI] [PubMed] [Google Scholar]
- 9.Scruggs BA, Sohn EH. Retinal Oxalosis in End-stage Renal Disease. JAMA ophthalmology 2018;136:e181523. [DOI] [PubMed] [Google Scholar]
- 10.Tang X, Voskoboev NV, Wannarka SL, Olson JB, Milliner DS, Lieske JC. Oxalate quantification in hemodialysate to assess dialysis adequacy for primary hyperoxaluria. American journal of nephrology 2014;39:376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padayatty SJ, Levine M. Vitamin C physiology: the known and the unknown and Goldilocks. Oral diseases 2016;22:463–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knight J, Madduma-Liyanage K, Mobley JA, Assimos DG, Holmes RP. Ascorbic acid intake and oxalate synthesis. Urolithiasis 2016;44:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ono K Secondary hyperoxalemia caused by vitamin C supplementation in regular hemodialysis patients. Clinical nephrology 1986;26:239–43. [PubMed] [Google Scholar]
- 14.Chapter 3: Use of ESAs and other agents * to treat anemia in CKD. Kidney International Supplements 2012;2:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson GL, Ortwerth BJ. The non-oxidative degradation of ascorbic acid at physiological conditions. Biochim Biophys Acta 2000;1501:12–24. [DOI] [PubMed] [Google Scholar]
- 16.Taylor EN, Curhan GC. Determinants of 24-hour urinary oxalate excretion. Clinical journal of the American Society of Nephrology : CJASN 2008;3:1453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rumsey SC, Levine M. Absorption, transport, and disposition of ascorbic acid in humans. The Journal of Nutritional Biochemistry 1998;9:116–30. [Google Scholar]
- 18.Perinpam M, Enders FT, Mara KC, et al. Plasma oxalate in relation to eGFR in patients with primary hyperoxaluria, enteric hyperoxaluria and urinary stone disease. Clin Biochem 2017;50:1014–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sangave AA, Gearinger MD, Diloreto DA. CRYSTALLINE RETINOPATHY FROM HYPEROXALURIA: LONG-TERM FOLLOW-UP. Retinal cases & brief reports 2017;11:1–3. [DOI] [PubMed] [Google Scholar]
- 20.Hopp K, Cogal AG, Bergstralh EJ, et al. Phenotype-Genotype Correlations and Estimated Carrier Frequencies of Primary Hyperoxaluria. Journal of the American Society of Nephrology : JASN 2015;26:2559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riedel TJ, Knight J, Murray MS, Milliner DS, Holmes RP, Lowther WT. 4-Hydroxy-2-oxoglutarate aldolase inactivity in primary hyperoxaluria type 3 and glyoxylate reductase inhibition. Biochimica et biophysica acta 2012;1822:1544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mydlik M, Derzsiova K, Svac J, Dlhopolcek P, Zemberova E. Peritoneal clearance and peritoneal transfer of oxalic acid, vitamin C, and vitamin B6 during continuous ambulatory peritoneal dialysis. Artificial organs 1998;22:784–8. [DOI] [PubMed] [Google Scholar]