Abstract
Inflammation is a hallmark of cancer. For pancreatic ductal adenocarcinoma (PDAC), malignant cells arise in the context of a brisk inflammatory cell infiltrate surrounded by dense fibrosis that is seen beginning at the earliest stages of cancer conception. This inflammatory and fibrotic milieu supports cancer cell escape from immune elimination and promotes malignant progression and metastatic spread to distant organs. Targeting this inflammatory reaction in PDAC by inhibiting or depleting pro-tumor elements and by engaging the potential of inflammatory cells to acquire anti-tumor activity has garnered strong research and clinical interest. Herein, we describe the current understanding of key determinants of inflammation in PDAC; mechanisms by which inflammation drives immune suppression; the impact of inflammation on metastasis, therapeutic resistance, and clinical outcomes; and strategies to intervene on inflammation for providing therapeutic benefit.
Keywords: inflammation, cancer, pancreatic ductal adenocarcinoma, immunotherapy, immune evasion, treatment paradigms, T cells, macrophages, neutrophils, tumor microenvironment, immunosurveillance, vaccines, therapeutic resistance, metastasis, clinical trials
1. Introduction
Virtually all cancers harbor an inflammatory reaction with potential to support cancer progression and metastasis. Inflammation is a complex and highly coordinated cellular and biochemical process intended to resolve tissue injury and protect the host. However, when this “wound healing” process becomes chronic, derangement of normal cellular mechanisms occurs and paradoxically, tissue injury and neoplasia can ensue. In this regard, chronic inflammation is a hallmark of cancer (Hanahan & Weinberg, 2011), and inflammation can impair normal cellular and organ physiology as well as the efficacy of therapeutic interventions (Gajewski, Schreiber, & Fu, 2013; Mantovani, Allavena, Sica, & Balkwill, 2008).
Inflammation in cancer is associated with poor clinical outcomes. The modified Glasgow Prognostic Score (mGPS) and the neutrophil-lymphocyte ratio are measures of systemic inflammation and in pancreatic ductal adenocarcinoma (PDAC), are predictive of prognosis (Liu, Kalbasi, & Beatty, 2017). Inflammation can manifest with fatigue, muscle wasting, malnourishment, and weight loss. Thus, identifying strategies to manage systemic inflammation is a major focus of efforts to improve the supportive care of patients with cancer (Hendifar, et al., 2018).
The inflammatory reaction to cancer is also a critical determinant of therapeutic outcomes. Cellular stress is a major stimulus of inflammation designed to support and protect tissues from injury. However, cancer cells co-opt this “wound healing” process to establish a microenvironment conducive to their survival. This inflammatory response can also enforce remarkable immunosuppressive properties and in doing so, subvert productive immune surveillance in cancer. As such, the local tumor microenvironment and the resonating systemic inflammatory response have emerged as necessary targets for realizing the potential of cancer immunotherapy in PDAC (Beatty & Gladney, 2015).
In this review, we summarize the cellular components of the inflammatory response in the tumor microenvironment of pancreatic cancer, as well as the systemic inflammation that characterizes this malignancy. We review mechanisms by which inflammation promotes immune suppression and metastasis, and focus on current strategies that have emerged from preclinical models and are being evaluated in clinical trials to derail components of inflammation for clinical benefit. In addition, we address challenges with manipulating cancer inflammation including the potential to unleash compensatory mechanisms of therapeutic resistance. Finally, we address the need for new treatment paradigms that challenge the current clinical dogma of “treatment till progression” with the mindset that rational combinations and appropriate sequencing of therapeutics will be needed to not only induce tumor responses but to maintain durable remissions. With the advent of immunotherapy as a standard of care for many cancers, the prospect of converting pancreatic cancer from an immunologically-resistant to -sensitive malignancy has strong clinical implications. To this end, inflammation in pancreas cancer is a major determinant of cancer cell biology, which makes the components of inflammation exciting targets for improving clinical outcomes in this lethal disease.
2. Inflammation in the tumor microenvironment of pancreatic ductal adenocarcinoma
The molecular determinants of PDAC are well-characterized with activating mutations in the KRAS oncogene recognized as almost universally fundamental to PDAC development (Hidalgo, 2010). Nearly all early-stage, low grade, pancreatic intraepithelial neoplasia (PanIN)-1 lesions, which are precursors of PDAC, contain mutations in Kras (Kanda, et al., 2012). Moreover, PanIN-1 lesions are detected in most middle-aged patients (Konstantinidis, et al., 2013) (Matsuda, et al., 2017), yet the average lifetime risk of pancreatic cancer is <2% (Siegel, Miller, & Jemal, 2019). Insight into the role of Kras in pancreatic carcinogenesis has been provided by genetic mouse models, which show that Kras mutations introduced during embryogenesis are sufficient to drive the development of PanIN lesions which progress to invasive PDAC (Hingorani, et al., 2003). However, pancreatic acinar cells of adult mice demonstrate remarkable resistance to oncogenic perturbations in Kras (Guerra, et al., 2007). These seemingly conflicted observations can be explained by a role for inflammation. For instance, in the setting of local inflammation within the pancreas, cellular transformation of adult acinar cells is observed, but only in the presence of Kras oncogenes (Guerra, et al., 2011). In addition, genetic deletion of the inflammatory mediator STAT3 in pancreatic epithelial cells inhibits Kras-induced PanIN formation and progression (Corcoran, et al., 2011; Lesina, et al., 2011). Thus, inflammation demonstrates a key role in awakening epithelial cells with malignant potential in the pancreas. Here, we describe the molecular and cellular determinants of inflammation in pancreatic cancer from conception to invasion and their potential implications in defining PDAC subtypes.
2.1. Inflammation co-evolves with pancreatic cancer development
The earliest stages of pancreatic cancer development are characterized by an inflammatory microenvironment with recruitment of myeloid cells and fibroblasts. This biology has been most extensively studied in preclinical mouse models. For example, in the established LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre (KPC) mouse model of pancreatic cancer (Hingorani, et al., 2005; Lee, Komar, Bengsch, Graham, & Beatty, 2016), local inflammation is detected from disease conception. This early inflammatory reaction that surrounds PanIN lesions is dominated by tumor-associated macrophages (TAMs), immature myeloid cells, neutrophils, and regulatory T cells (Treg) and persists through invasive cancer (Clark, et al., 2007). Although merely cellular precursors of carcinoma, single cells from PanIN lesions in mouse models have shown their capacity to migrate into the surrounding stroma, escape into the bloodstream, and seed the liver (Rhim, et al., 2012). This process is exacerbated by inflammation in the pancreas and thus, defines a role for inflammation in driving early dissemination of pancreatic cells with malignant potential.
The development of pancreatic cancer is a step-wise process that occurs over many years. Mathematical models suggest that the time from the initiating mutation to detection of the parental non-metastatic founder cell can be more than a decade, and a further five years may then elapse before metastatic spread (Yachida, et al., 2010). During this period of cancer evolution, systemic inflammation from co-morbidities can play a supporting role in cancer progression. For example, chronic pancreatitis is a known risk factor for pancreatic cancer (Kirkegard, Mortensen, & Cronin-Fenton, 2017; Midha, Chawla, & Garg, 2016). There is also an emerging understanding that obesity, another risk factor for pancreatic cancer (Renehan, Tyson, Egger, Heller, & Zwahlen, 2008), causes inflammation, including increased levels of cytokines IL-1β, IL-6, IL-8, CCL2, CCL5, and TNFα (Kolb, Sutterwala, & Zhang, 2016). In particular, in mouse models, obesity promotes IL-1β-dependent neutrophil infiltration of pancreatic tumors and is associated with increased desmoplasia and resistance to chemotherapy (Incio, et al., 2016). In addition to being an independent risk factor for pancreatic cancer, obesity is also associated with type II diabetes. Long-term diabetes is also a risk factor for pancreatic cancer, while new-onset diabetes can be an early sign of the disease (Wolfgang, et al., 2013) and a result of the cancer itself (Gong, et al., 2014). Type 2 diabetes is also associated with inflammatory biomarkers produced by adipose tissue, and this inflammatory response can result in insulin resistance (Lontchi-Yimagou, Sobngwi, Matsha, & Kengne, 2013). Other lifestyle factors that both increase systemic inflammation and are linked with pancreatic cancer include tobacco smoking and alcohol use (Anderson, et al., 2012; Korc, Jeon, Edderkaoui, Pandol, & Petrov, 2017). Overall, systemic inflammation can come from a myriad of sources with each capable of increasing the risk of pancreatic cancer development over many years.
Inflammation within the tumor microenvironment co-evolves with cancer development. To this end, the inflammatory phenotype of PDAC is, at least initially, directed by malignant cells. For instance, Kras activation induces the upregulation of GM-CSF in pancreatic ductal epithelial cells which then summons Gr-1+CD11b+ myeloid cells (Bayne, et al., 2012; Pylayeva-Gupta, Lee, Hajdu, Miller, & Bar-Sagi, 2012). Genetic knockdown of GM-CSF in pancreatic epithelial cells blocks this myeloid recruitment with a compensatory influx of T cells with tumor-inhibitory activity. Early in pancreatic tumorigenesis, oncogenic Kras expression in pancreatic acinar cells can also induce expression of intercellular adhesion molecule-1 (ICAM-1), which attracts macrophages to remodel the surrounding extracellular matrix to favor progression from acinar-to-ductal metaplasia to PanIN formation (Liou, et al., 2015). With PanIN progression, the biology of infiltrating macrophages evolves, driven by interleukin-13 (IL-13) signaling, to support fibrosis and PanIN growth (Liou, et al., 2017). This progression in macrophage phenotype with tumor development has also been observed in lung cancer (Singhal, et al., 2019). Thus, the phenotypes of inflammation and cancer are interdependent and defined by bidirectional cellular communication.
2.2. The complexity of the inflammatory reaction to PDAC
A diversity of cellular components forms the inflammatory response to PDAC and contribute to the pro-tumor phenotype of the immune microenvironment in tumors. Infiltrating immune cells are dominated by macrophages, immature myeloid cells, neutrophils, and T cells. While each of these cell types has normal functions in wound healing and immunosurveillance, in the setting of cancer they can also adopt immune-suppressive or pro-tumor roles. Understanding the phenotype and function of these cell types and how they can be therapeutically depleted or re-polarized is a fundamental area of investigation. Here, we will review each of the main cellular components of the PDAC immune microenvironment, focusing on macrophages, other myeloid cells, T cells, and the pro- or anti-tumor subsets of each.
Macrophages are the most abundant leukocyte detected within mouse and human PDAC (Beatty, Eghbali, & Kim, 2017). It has been shown that tumor-associated macrophages in PDAC originate from bone-marrow derived inflammatory monocytes (Sanford, et al., 2013) as well as embryonic-derived tissue-resident macrophages (Zhu, et al., 2017). Interestingly, tissue-resident macrophages can expand during tumor progression by in situ proliferation (Zhu, et al., 2017) whereas the frequency of bone-marrow derived macrophages is regulated by their recruitment (Sanford, et al., 2013). These two distinct macrophage subsets contribute independently to tumor biology. For instance, whereas tissue-resident macrophages display a pro-fibrotic transcriptional signature, bone-marrow derived macrophages demonstrate a transcriptional profile suggestive of their role in antigen presentation. Further, tissue-resident macrophages are supportive of tumor growth, while bone-marrow derived macrophages have well-recognized roles in defining therapeutic resistance (Kalbasi, et al., 2017; Mitchem, et al., 2013; Nywening, et al., 2018; Sanford, et al., 2013). Multiple other subsets of macrophages derived from these two tissue origins are expected given the importance of microenvironmental cues in defining macrophage phenotype in tissues. Thus, macrophages within the tumor microenvironment may acquire distinct and non-redundant roles.
Bone marrow-derived myeloid cells are recruited to tumors via GM-CSF, G-CSF, IL-3, VEGF, and the interaction of CXCL12/CXCR4 or CCL2/CCR2, among others (Goedegebuure, et al., 2011; Pergamo & Miller, 2017). These factors can be produced by both malignant and non-malignant cells within tumors. The function of tumor-infiltrating myeloid cells is, at least in part, determined by the expression of oncogenic Kras in epithelial cells. For instance, myeloid cells support carcinogenesis in the setting of Kras activation, but upon inactivation of oncogenic Kras, myeloid cells effectively remodel the fibrotic stroma and enforce tissue repair (Y. Zhang, et al., 2017). Further, within non-malignant tissues, infiltrating myeloid cells can differentiate into macrophages or dendritic cells (Chomarat, Banchereau, Davoust, & Palucka, 2000; Rosenzwajg, Jourquin, Tailleux, & Gluckman, 2002). However, in PDAC, tumor-infiltrating myeloid cells appear to predominantly skew toward the macrophage-lineage and acquire an immunosuppressive phenotype that curtails the potential of T cell immunosurveillance (Pergamo & Miller, 2017). Thus, the biology of tumor-infiltrating bone marrow-derived myeloid cells in PDAC is dysregulated, yet pliable.
In contrast to myeloid cells, effector CD8+ T cells are significantly less abundant in the PDAC microenvironment in both humans and mice (Beatty, Eghbali, et al., 2017). In mouse models, CD3+ T cells are most readily detected at the periphery of developing PanIN lesions (Hiraoka, Onozato, Kosuge, & Hirohashi, 2006; Zhang, et al., 2014). In the context of pancreatitis, Kras oncogene activation induces the recruitment of CD4+ T cells to PanIN which then repress the anti-tumor potential of CD8+ effector T cells (Zhang, et al., 2014). The recruitment of IL-17 producing CD4+ T cells and γδT cells also supports the initiation and progression of early pancreatic cancer (McAllister, et al., 2014). In patients with surgically-resected PDAC, T cell infiltration can also be observed and correlates with improved outcomes suggesting the anti-tumor potential of tumor-infiltrating T cells (Balachandran, et al., 2017). However, T cells present within the tumor microenvironment of mouse and human PDAC rarely display evidence of activation (Bailey, Chang, et al., 2016) and tumor-infiltrating T cells commonly are excluded from direct interaction with malignant cells and appear trapped within the surrounding fibrotic stroma (Beatty, Eghbali, et al., 2017; Carstens, et al., 2017; Stromnes, Hulbert, Pierce, Greenberg, & Hingorani, 2017; Tsujikawa, et al., 2017). In KPC mice, pharmacologic depletion of both CD4+ and CD8+ T cells was not found to impact the natural history of PDAC (Evans, et al., 2016). However, depletion of CD4+ T cells alone inhibits pancreatitis-induced carcinogenesis in an inducible Kras model of pancreatic cancer (Zhang, et al., 2014). While CD4+ T cells may contribute to carcinogenesis by promoting myeloid inflammation and releasing pro-tumorigenic cytokines, CD4+ T regulatory cells are also a prominent feature of the immune infiltrate in PDAC, and can inhibit anti-tumor immunity (Helm, et al., 2014; Jang, et al., 2017). In addition, tumor-infiltrating γδT cells have been found to suppress αβ T cell activation in a mouse model of PDAC (Daley, et al., 2016). Thus, the pro-tumorigenic potential of the microenvironment that surrounds PDAC is defined by an intricate intercellular network directed by myeloid and lymphoid cell subsets with immunosuppressive properties.
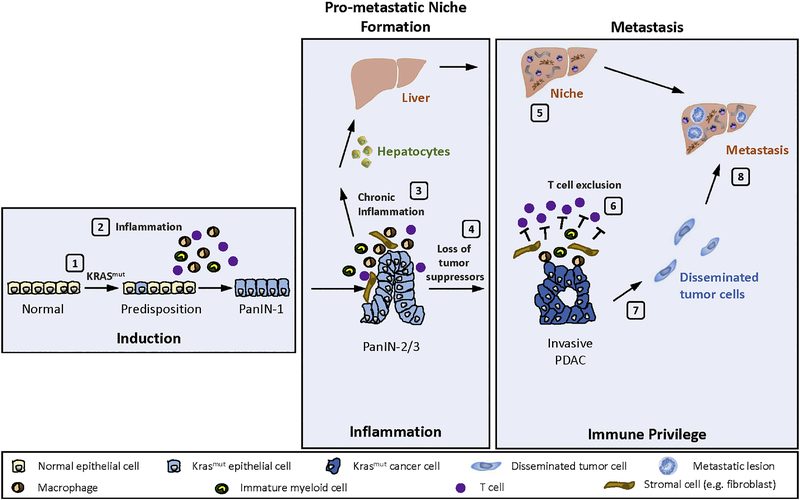
Together, findings investigating the immune contexture of the tumor microenvironment in PDAC support a conceptual model (Figure 1) in which pre-malignant inflammation and subsequent oncogene activation coordinate the induction of carcinogenesis. Epithelial cells with malignant potential then initiate the orchestration of an inflammatory reaction, which co-evolves with tumor development, acquires immunosuppressive properties, and ultimately establishes an immune-privileged microenvironment that protects the neo-organ from cytotoxic- and immune-based therapies (Dibra, Mishra, & Li, 2014). As such, it is hypothesized that disruption of key immunosuppressive components within the tumor microenvironment may unveil remarkable vulnerability of PDAC to immune-mediated elimination.
Figure 1.
Conceptual model of carcinogenesis and metastasis in pancreatic ductal adenocarcinoma. A multi-step process coordinates carcinogenesis and metastasis in pancreatic ductal adenocarcinoma.
Step 1, normal epithelial cells acquire mutation in the KRAS oncogene.
Step 2, pre-malignant inflammation leads to KRAS oncogene activation and formation of PanIN-1 lesions. In the setting of continued chronic inflammation, PanIN progression occurs.
Step 3, inflammatory mediators (e.g. IL-6) are released to initiate a systemic response that activates hepatocytes.
Step 4, loss of tumor suppressor genes (e.g. TRP53, CDKN2A) promote progression to invasive PDAC.
Step 5, inflammatory mediators released by the liver establish a pro-metastatic niche.
Step 6, T cells are excluded from direct interaction with malignant cells (T cell exclusion).
Step 7, malignant epithelial cells invade and intravasate.
Step 8, disseminated tumor cells seed and colonize distant organs (e.g. liver).
2.3. Cancer intrinsic properties dictate the inflammatory response
The immune microenvironment in PDAC is defined and supported by factors intrinsic to malignant epithelial cells. Activation of oncogenic KRAS, which is present in over 90% of PDAC tumors (BaileyChang, et al., 2016; Hidalgo, 2010), initiates an inflammatory cascade via signaling proteins including STAT3 and NFκB, which in turn induce the release of inflammatory cytokines, including GM-CSF, IL-6 and CCL5. These cytokines promote tumorigenesis and recruit immunosuppressive cells to the tumor microenvironment (Kitajima, Thummalapalli, & Barbie, 2016). However, the pro-inflammatory potential of mutant Kras relies on its activation. To this end, additional pre-inflammatory signals may shape the susceptibility of epithelial cells to malignant transformation. For instance, haploinsufficiency of the orphan nuclear receptor Nr5a2 in pancreatic epithelial cells establishes a basal level of early inflammatory signals, including chemokines and complement components, that are able to provoke the oncogenic activity of mutant Kras (Cobo, et al., 2018; Flandez, et al., 2014). These findings illustrate the remarkable capacity of epithelial cells to regulate local inflammation and support the notion that intrinsic defects in epithelial cells beyond those with oncogenic potential can contribute to carcinogenesis.
Cancer cell intrinsic factors also impact the immune microenvironment and the efficacy of immunotherapy. For instance, cancer cell expression of CXCL1 has been shown in mouse models to be a potential factor involved in defining T cell exclusion in PDAC and resistance to immunotherapy (Li, et al., 2018). Similarly, cancer cell intrinsic activation of the WNT/β-catenin signaling pathway in melanoma correlates with T cell exclusion by suppressing the recruitment of dendritic cells (Spranger, Bao, & Gajewski, 2015). It remains unclear, though, whether this pathway impacts T cell exclusion in PDAC. Mismatch repair deficiency (MMR-D) in malignant epithelial cells also inversely correlates with T cell exclusion (Llosa, et al., 2015) and its presence is associated with increased sensitivity to immune checkpoint blockade (Le, et al., 2015). Although MMR-D tumors represent <1% of pancreatic cancers (Hu, et al., 2018), patients with MMR-D PDAC can respond to PD-1 checkpoint blockade and thus, PD-1 immunotherapy is an FDA approved treatment option. Finally, the immunogenic quality of neoantigens expressed by PDAC has been proposed as a key determinant of productive immunosurveillance in patients with surgically-resected disease (Beatty, Eghbali, et al., 2017). Although CD8+ T cell infiltration alone positively correlates with long-term survival in this patient population, the quality of neoantigens defined by a fitness model, rather than the quantity, was shown to combine with CD8+ T cell abundance to most effectively stratify patients with long-term survival (Balachandran, et al., 2017). Together, these findings support a role for cancer cell intrinsic properties in shaping local inflammation, PDAC immunogenicity, and cancer cell susceptibility to immunotherapy.
2.4. Cancer extrinsic mechanisms regulating the inflammatory response
Due to its anatomical location, the pancreas is intimately linked with the gut microbiota (Cani & Jordan, 2018). Bacteria in the gut can directly access the pancreas, implying that intestinal bacteria may regulate the pancreatic microenvironment (Pushalkar, et al., 2018). In both mice and humans, the microbiome of PDAC differs substantially from the normal pancreas (Pushalkar, et al., 2018). In models of PDAC, broad spectrum oral antibiotics that deplete the gut microbiome significantly reduce tumor burden and liver metastasis (Sethi, et al., 2018). This outcome is dependent on mature T and B cells and associated with an increase in IFNγ+ T cells in tumors, with a corresponding decrease in T cells producing IL-17A and IL-10 production. Further, bacterial ablation can shift the cellular composition of the tumor microenvironment by reducing immature myeloid cells, altering the differentiation status of macrophages, and promoting T activation (Pushalkar, et al., 2018). These effects of the microbiome appear mediated through tolerogenic immune signaling via select Toll-like receptors in monocytic cells. Bacteria found within the tumor microenvironment in PDAC patients have also been implicated in resistance to chemotherapy via their expression of cytidine deaminase that metabolizes gemcitabine (Geller, et al., 2017). Together, the gut microbiome has emerged as a therapeutic target in cancer due to its capacity to affect inflammation, regulate tumor progression, and define chemoresistance.
2.5. Inflammation as a classification strategy for PDAC subtypes
Dysregulation in defined core cellular signaling pathways characterize pancreatic cancer (Jones, et al., 2008). For instance, integrated genomic analyses show that mutations in recurrent genes aggregate into pathways that define subtypes of PDAC with potential therapeutic implications (BaileyChang, et al., 2016; Collisson, et al., 2011; Moffitt, et al., 2015; Puleo, et al., 2018). Amongst these subtypes, it has been proposed that a subset of PDAC tumors display immunogenic features defined by upregulation of networks associated with immunoregulation (BaileyChang, et al., 2016). Consistent with this observation, a subset of human PDAC also demonstrates high cytolytic T cell activity, which is inversely correlated with genomic alterations in the tumor (Balli, Rech, Stanger, & Vonderheide, 2017). Notably, these tumors exhibit increased expression of multiple immune checkpoint genes other than PD-L1 (Balli, et al., 2017). This finding is consistent with clinical observations and supports a role for alternative mechanisms of adaptive and acquired immune resistance beyond PD-1 and CTLA-4.
The immune microenvironment in cancer is dynamic and diverse. In-depth analyses using six molecular platforms designed to interrogate RNA, DNA, and protein signatures have revealed six immune subtypes across a wide range of malignancies, including (i) wound healing, (ii) IFNγ dominant, (iii) inflammatory, (iv) lymphocyte depleted, (v) immunologically quiet, and (vi) TGF-β dominant (Thorsson, et al., 2018). In PDAC, the wound healing and inflammatory subtypes were the most common. However, tissue-based microscopy of human PDAC reveals heterogeneous cellular subpopulations of immune cells arranged spatially within the tumor microenvironment suggesting increased complexity to the interpretation of genetic-based profiles using whole tissues (Beatty, Eghbali, et al., 2017; Carstens, et al., 2017; Stromnes, et al., 2017; Tsujikawa, et al., 2017). Microscopy-based analysis also shows that PDAC tumors with cytotoxic T cells located adjacent to cancer cells significantly correlate with improved survival in patients (Carstens, et al., 2017). In addition, tissue-based microscopy in combination with genetic analyses has revealed four PDAC subtypes based on genetic, stromal and immunologic diversity (Knudsen, et al., 2017). This suggests that the inflammatory reaction to PDAC assumes non-random patterns which has implications for the potential to shift tumors from therapeutically-resistant to -sensitive. However, an understanding of the mechanisms driving these immunologically-distinct microenvironments is lacking. Nonetheless, tissue analyses of human PDAC are providing an initial framework toward understanding the cellular and molecular pathways that might be used to immunologically define PDAC with the aim to identify a foothold for immunotherapy.
3. Pancreatic carcinoma induces systemic inflammation
Cancer can be a systemic process even prior to its dissemination to distant organs. In particular, malignant cells can provoke systemic inflammation that in turn, supports tumor growth and portends a poor prognosis. In patients with surgically-resectable PDAC, inflammatory monocytes are increased in the peripheral blood and decreased in the bone marrow, indicative of cellular mobilization (Sanford, et al., 2013). This biology is reproduced in mouse models of cancer where it has been found that bone marrow mobilization in PDAC is driven by the CCR2/CCL2 axis (Sanford, et al., 2013).
The systemic inflammatory response to cancer is detected in patients by an increase in the neutrophil:lymphocyte ratio and by increased peripheral blood inflammatory cytokines, including C-reactive protein (CRP), interleukin-6 (IL-6), soluble tumor necrosis factor receptor type II (sTNF-RII) and macrophage inhibitory cytokine-1 (MIC-1) (Olive, 2017). High circulating concentrations of these proteins are associated with significantly higher mortality and a poor prognosis (Babic, et al., 2018).
The systemic inflammatory response to PDAC also results in a reprogramming of host metabolism. Specifically, in patients with PDAC, inflammation can be associated with a wasting syndrome, cachexia, that directs loss of both adipose and muscle tissue (Hendifar, et al., 2018). This process appears to be driven by local tumor growth in the pancreatic environment leading to decreased exocrine pancreatic function (Danai, et al., 2018). IL-6 produced in response to PDAC development also reduces the ketogenic potential of the liver (Flint, et al., 2016). In doing so, IL-6 triggers increased glucocorticoid levels which may even impair immunosurveillance and thereby, limit the potential of immunotherapy (Flint, et al., 2016). Thus, local PDAC development can invoke systemic biology with clinical implications.
4. Inflammation supports cancer metastasis
Inflammation is a key determinant of cancer metastasis. In mouse models of PDAC, depletion of macrophages using clodronate-encapsulated liposomes significantly diminishes the liver and lung metastatic potential of spontaneously arising PDAC (Griesmann, et al., 2017). Similarly, in an orthotopic injection model of PDAC, inflammatory monocytes mobilized from the bone marrow are recruited to the liver during cancer progression where they establish a niche supportive of metastasis (Sanford, et al., 2013). Thus, a systemic myeloid reaction is provoked by cancer to support the spread of PDAC to distant organs.
Disrupting monocyte recruitment to the liver by blocking CCR2 protects against liver metastasis (Sanford, et al., 2013). This finding illustrates the importance of chemotactic factors in defining the pro-metastatic potential of myeloid cells. Once in the liver, inflammatory monocytes secrete granulin which activates resident hepatic stellate cells, stimulating their secretion of periostin and formation of a fibrotic microenvironment that facilitates PDAC liver metastasis (Nielsen, et al., 2016). Thus, macrophages recruited to distant organs can initiate a cascade of signaling events that culminate in formation of a pro-metastatic niche.
The pro-metastatic niche that forms in the liver during cancer development may be instructed by multiple factors. For example, exosomes released by malignant cells can induce formation of the niche by stimulating Kupffer cells (resident liver macrophages) to release TGF-β which induces fibronectin production by hepatic stellate cells (Costa-Silva, et al., 2015; Hoshino, et al., 2015). In contrast, expression of TIMP1 by premalignant lesions can facilitate formation of a pro-metastatic niche by first activating hepatic stellate cells via CD63 and phosphatidylinositol 3-kinase (PI3K), which in turn lead to increased hepatic CXCL12 (also known as stromal-derived factor-1), an inducer of neutrophil migration (Grunwald, et al., 2016; Seubert, et al., 2015). Thus, it is possible for macrophages or stellate cells to serve as the initial orchestrators of a pro-metastatic niche in the liver.
The environment of distant organs is a key determinant of the metastatic potential of disseminated tumor cells. Formation of a niche environment in distant organs can occur early during carcinogenesis (Lee, et al., 2019). The mechanistic underpinnings that direct formation of the niche may also differ between organs. In the lung, a pro-metastatic niche can form in response to primary tumor development. This biology involves release of lung-derived chemoattractants (e.g. S100A8 and S100A9) that recruit myeloid cells (Hiratsuka, Watanabe, Aburatani, & Maru, 2006; Hiratsuka, et al., 2008). Disseminated cancer cells entering the lung can also recruit inflammatory monocytes via CCL2-CCR2 which then support cancer cell extravasation through their release of VEGF (Qian, et al., 2011). Cancer cells can also activate myeloid cells via TLR2 signaling to release TNF that supports lung metastasis (Kim, et al., 2009). In general, sustained inflammation facilitates cancer cell metastasis but can also awaken dormant cancer cells by inducing neutrophil recruitment to metastatic sites where they remodel extracellular matrix components and stimulate cancer cell proliferation (Albrengues, et al., 2018).
In the liver, macrophages and hepatic stellate cells are implicated in orchestrating a pro-metastatic niche and in promoting the outgrowth of disseminated tumor cells. This biology can be instructed by hepatocytes, the chief functional cells of the liver, which coordinate formation of the niche through their secretion of acute phase reactants, namely serum amyloid A (SAA) proteins (Lee, et al., 2019). Importantly, hepatocytes are not implicated in formation of a pro-metastatic niche in the lung. This observation identifies a role for organ-specific mechanisms in regulating metastatic spread in cancer. In the liver, the pro-metastatic niche is instructed by IL-6 released early during carcinogenesis by non-malignant stromal cells residing in primary tumors (Figure 1). Hepatocytes respond to IL-6 by activating STAT3 which induces the release of SAA that then orchestrate a niche environment in the liver supportive of cancer seeding and growth. IL-6 released in non-malignant settings can reproduce the same biology implying that other inflammatory conditions, such as obesity and cardiovascular disease, which are associated with elevated IL-6 may influence the metastatic potential of cancer. Blocking IL-6 activation of STAT3, genetic deletion of STAT3 in hepatocytes, or genetic knockout of SAA prevents formation of a pro-metastatic niche (Lee, et al., 2019). Together, these findings illustrate the inherent role of hepatocytes in defining cancer metastasis to the liver due to their sensitivity to inflammatory mediators.
5. Inflammation is a therapeutic barrier in PDAC
Cancer inflammation is a key determinant of therapeutic efficacy. For instance, tumor-infiltrating myeloid cells can mediate resistance to both cytotoxic- and immune-based therapies. Myeloid cells facilitate treatment resistance by suppressing the induction and activity of anti-tumor adaptive immunity, metabolizing chemotherapy, providing growth factors, and supporting neovascularization (De Palma & Lewis, 2013). However, myeloid cell phenotype is pliable and dependent on cues received from the surrounding microenvironment, such that while myeloid cells most commonly acquire pro-tumor properties, they can be induced with anti-tumor activity (Long & Beatty, 2013). As such, strategies to intervene on inflammation include not only approaches to disrupt the intra-tumoral recruitment and activity of myeloid cells in cancer but also methods to redirect myeloid cells with tumor-suppressive and immune-stimulatory properties (Liu, et al., 2017).
5.1. Inhibiting myeloid cell recruitment as a therapeutic strategy
Peripheral blood myeloid cells are recruited to the tumor microenvironment by an array of chemokines. For instance, the CCL2/CCR2 chemokine pathway recruits a subset of peripheral blood monocytes to tumor tissues where they then differentiate into macrophages (Mitchem, et al., 2013; Nywening, et al., 2018; Sanford, et al., 2013). In mouse models of PDAC, CCR2 inhibition blocks monocyte recruitment, improves the efficacy of chemotherapy, inhibits metastasis, and increases T cell immune infiltration and anti-tumor activity (Mitchem, et al., 2013; Nywening, et al., 2018; Sanford, et al., 2013). Inhibiting the CCL2/CCR2 axis also suppresses radiation-induced neovascularization by blocking monocyte recruitment to tumors and in doing so, enhances the efficacy of radiotherapy in a mouse model of PDAC (Kalbasi, et al., 2017). Thus, monocyte recruitment to tumors can be targeted for therapeutic benefit.
Neutrophils can also limit the efficacy of cytotoxic therapies and restrain the anti-tumor activity of T cells. Neutrophils are recruited to tumors via their expression of CXCR2. Inhibition of CXCR2 modestly improves T cell infiltration into spontaneously-arising PDAC tumors in mice (Steele, et al., 2016) and delays tumor engraftment in an implantable model of PDAC (Chao, Furth, & Vonderheide, 2016). In combination with chemotherapy or PD1 blockade, CXCR2 inhibition also produces a survival benefit in mice with spontaneous PDAC (Chao, et al., 2016). These findings support a role for neutrophils in regulating T cell immunosurveillance and the efficacy of cytotoxic chemotherapy.
Disrupting the recruitment of monocytes or neutrophils to tumors can cause a compensatory increase in the non-targeted myeloid population. For instance, CXCR2 inhibition invokes a compensatory increase in macrophages, whereas CCR2 inhibition stimulates an increase in neutrophil recruitment to tumors (Nywening, et al., 2018). This observation provides rationale to intervene on the trafficking of both myeloid cell types. To this end, blockade of CCR2 and CXCR2 was tested in an orthotopic PDAC model and found to improve the efficacy of chemotherapy that was dependent on T cells (Nywening, et al., 2018). Similarly, CXCR2 inhibition was found to combine with CSF1R inhibition and PD1 blockade to induce T cell dependent anti-tumor immunity in subcutaneous cancer models (Kumar, et al., 2017). Together, these findings illustrate the dynamic interplay between multiple myeloid cell populations that compensate for each other to restrict T cell immunosurveillance in cancer.
The recruitment of inflammatory cells to the tumor microenvironment is supported by both cancer cell intrinsic and extrinsic mechanisms. For instance, CXCL1 expression by tumor cells has been posited as a mechanism of recruitment for neutrophils which then restrict T cell entry (Li, et al., 2018). Similarly, hyperactivation of focal adhesion kinase (FAK) in neoplastic cells induces a chemokine and cytokine network that can attract inflammatory cells to the tumor microenvironment (Jiang, et al., 2016). Disruption of FAK activity can remodel the inflammatory and fibrotic environment of tumors and in doing so, sensitize PDAC in mouse models to chemotherapy and T cell immunotherapy (Jiang, et al., 2016). However, nonmalignant cells can also regulate T cell immunosurveillance in PDAC. For example, macrophages residing outside of the tumor microenvironment have been implicated in restricting T cell entry and the efficacy of cancer immunotherapy (Beatty, et al., 2015). In addition, cancer-associated fibroblasts via their secretion of CXCL12 have been found to regulate T cell entry into PDAC (Feig, et al., 2013; Garg, et al., 2018). CXCL12 interacts with CXCR4 and can promote tumor survival and invasion (Sleightholm, et al., 2017) as well as the recruitment of myeloid cells (Obermajer, Muthuswamy, Odunsi, Edwards, & Kalinski, 2011). Inhibition of CXCR4 alone was found to rapidly facilitate the infiltration of T cells in a spontaneous model of PDAC and to combine with PDL1 blockade to provide short-term control of tumor growth (Feig, et al., 2013). Together, these data demonstrate that the T cell immune privileged status of PDAC is an actively regulated process coordinated by both malignant and non-malignant cells.
5.2. Disrupting signaling pathways to redirect myeloid cell biology in cancer
Within the tumor microenvironment, multiple signaling pathways are involved in defining the immunosuppressive activity of macrophages. For instance, Fcγ receptor signaling via PI3Kγ activation of the BTK signaling pathway polarizes macrophages with immunosuppressive properties (Gunderson, et al., 2016). Disruption of either PI3Kγ or BTK in an orthotopic mouse model of PDAC was shown to promote T cell infiltration and enhanced efficacy of chemotherapy in a T cell dependent manner (Gunderson, et al., 2016). Blockade of inflammatory mediators, such as IL-6, that drive STAT3 activation can also improve the efficacy of chemotherapy (Long, Tooker, et al., 2017; Wormann, et al., 2016). Similarly, colony stimulating factor 1 (CSF1) is a growth factor important for macrophage survival and in defining the polarity of tumor-associated macrophages (Pyonteck, et al., 2013). In a mouse model of spontaneous PDAC, inhibition of CSF1R produced a depletion of tumor-associated macrophages and improved overall survival (Candido, et al., 2018). In an orthotopic model of PDAC, CSF1R blockade also shifted the phenotype of tumor-associated macrophages seen by a decrease in macrophage expression of immunosuppressive genes (e.g. Il10, Arg1) and an increase of antitumor immunity genes (e.g. Il12p35, Ifnb, Nos2) (Zhu, et al., 2014). This “reprogramming” of macrophages in the tumor by targeting CSF1R was associated with an increase in activated T cells, PDL1 expression on tumor cells, and enhanced responsiveness to immune checkpoint blockade with anti-PD1 and anti-CTLA4 antibodies (Zhu, et al., 2014). Together, these findings support a role for signaling pathways in macrophages in shaping the complexity of the immune reaction to PDAC.
An understanding of the drivers of myeloid cell biology in the tumor microenvironment has important therapeutic implications. Innate immune receptors specific for damage-associated molecular patterns (DAMPs) can define the activity of macrophages. For instance, ligation of dectin 1 in macrophages stimulates their infiltration and induction of pancreatic carcinogenesis in mice (Daley, et al., 2017). Although acute activation of TLR signaling can stimulate an antitumor response (Liu, et al., 2019), chronic stimulation promotes immune suppression (Zambirinis & Miller, 2013). TLR7 (Ochi, Graffeo, et al., 2012) and TLR9 ligands (Zambirinis, et al., 2015) produced in the setting of pancreatic carcinogenesis and pancreatitis promote tumor progression and upregulate inflammatory pathways such as STAT3, Notch, and NFκB. Similarly, TLR9 activation induces pancreatic stellate cells to secrete CCL11 which produces immune suppressive effects via regulatory T cell recruitment and immature myeloid cell proliferation. TLR4 agonism in myeloid cells also accelerates pancreatic carcinogenesis in mice (Ochi, Nguyen, et al., 2012). Similarly, RIP1K expression in tumor-associated macrophages suppresses the STAT1 pathway in macrophages and in doing so, endows them with immunosuppressive properties. Inhibition of RIP1K redirects macrophages with increased STAT1 signaling and immunostimulatory properties that support the activity of T cell immunotherapy (Wang, et al., 2018). It is noteworthy that central to the activity of dectin 1, TLRs, and RIP1K is the downstream activation of NFkB pathway. NFkB transcription factors can also be activated by interleukin-1 receptor-associated kinases (IRAKs). Specifically, IRAK4 is a central regulator of TLR signaling and inhibition of IRAK4 activation reduces NFkB activity in PDAC and enhances the efficacy of chemotherapy (D. Zhang, et al., 2017). Together, these data demonstrate the impact of chronic inflammatory signals in driving immunosuppression and therapeutic resistance.
5.3. Inducing inflammation with anti-tumor activity
The inherent plasticity of the myeloid compartment has emerged as a therapeutic opportunity in cancer. Myeloid cell infiltration in cancer is nearly universal (Qian & Pollard, 2010) and although these cells commonly contribute to tumor development and metastasis, tumor-infiltrating myeloid cells can be manipulated (Long, Collier, & Beatty, 2017). This potential to harness the myeloid compartment for therapeutic benefit is seen with a CD40 agonist. CD40 is expressed on myeloid cells, including monocytes, macrophages and dendritic cells. Although CD40 ligation is a critical step in licensing dendritic cells to effectively present antigen and activate CD8+ T cells, systemic CD40 activation also induces an acute and systemic cytokine release of CCL2 and IFN-γ, among several other cytokines (Beatty, Li, & Long, 2017). In response to this systemic immune reaction induced by a CD40 agonist, monocytes are recruited to tumors by CCL2 where they mediate IFNγ dependent anti-fibrotic activity. IFNγ-activated monocytes facilitate intra-tumoral release of matrix metalloproteinases which in turn, degrade components of the extracellular matrix within tumors, including collagen and fibronectin (Long, et al., 2016). Together, this anti-fibrotic inflammatory response mediated by IFNγ-activated monocytes conditions tumors with increased sensitivity to chemotherapy.
CD40 agonists can promote macrophage-dependent anti-tumor activity in both mice and humans that is independent of T cells (Beatty, et al., 2011; Buhtoiarov, Lum, Berke, Sondel, & Rakhmilevich, 2006). However, CD40 agonists can also re-invigorate T cells in lymph nodes surrounding PDAC in mice (Beatty, et al., 2011), thereby providing rationale for CD40 agonists to potentially stimulate productive T cell immunosurveillance. To this end, CD40 agonists have been tested in mouse models of PDAC in combination with radiation and chemotherapy (Beatty, et al., 2015; Byrne & Vonderheide, 2016; Rech, et al., 2018; Winograd, et al., 2015; Yasmin-Karim, et al., 2018). These combination strategies aim to leverage the immunostimulatory activity of a CD40 agonist with the potential of cytotoxic therapy to induce immunogenic cell death and the release of tumor antigens (Beatty, Li, et al., 2017). In addition, immune checkpoint blockade has been combined with a CD40 agonist in mouse models to produce enhanced anti-tumor T cell dependent responses (Winograd, et al., 2015). Thus, myeloid agonists show potential for stimulating both innate and adaptive immunosurveillance for improving therapeutic outcomes with standard therapies.
5.4. Targeting immune checkpoint pathways regulating innate immunosurveillance in cancer
The biology of myeloid cells is defined by a balance of stimulatory and inhibitory signals. For instance, macrophage phagocytosis of tumor cells is determined by both pro-phagocytic and anti-phagocytic signals that are communicated to macrophages by malignant cells (Long & Beatty, 2013). In mouse models of hematologic cancers, blockade of CD47, a “don’t eat me signal”, promotes macrophage phagocytosis of tumor cells and improves the therapeutic activity of tumor-specific antibodies (e.g. anti-CD20) (Jaiswal, et al., 2009; Weiskopf, et al., 2013). CD47 suppresses the phagocytic activity of macrophages by binding SIRPα (signal regulatory protein α)-receptor expressed on macrophages. However, in solid cancers, CD47 blocking antibodies have shown minimal activity in promoting macrophage phagocytosis of malignant epithelial cells (Barkal, et al., 2018; Feng, et al., 2015; Liu, et al., 2019; Michaels, et al., 2018). This finding is thought to reflect additional negative regulatory signals beyond CD47 that govern macrophage activity. For instance, LILRB1 is an inhibitory receptor upregulated on the surface of tumor-associated macrophages which binds MHC class I to suppress macrophage phagocytosis of tumor cells (Barkal, et al., 2018). Loss of MHC class I expression on tumor cells was found to sensitize tumors to macrophage-dependent immunosurveillance (Barkal, et al., 2018). Thus, macrophages have the capacity to survey tissues for loss of self-defining markers, such as MHC molecules.
Although chronic TLR agonism is associated with tumor progression, acute stimulation of TLR pathways in macrophages invokes anti-tumor activity (Buhtoiarov, et al., 2006; Liu, et al., 2019). This appears to be due, at least in part, to the capacity of macrophage-stimulatory pathways to influence the metabolic state of macrophages, a key determinant of their biology. Acute TLR agonism alters the central carbon metabolism of macrophages to enhance their capacity to phagocytose tumor cells and circumvent inhibitory signals (e.g. CD47) (Liu, et al., 2019). Thus, macrophage-dependent immunosurveillance in cancer can be harnessed by disrupting immune checkpoint signals (e.g. CD47 and LILRB1) as well as by providing stimulatory signals (e.g. TLR agonism). Overall, the plasticity and therapeutic potential of the inflammatory reaction to cancer offers a novel approach to intervening on the natural history of tumors. However, this therapeutic potential is tempered by the substantial complexity and dynamics of inflammation, as well as the remarkable resilience of malignant epithelial cells to rapidly adapt to immune pressure.
6. Clinical studies targeting inflammation in pancreatic cancer
The remarkable success seen with immune checkpoint blockade targeting the PD-1/PD-L1 pathway in solid tumors has provided the scientific and clinical rationale for investigation of immunotherapy in PDAC. Efforts to translate immunotherapy to PDAC, though, have been met with significant challenge. For instance, PD1/PDL1 immune checkpoint blockade as monotherapy has not produced clinical benefit for the majority of patients with the exception of a subset (<1%) with microsatellite instability (MSI)-high tumors (Brahmer, et al., 2012; Le, et al., 2017; Ott, et al., 2019). Similarly, CTLA-4 antibodies as monotherapy and in combination with chemotherapy have not shown overt clinical activity (Aglietta, et al., 2014; Royal, et al., 2010). Vaccines and chimeric antigen receptor (CAR)-modified autologous T cells have also not demonstrated reproducible clinical responses (Beatty, et al., 2014; Beatty, et al., 2018). Together, these clinical data signify that multiple mechanisms likely underpin immune evasion in PDAC and reinforce the need for rational treatment combinations. To this end, trials with combination immunotherapy are being tested in pancreatic cancer (Table 1). While the response rate in these trials is low, it is notable that responses to combination immunotherapy (in the absence of cytotoxic therapy) are occasionally observed in a subset of patients with good performance status and who have received multiple prior lines of therapy (Table 1). These responses illustrate the potential of pancreatic cancer to be immune-sensitive.
Table 1.
Activity of immunotherapy combinations in advanced pancreatic ductal adenocarcinoma
| Targets | Treatment | Prior Therapies | # of Patients | Tumor Response | Reference |
|---|---|---|---|---|---|
| BTK + PDL1 | ibrutinib + durvalumab | >1 | 43 | 1 PR | Hong, DS et al.“A multicenter study of the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib plus durvalumab in patients with relapsed/refractory (R/R) solid tumors.” Journal of Clinical Oncology 36, no. 15_suppl (May 20 2018) 2578–2578. |
| CD73 + PDL1 | oleclumab + durvalumab | 2–3 | 20 | 2 PR | Overman, MJ et al. “Safety, efficacy and pharmacodynamics (PD) of MEDI9447 (oleclumab) alone or in combination with durvalumab in advanced colorectal cancer (CRC) or pancreatic cancer (panc).”Journal of Clinical Oncology 36, no. 15_suppl (May 20 2018) 4123–4123. |
| CSF1R + PD1 | cabiralizumab + nivolumab | >1 | 33 | 4 PR | Wainberg, Z et al. “First-in-human phase 1 dose escalation and expansion of a novel combination, anti–CSF-1 receptor (cabiralizumab) plus anti–PD-1 (nivolumab), in patients with advanced solid tumors” J Immunother Cancer. 2017 [abst O42] |
| CXCR4 + PD1 | BL-8040 + pembrolizumab | >1 | 37 | 1 PR | Sun, D et al. “Anti-PD-1 therapy combined with chemotherapy or target therapy in patients with advanced biliary tract cancer in real-world clinical setting.” Annals of Oncology (2018) 29 (suppl_8): viii400viii441. |
| IDO + PDL1 | epacadostat + durvalumab | >1 | 15 | 0 PR | Naing, A et al. “Epacadostat plus durvalumab in patients with advanced solid tumors: preliminary results of the ongoing, open-label, phase I/II ECHO-203 study.”Cancer Res 2018;78(13 Suppl):Abstract nr CT177. |
| Total | 178 | 9 (5%) |
Discoveries from preclinical models are informing the next generation of therapeutic strategies in PDAC. Ongoing clinical trials for patients with pancreatic cancer, including all stages of disease, are investigating drugs that target inflammation in combination with cytotoxic therapy (chemotherapy and radiation) and with PD-1/PD-L1 checkpoint blockade (Table 2). Inflammatory pathways being targeted include CD40, ICOS, IL-6R, Vitamin D receptor, IL-1, FAK, CD73, Adenosine A2A receptor, CCR2/5, CXCR4, PEGPH20, CSF1R, and BTK. In addition, vaccines are being combined with immune checkpoint blockade directed against CTLA-4, IDO, and PD1/PDL1 as well as with radiation and CSF1R blockade. Multiple CAR T cell studies have been initiated to identify the potential safety and relevance of CAR targets and to understand mechanisms that regulate T cell trafficking and anti-tumor activity. Novel cell-based therapies involving the adoptive transfer of NK cells as well as oncolytic viruses are under investigation in early phase studies. Moreover, to rapidly advance the evaluation of novel therapeutics and combinations, platform trial designs are being developed that are conducted across multiple institutional sites (NCT03214250, NCT03193190). Together, these “signal-seeking” studies aim to rapidly screen promising treatment strategies and to identify subsets of patients who may achieve clinical benefit.
Table 2.
Active immunotherapy-based trials in pancreatic ductal adenocarcinoma*
| Treatment Type | Active and recruiting trials (n) | Treatment Targets |
|---|---|---|
| ADC | 10 | AG7, AXL, CA19–9, CD71, GCC, Her2, mesothelin, PSMA, tissue factor |
| Antibody | 4 | CA19–9, CD25, CEACAM5/6, LIF |
| CAR T cell | 10 | CD70, CEA, EPCAM, Her2, mesothelin, MUC1, PSCA |
| Cell-based (non-CAR T cell) | 15 | NK cells, T cells, TIL |
| Cytotoxic Therapy + ADC | 2 | Mesothelin |
| Cytotoxic Therapy + Antibody | 9 | CD40, Claudin-18.2, CTLA4, HGF, IL-1alpha, IL-6R, PDGFRalpha, VEGF |
| Cytotoxic Therapy + PD1/PDL1 + Other | 17 | Angiotensin II receptor, CCR2/5, CD40, CD73, CSF1R, CTLA4, FAK, HCQ, Vaccine, VEGF |
| Cytotoxic Therapy + Oncolytic Virus | 1 | N/A |
| IO (Other) | 3 | DART (B7H3-CD3), DR5 agonist, Pegylated IL10 |
| Oncolytic virus | 6 | N/A |
| PD1/PDL1 blockade + Other | 26 | 41BB, adenosine A2A receptor, CD73, CTLA4, CXCR4, DNA methyltransferase, FAK, HA, HDAC, Hsp90, ICOS, JAK1, MCSF, MEK, Paricalcitol, PARP, PI3Kdelta, PORCN, RIPK1, SBRT, SEMA4D, TGF-beta, vaccine, VEGFR2 |
| Vaccine | 8 | DC, Peptide, Whole Cell |
| Vaccine + Other | 3 | CSF1R, CTLA4, IDO, PD1 |
ADC, antibody drug conjugate; CAR, chimeric antigen receptor; Cytotoxic therapy, includes chemotherapy and radiation; DART, dual-affinity re-targeting; DC, dendritic cell; IO, immuno-oncology drug; SBRT, stereotactic body radiation
Includes studies for all stages of pancreatic cancer
In other solid malignancies, new treatment paradigms are beginning to emerge that have relevance to PDAC. For instance, maintenance therapy with PDL1 blocking antibodies is a treatment option for stage III non-small cell lung cancer patients after definitive concurrent chemoradiation (Antonia, et al., 2018; Antonia, et al., 2017). As treatment responses to chemotherapy in PDAC are almost uniformly incomplete and transient, strategies to consolidate and maintain responses would be of significant benefit. To this end, PARP inhibitors, which block DNA repair of double stranded breaks leading to synthetic lethality in BRCA mutant cells, are being applied as maintenance therapy in other solid malignancies (Mirza, et al., 2016). This approach is also being investigated in patients with BRCA mutant PDAC and next generation strategies are already being studied in which PARP inhibitors are combined with immunotherapy in the maintenance setting (NCT03404960). We propose that strategies that target inflammation and are agnostic to BRCA mutational status may also be incorporated as maintenance strategies and would spare the need for continued cytotoxic therapy which could then be reserved as a salvage treatment.
Immunotherapy in PDAC is also being investigated in the neoadjuvant and adjuvant settings. For patients with surgically-resected PDAC, the standard of care remains surgery followed by adjuvant chemotherapy. However, accumulating evidence is suggesting a role for neoadjuvant therapy in PDAC (Tienhoven, et al., 2018; Versteijne, et al., 2018). The neoadjuvant setting also offers an opportunity to more rigorously interrogate mechanisms of treatment response and resistance. In other solid tumors, neoadjuvant immunotherapy has demonstrated activity (Blank, et al., 2018; Forde, Chaft, & Pardoll, 2018; Huang, et al., 2019). In PDAC, neoadjuvant vaccination has shown the capacity to induce the formation of tertiary lymphoid aggregates in primary tumors (Lutz, et al., 2014). As T cell infiltration in surgically-resected PDAC specimens correlates with improved outcomes (Balachandran, et al., 2017), a logical next step to impact survival in resectable PDAC is to invoke a productive T cell immune response prior to surgical resection.
A major focus of immunotherapy in PDAC is to identify strategies that can convert tumors from immunologically-resistant to -sensitive. For instance, whole cell vaccination has demonstrated the capacity to trigger the formation of T cell-rich tertiary lymphoid structures within pancreatic tumors that correlate with improved outcomes (Lutz, et al., 2014). Overall, there is growing evidence that successful immune responses can be mounted against pancreatic cancer. For instance, long-term survivors have been characterized as having high CD8+ T cell infiltrates as well as high-quality neoantigens (Balachandran, et al., 2017). Furthermore, checkpoint blockade can produce remarkable responses in patients with mismatch repair-deficient pancreatic cancer(Le, et al., 2017). Finally, clinical responses in combination immunotherapy trials, that are targeting both the myeloid reaction and T cell immune checkpoints, have been observed suggesting the potential to convert even mismatch repair-proficient PDAC into an immune-sensitive cancer (Table 1). Thus far, though, it is evident that enhancing the efficacy of immunotherapy in PDAC will require a multipronged approach that incorporates multiple immunotherapeutic targets. Stimulating productive innate and adaptive immunosurveillance in PDAC will need to consider strategies that improve cancer cell immunogenicity, resolve local and systemic immunosuppression, and invoke and maintain antitumor immunity.
7. Concluding Remarks
Pancreatic cancer is notable for its remarkable therapeutic resistance. Preclinical models have revealed the importance of inflammation as a key determinant of not only immunotherapy, but also cytotoxic therapies such as chemotherapy and radiation. In this review, we described the cellular components of inflammation in pancreatic cancer; we reviewed mechanisms by which inflammatory cells regulate tumorigenesis, immune evasion, and metastasis; and we discussed strategies to derail mechanisms of treatment resistance imposed by inflammation. Inflammation is tightly regulated by both cancer cell intrinsic and extrinsic mechanisms. Consistent with this, patterns of inflammation in cancer, that are detected amidst an otherwise heterogeneous and seemingly disorganized microenvironment, are emerging via high-dimensional genomic and single cell analyses. This biology is expected to inform strategies to shift tumors from treatment-resistant to -sensitive.
Inflammation is a key determinant of cancer cell survival, immune evasion, and metastasis. Disrupting mechanisms central to inflammatory cell recruitment and biology as well shifting the phenotype of the inflammatory reaction from pro- to anti-tumor are therapeutic strategies that are under investigation. Ongoing research aims to expand our understanding of the intercellular networks that coordinate the inflammatory reaction to cancer. Next generation treatment strategies are expected to focus on rational drug combinations and sequencing with the goal to produce deep and durable treatment responses. In summary, cancer cell biology is intimately dependent on instructional cues received from inflammatory cells and thus, inflammation holds much promise as a therapeutic target in PDAC should it be capable of being tamed.
Acknowledgements
This work was supported by National Institutes of Health grants R01 CA197916 (G.L.B.), T32 CA009140 (M.L.S.), and U01 CA224193 (G.L.B), the 2017 Stand Up to Cancer (SU2C) Innovative Research Grant SU2C-AACR-IRG 13-17 (G.L.B.) and grant support from the Robert L. Fine Cancer Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Robert L. Fine Cancer Research Foundation.
Disclosure of Potential Conflicts of Interest:
G.L.B. is a consultant/advisory board member for Seattle Genetics, Aduro Biotech, AstraZeneca, Bristol-Myers Squibb, Genmab, Merck, and BiolineRx; reports receiving commercial research grants from Incyte, Bristol-Myers Squibb, Verastem, Halozyme, Biothera, Newlink, Novartis, and Janssen; and is an inventor of intellectual property and recipient of royalties related to CAR T cells that is licensed by the University of Pennsylvania to Novartis. No potential conflicts of interest were disclosed by the other authors.
Abbreviations
- Arg1
arginase 1
- BRCA
breast cancer gene
- BTK
Bruton’s tyrosine kinase
- CAR
chimeric antigen receptor
- CCL
C-C motif ligand
- CCR
C-C chemokine receptor
- CD
Cluster of differentiation
- CRP
c-reactive protein
- CSF1R
colony stimulating factor 1 receptor
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- CXCL
C-X-C motif chemokine
- DAMP
damage-associated molecular patterns
- DNA
deoxyribonucleic acid
- FAK
focal adhesion kinase
- G-CSF
granulocyte-colony stimulating factor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- ICAM1
intercellular adhesion molecule 1
- ICOS
inducible T-cell costimulator
- IDO
indoleamine 2,3-dioxygenase
- Ifnb
interferon beta
- IFNγ
interferon gamma
- IL-
interleukin-
- IRAK4
interleukin-1 receptor associated kinase 4
- KPC
LSL-KrasG12D/+;LSL-Trp53R127H/+;Pdx-1-Cre
- KRAS
Kirsten rat sarcoma
- LILRB1
leukocyte immunoglobulin like receptor B1
- mGPS
modified Glasgow Prognostic Score
- MHC
major histocompatibility complex
- MIC-1
macrophage inhibitory cytokine-1
- MMR-D
mismatch repair deficiency
- NFκB
nuclear factor kappa-light-chain-enhanced of activated B cells
- NK
natural killer
- Nos2
nitric oxidase synthase 2
- PanIN
pancreatic intraepithelial neoplasia
- PARP
poly ADP ribose polymerase
- PDAC
pancreatic ductal adenocarcinoma
- PD1
programmed cell death protein 1
- PDL1
programmed death-ligand 1
- PEGPH20
pegvorhyaluronidase alfa
- PI3Kγ
phosphoinositide 3-kinase
- RIP1K
receptor-interacting protein 1 kinase
- SIRPα
signal regulatory protein alpha
- STAT3
signal transducer and activator of transcription 3
- TAMs
tumor associated macrophages
- TIMP1
tissue inhibitor of metalloproteinase 1
- Tregs
T regulatory cells
- PD-1
programmed cell death protein 1
- PD-L1
programmed death-ligand 1
- RNA
ribonucleic acid
- SAA
serum amyloid A
- TGFβ
transforming growth factor beta
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- sTNF-RII
soluble tumor necrosis factor receptor type II
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aglietta M, Barone C, Sawyer MB, Moore MJ, Miller WH Jr., Bagala C, Colombi F, Cagnazzo C, Gioeni L, Wang E, Huang B, Fly KD, & Leone F (2014). A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol, 25, 1750–1755. [DOI] [PubMed] [Google Scholar]
- Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Kuttner V, Bruzas E, Maiorino L, Bautista C, Carmona EM, Gimotty PA, Fearon DT, Chang K, Lyons SK, Pinkerton KE, Trotman LC, Goldberg MS, Yeh JT, & Egeblad M (2018). Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Zolotarevsky E, Cooper KL, Sherman S, Shats O, Whitcomb DC, Lynch HT, Ghiorzo P, Rubinstein WS, Vogel KJ, Sasson AR, Grizzle WE, Ketcham MA, Lee SY, Normolle D, Plonka CM, Mertens AN, Tripon RC, & Brand RE (2012). Alcohol and tobacco lower the age of presentation in sporadic pancreatic cancer in a dose-dependent manner: a multicenter study. Am J Gastroenterol, 107, 1730–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeno J, Faivre-Finn C, Reck M, Vansteenkiste J, Spigel DR, Wadsworth C, Melillo G, Taboada M, Dennis PA, & Ozguroglu M (2018). Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med, 379, 2342–2350. [DOI] [PubMed] [Google Scholar]
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeno J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, & Ozguroglu M (2017). Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med, 377, 1919–1929. [DOI] [PubMed] [Google Scholar]
- Babic A, Schnure N, Neupane NP, Zaman MM, Rifai N, Welch MW, Brais LK, Rubinson DA, Morales-Oyarvide V, Yuan C, Zhang S, Poole EM, Wolpin BM, Kulke MH, Barbie DA, Wong K, Fuchs CS, & Ng K (2018). Plasma inflammatory cytokines and survival of pancreatic cancer patients. Clin Transl Gastroenterol, 9, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey P, Chang DK, Forget MA, Lucas FA, Alvarez HA, Haymaker C, Chattopadhyay C, Kim SH, Ekmekcioglu S, Grimm EA, Biankin AV, Hwu P, Maitra A, & Roszik J (2016). Exploiting the neoantigen landscape for immunotherapy of pancreatic ductal adenocarcinoma. Sci Rep, 6, 35848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grutzmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Biankin AV, & Grimmond SM (2016). Genomic analyses identify molecular subtypes of pancreatic cancer. Nature, 531, 47–52. [DOI] [PubMed] [Google Scholar]
- Balachandran VP, Luksza M, Zhao JN, Makarov V, Moral JA, Remark R, Herbst B, Askan G, Bhanot U, Senbabaoglu Y, Wells DK, Cary CIO, Grbovic-Huezo O, Attiyeh M, Medina B, Zhang J, Loo J, Saglimbeni J, Abu-Akeel M, Zappasodi R, Riaz N, Smoragiewicz M, Kelley ZL, Basturk O, Gonen M, Levine AJ, Allen PJ, Fearon DT, Merad M, Gnjatic S, Iacobuzio-Donahue CA, Wolchok JD, DeMatteo RP, Chan TA, Greenbaum BD, Merghoub T, & Leach SD (2017). Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature, 551, 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balli D, Rech AJ, Stanger BZ, & Vonderheide RH (2017). Immune Cytolytic Activity Stratifies Molecular Subsets of Human Pancreatic Cancer. Clin Cancer Res, 23, 3129–3138. [DOI] [PubMed] [Google Scholar]
- Barkal AA, Weiskopf K, Kao KS, Gordon SR, Rosental B, Yiu YY, George BM, Markovic M, Ring NG, Tsai JM, McKenna KM, Ho PY, Cheng RZ, Chen JY, Barkal LJ, Ring AM, Weissman IL, & Maute RL (2018). Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol, 19, 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, & Vonderheide RH (2012). Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell, 21, 822–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun WJ, Huhn RD, Song WR, Li DG, Sharp LL, Torigian DA, O’Dwyer PJ, & Vonderheide RH (2011). CD40 Agonists Alter Tumor Stroma and Show Efficacy Against Pancreatic Carcinoma in Mice and Humans. Science, 331, 1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Eghbali S, & Kim R (2017). Deploying Immunotherapy in Pancreatic Cancer: Defining Mechanisms of Response and Resistance. Am Soc Clin Oncol Educ Book, 37, 267–278. [DOI] [PubMed] [Google Scholar]
- Beatty GL, & Gladney WL (2015). Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res, 21, 687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, Kalos M, & June CH (2014). Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res, 2, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Li Y, & Long KB (2017). Cancer immunotherapy: activating innate and adaptive immunity through CD40 agonists. Expert Review of Anticancer Therapy, 17, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, O’Hara MH, Lacey SF, Torigian DA, Nazimuddin F, Chen F, Kulikovskaya IM, Soulen MC, McGarvey M, Nelson AM, Gladney WL, Levine BL, Melenhorst JJ, Plesa G, & June CH (2018). Activity of Mesothelin-Specific Chimeric Antigen Receptor T Cells Against Pancreatic Carcinoma Metastases in a Phase 1 Trial. Gastroenterology, 155, 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, Lee JW, Clendenin C, Gladney WL, Knoblock DM, Guirnalda PD, & Vonderheide RH (2015). Exclusion of T Cells From Pancreatic Carcinomas in Mice Is Regulated by Ly6C(low) F4/80(+) Extratumoral Macrophages. Gastroenterology, 149, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, Krijgsman O, van den Braber M, Philips D, Broeks A, van Thienen JV, Mallo HA, Adriaansz S, Ter Meulen S, Pronk LM, Grijpink-Ongering LG, Bruining A, Gittelman RM, Warren S, van Tinteren H, Peeper DS, Haanen J, van Akkooi ACJ, & Schumacher TN (2018). Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med, 24, 1655–1661. [DOI] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, & Wigginton JM (2012). Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med, 366, 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhtoiarov IN, Lum HD, Berke G, Sondel PM, & Rakhmilevich AL (2006). Synergistic activation of macrophages via CD40 and TLR9 results in T cell independent antitumor effects. Journal of Immunology, 176, 309–318. [DOI] [PubMed] [Google Scholar]
- Byrne KT, & Vonderheide RH (2016). CD40 Stimulation Obviates Innate Sensors and Drives T Cell Immunity in Cancer. Cell Rep, 15, 2719–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candido JB, Morton JP, Bailey P, Campbell AD, Karim SA, Jamieson T, Lapienyte L, Gopinathan A, Clark W, McGhee EJ, Wang J, Escorcio-Correia M, Zollinger R, Roshani R, Drew L, Rishi L, Arkell R, Evans TRJ, Nixon C, Jodrell DI, Wilkinson RW, Biankin AV, Barry ST, Balkwill FR, & Sansom OJ (2018). CSF1R(+) Macrophages Sustain Pancreatic Tumor Growth through T Cell Suppression and Maintenance of Key Gene Programs that Define the Squamous Subtype. Cell Rep, 23, 1448–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, & Jordan BF (2018). Gut microbiota-mediated inflammation in obesity: a link with gastrointestinal cancer. Nat Rev Gastroenterol Hepatol, 15, 671–682. [DOI] [PubMed] [Google Scholar]
- Carstens JL, Correa de Sampaio P, Yang D, Barua S, Wang H, Rao A, Allison JP, LeBleu VS, & Kalluri R (2017). Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat Commun, 8, 15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao T, Furth EE, & Vonderheide RH (2016). CXCR2-Dependent Accumulation of Tumor-Associated Neutrophils Regulates T-cell Immunity in Pancreatic Ductal Adenocarcinoma. Cancer Immunol Res, 4, 968–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomarat P, Banchereau J, Davoust J, & Palucka AK (2000). IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol, 1, 510–514. [DOI] [PubMed] [Google Scholar]
- Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, & Vonderheide RH (2007). Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res, 67, 9518–9527. [DOI] [PubMed] [Google Scholar]
- Cobo I, Martinelli P, Flandez M, Bakiri L, Zhang M, Carrillo-de-Santa-Pau E, Jia J, Sanchez-Arevalo Lobo VJ, Megias D, Felipe I, Del Pozo N, Millan I, Thommesen L, Bruland T, Olson SH, Smith J, Schoonjans K, Bamlet WR, Petersen GM, Malats N, Amundadottir LT, Wagner EF, & Real FX (2018). Transcriptional regulation by NR5A2 links differentiation and inflammation in the pancreas. Nature, 554, 533–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, Cooc J, Weinkle J, Kim GE, Jakkula L, Feiler HS, Ko AH, Olshen AB, Danenberg KL, Tempero MA, Spellman PT, Hanahan D, & Gray JW (2011). Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med, 17, 500–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran RB, Contino G, Deshpande V, Tzatsos A, Conrad C, Benes CH, Levy DE, Settleman J, Engelman JA, & Bardeesy N (2011). STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res, 71, 5020–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, GarciaSantos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, & Lyden D (2015). Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol, 17, 816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley D, Mani VR, Mohan N, Akkad N, Ochi A, Heindel DW, Lee KB, Zambirinis CP, Pandian GSB, Savadkar S, Torres-Hernandez A, Nayak S, Wang D, Hundeyin M, Diskin B, Aykut B, Werba G, Barilla RM, Rodriguez R, Chang S, Gardner L, Mahal LK, Ueberheide B, & Miller G (2017). Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat Med, 23, 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley D, Zambirinis CP, Seifert L, Akkad N, Mohan N, Werba G, Barilla R, TorresHernandez A, Hundeyin M, Mani VRK, Avanzi A, Tippens D, Narayanan R, Jang JE, Newman E, Pillarisetty VG, Dustin ML, Bar-Sagi D, Hajdu C, & Miller G (2016). gammadelta T Cells Support Pancreatic Oncogenesis by Restraining alphabeta T Cell Activation. Cell, 166, 1485–1499 e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danai LV, Babic A, Rosenthal MH, Dennstedt EA, Muir A, Lien EC, Mayers JR, Tai K, Lau AN, Jones-Sali P, Prado CM, Petersen GM, Takahashi N, Sugimoto M, Yeh JJ, Lopez N, Bardeesy N, Fernandez-Del Castillo C, Liss AS, Koong AC, Bui J, Yuan C, Welch MW, Brais LK, Kulke MH, Dennis C, Clish CB, Wolpin BM, & Vander Heiden MG (2018). Altered exocrine function can drive adipose wasting in early pancreatic cancer. Nature, 558, 600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M, & Lewis CE (2013). Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell, 23, 277–286. [DOI] [PubMed] [Google Scholar]
- Dibra D, Mishra L, & Li S (2014). Molecular mechanisms of oncogene-induced inflammation and inflammation-sustained oncogene activation in gastrointestinal tumors: an under-appreciated symbiotic relationship. Biochim Biophys Acta, 1846, 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RA, Diamond MS, Rech AJ, Chao T, Richardson MW, Lin JH, Bajor DL, Byrne KT, Stanger BZ, Riley JL, Markosyan N, Winograd R, & Vonderheide RH (2016). Lack of immunoediting in murine pancreatic cancer reversed with neoantigen. JCI Insight, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, & Fearon DT (2013). Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A, 110, 20212–20217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, Chen JY, Weissman-Tsukamoto R, Volkmer JP, Ho PY, McKenna KM, Cheshier S, Zhang M, Guo N, Gip P, Mitra SS, & Weissman IL (2015). Macrophages eat cancer cells using their own calreticulin as a guide: roles of TLR and Btk. Proc Natl Acad Sci U S A, 112, 2145–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandez M, Cendrowski J, Canamero M, Salas A, del Pozo N, Schoonjans K, & Real FX (2014). Nr5a2 heterozygosity sensitises to, and cooperates with, inflammation in KRas(G12V)-driven pancreatic tumourigenesis. Gut, 63, 647–655. [DOI] [PubMed] [Google Scholar]
- Flint TR, Janowitz T, Connell CM, Roberts EW, Denton AE, Coll AP, Jodrell DI, & Fearon DT (2016). Tumor-Induced IL-6 Reprograms Host Metabolism to Suppress Anti-tumor Immunity. Cell Metab, 24, 672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde PM, Chaft JE, & Pardoll DM (2018). Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med, 379, e14. [DOI] [PubMed] [Google Scholar]
- Gajewski TF, Schreiber H, & Fu YX (2013). Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol, 14, 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg B, Giri B, Modi S, Sethi V, Castro I, Umland O, Ban Y, Lavania S, Dawra R, Banerjee S, Vickers S, Merchant NB, Chen SX, Gilboa E, Ramakrishnan S, Saluja A, & Dudeja V (2018). NFkappaB in Pancreatic Stellate Cells Reduces Infiltration of Tumors by Cytotoxic T Cells and Killing of Cancer Cells, via Upregulation of CXCL12. Gastroenterology, 155, 880–891 e888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee K, Thaiss CA, Reuben A, Livny J, Avraham R, Frederick DT, Ligorio M, Chatman K, Johnston SE, Mosher CM, Brandis A, Fuks G, Gurbatri C, Gopalakrishnan V, Kim M, Hurd MW, Katz M, Fleming J, Maitra A, Smith DA, Skalak M, Bu J, Michaud M, Trauger SA, Barshack I, Golan T, Sandbank J, Flaherty KT, Mandinova A, Garrett WS, Thayer SP, Ferrone CR, Huttenhower C, Bhatia SN, Gevers D, Wargo JA, Golub TR, & Straussman R (2017). Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science, 357, 1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedegebuure P, Mitchem JB, Porembka MR, Tan MC, Belt BA, Wang-Gillam A, Gillanders WE, Hawkins WG, & Linehan DC (2011). Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer. Curr Cancer Drug Targets, 11, 734–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Robbins LA, Lugea A, Waldron RT, Jeon CY, & Pandol SJ (2014). Diabetes, pancreatic cancer, and metformin therapy. Front Physiol, 5, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesmann H, Drexel C, Milosevic N, Sipos B, Rosendahl J, Gress TM, & Michl P (2017). Pharmacological macrophage inhibition decreases metastasis formation in a genetic model of pancreatic cancer. Gut, 66, 1278–1285. [DOI] [PubMed] [Google Scholar]
- Grunwald B, Harant V, Schaten S, Fruhschutz M, Spallek R, Hochst B, Stutzer K, Berchtold S, Erkan M, Prokopchuk O, Martignoni M, Esposito I, Heikenwalder M, Gupta A, Siveke J, Saftig P, Knolle P, Wohlleber D, & Kruger A (2016). Pancreatic Premalignant Lesions Secrete Tissue Inhibitor of Metalloproteinases-1, Which Activates Hepatic Stellate Cells Via CD63 Signaling to Create a Premetastatic Niche in the Liver. Gastroenterology, 151, 1011–1024 e1017. [DOI] [PubMed] [Google Scholar]
- Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernandez-Porras I, Canamero M, Rodriguez-Justo M, Serrano M, & Barbacid M (2011). Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell, 19, 728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, & Barbacid M (2007). Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell, 11, 291–302. [DOI] [PubMed] [Google Scholar]
- Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, Kim G, Hanahan D, Tempero MA, Sheppard B, Irving B, Chang BY, Varner JA, & Coussens LM (2016). Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov, 6, 270–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, & Weinberg RA (2011). Hallmarks of cancer: the next generation. Cell, 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Helm O, Mennrich R, Petrick D, Goebel L, Freitag-Wolf S, Roder C, Kalthoff H, Rocken C, Sipos B, Kabelitz D, Schafer H, Oberg HH, Wesch D, & Sebens S (2014). Comparative characterization of stroma cells and ductal epithelium in chronic pancreatitis and pancreatic ductal adenocarcinoma. PLoS One, 9, e94357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendifar AE, Petzel MQB, Zimmers TA, Denlinger CS, Matrisian LM, Picozzi VJ, & Rahib L (2018). Pancreas Cancer-Associated Weight Loss. Oncologist. Hidalgo, M. (2010). Pancreatic cancer. N Engl J Med, 362, 1605–1617. [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, & Tuveson DA (2003). Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell, 4, 437–450. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, & Tuveson DA (2005). Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell, 7, 469–483. [DOI] [PubMed] [Google Scholar]
- Hiraoka N, Onozato K, Kosuge T, & Hirohashi S (2006). Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res, 12, 5423–5434. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Aburatani H, & Maru Y (2006). Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol, 8, 1369–1375. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, Shibuya M, Akira S, Aburatani H, & Maru Y (2008). The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol, 10, 1349–1355. [DOI] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, & Lyden D (2015). Tumour exosome integrins determine organotropic metastasis. Nature, 527, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu ZI, Shia J, Stadler ZK, Varghese AM, Capanu M, Salo-Mullen E, Lowery MA, Diaz LA Jr., Mandelker D, Yu KH, Zervoudakis A, Kelsen DP, Iacobuzio-Donahue CA, Klimstra DS, Saltz LB, Sahin IH, & O’Reilly EM (2018). Evaluating Mismatch Repair Deficiency in Pancreatic Adenocarcinoma: Challenges and Recommendations. Clin Cancer Res, 24, 1326–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AC, Orlowski RJ, Xu X, Mick R, George SM, Yan PK, Manne S, Kraya AA, Wubbenhorst B, Dorfman L, D’Andrea K, Wenz BM, Liu S, Chilukuri L, Kozlov A, Carberry M, Giles L, Kier MW, Quagliarello F, McGettigan S, Kreider K, Annamalai L, Zhao Q, Mogg R, Xu W, Blumenschein WM, Yearley JH, Linette GP, Amaravadi RK, Schuchter LM, Herati RS, Bengsch B, Nathanson KL, Farwell MD, Karakousis GC, Wherry EJ, & Mitchell TC (2019). A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med, 25, 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incio J, Liu H, Suboj P, Chin SM, Chen IX, Pinter M, Ng MR, Nia HT, Grahovac J, Kao S, Babykutty S, Huang Y, Jung K, Rahbari NN, Han X, Chauhan VP, Martin JD, Kahn J, Huang P, Desphande V, Michaelson J, Michelakos TP, Ferrone CR, Soares R, Boucher Y, Fukumura D, & Jain RK (2016). Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov, 6, 852–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, & Weissman IL (2009). CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell, 138, 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JE, Hajdu CH, Liot C, Miller G, Dustin ML, & Bar-Sagi D (2017). Crosstalk between Regulatory T Cells and Tumor-Associated Dendritic Cells Negates Anti-tumor Immunity in Pancreatic Cancer. Cell Rep, 20, 558–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, Nywening TM, Hawkins WG, Shapiro IM, Weaver DT, Pachter JA, Wang-Gillam A, & DeNardo DG (2016). Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med, 22, 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, & Kinzler KW (2008). Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science, 321, 1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbasi A, Komar C, Tooker GM, Liu M, Lee JW, Gladney WL, Ben-Josef E, & Beatty GL (2017). Tumor-Derived CCL2 Mediates Resistance to Radiotherapy in Pancreatic Ductal Adenocarcinoma. Clin Cancer Res, 23, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B, & Goggins M (2012). Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology, 142, 730–733 e739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, & Karin M (2009). Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature, 457, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegard J, Mortensen FV, & Cronin-Fenton D (2017). Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am J Gastroenterol, 112, 1366–1372. [DOI] [PubMed] [Google Scholar]
- Kitajima S, Thummalapalli R, & Barbie DA (2016). Inflammation as a driver and vulnerability of KRAS mediated oncogenesis. Semin Cell Dev Biol, 58, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen ES, Vail P, Balaji U, Ngo H, Botros IW, Makarov V, Riaz N, Balachandran V, Leach S, Thompson DM, Chan TA, & Witkiewicz AK (2017). Stratification of Pancreatic Ductal Adenocarcinoma: Combinatorial Genetic, Stromal, and Immunologic Markers. Clin Cancer Res, 23, 4429–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb R, Sutterwala FS, & Zhang W (2016). Obesity and cancer: inflammation bridges the two. Curr Opin Pharmacol, 29, 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidis IT, Vinuela EF, Tang LH, Klimstra DS, D’Angelica MI, Dematteo RP, Kingham TP, Fong Y, Jarnagin WR, & Allen PJ (2013). Incidentally discovered pancreatic intraepithelial neoplasia: what is its clinical significance? Ann Surg Oncol, 20, 3643–3647. [DOI] [PubMed] [Google Scholar]
- Korc M, Jeon CY, Edderkaoui M, Pandol SJ, & Petrov MS (2017). Tobacco and alcohol as risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol, 31, 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, Hashimoto A, Vonteddu P, Behera R, Goins MA, Mulligan C, Nam B, Hockstein N, Denstman F, Shakamuri S, Speicher DW, Weeraratna AT, Chao T, Vonderheide RH, Languino LR, Ordentlich P, Liu Q, Xu X, Lo A, Pure E, Zhang C, Loboda A, Sepulveda MA, Snyder LA, & Gabrilovich DI (2017). Cancer-Associated Fibroblasts Neutralize the Anti-tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors. Cancer Cell, 32, 654–668 e655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, & Diaz LA Jr. (2017). Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science, 357, 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, & Diaz LA Jr. (2015). PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med, 372, 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Komar CA, Bengsch F, Graham K, & Beatty GL (2016). Genetically Engineered Mouse Models of Pancreatic Cancer: The KPC Model (LSL-Kras(G12D/+);LSL-Trp53(R172H/+) ;Pdx-1-Cre), Its Variants, and Their Application in Immunooncology Drug Discovery. Curr Protoc Pharmacol, 73, 14 39 11–14 39 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Stone ML, Porrett PM, Thomas SK, Komar CA, Li JH, Delman D, Graham K, Gladney WL, Hua X, Black TA, Chien AL, Majmundar KS, Thompson JC, Yee SS, O’Hara MH, Aggarwal C, Xin D, Shaked A, Gao M, Liu D, Borad MJ, Ramanathan RK, Carpenter EL, Ji A, de Beer MC, de Beer FC, Webb NR, & Beatty GL (2019). Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature, 567, 249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, Yoshimura A, Reindl W, Sipos B, Akira S, Schmid RM, & Algul H (2011). Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell, 19, 456–469. [DOI] [PubMed] [Google Scholar]
- Li J, Byrne KT, Yan F, Yamazoe T, Chen Z, Baslan T, Richman LP, Lin JH, Sun YH, Rech AJ, Balli D, Hay CA, Sela Y, Merrell AJ, Liudahl SM, Gordon N, Norgard RJ, Yuan S, Yu S, Chao T, Ye S, Eisinger-Mathason TSK, Faryabi RB, Tobias JW, Lowe SW, Coussens LM, Wherry EJ, Vonderheide RH, & Stanger BZ (2018). Tumor Cell-Intrinsic Factors Underlie Heterogeneity of Immune Cell Infiltration and Response to Immunotherapy. Immunity, 49, 178–193 e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou GY, Bastea L, Fleming A, Doppler H, Edenfield BH, Dawson DW, Zhang L, Bardeesy N, & Storz P (2017). The Presence of Interleukin-13 at Pancreatic ADM/PanIN Lesions Alters Macrophage Populations and Mediates Pancreatic Tumorigenesis. Cell Rep, 19, 1322–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou GY, Doppler H, Necela B, Edenfield B, Zhang L, Dawson DW, & Storz P (2015). Mutant KRAS-induced expression of ICAM-1 in pancreatic acinar cells causes attraction of macrophages to expedite the formation of precancerous lesions. Cancer Discov, 5, 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Kalbasi A, & Beatty GL (2017). Functio Laesa: Cancer Inflammation and Therapeutic Resistance. J Oncol Pract, 13, 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, O’Connor RS, Trefely S, Graham K, Snyder NW, & Beatty GL (2019). Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated ‘don’t-eat-me’ signal. Nat Immunol, 20, 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, Zhang M, Papadopoulos N, Kinzler KW, Vogelstein B, Sears CL, Anders RA, Pardoll DM, & Housseau F (2015). The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov, 5, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KB, & Beatty GL (2013). Harnessing the antitumor potential of macrophages for cancer immunotherapy. Oncoimmunology, 2, e26860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KB, Collier AI, & Beatty GL (2017). Macrophages: Key orchestrators of a tumor microenvironment defined by therapeutic resistance. Mol Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KB, Gladney WL, Tooker GM, Graham K, Fraietta JA, & Beatty GL (2016). IFN gamma and CCL2 Cooperate to Redirect Tumor-Infiltrating Monocytes to Degrade Fibrosis and Enhance Chemotherapy Efficacy in Pancreatic Carcinoma. Cancer Discovery, 6, 400–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KB, Tooker G, Tooker E, Luque SL, Lee JW, Pan X, & Beatty GL (2017). IL6 Receptor Blockade Enhances Chemotherapy Efficacy in Pancreatic Ductal Adenocarcinoma. Mol Cancer Ther, 16, 1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lontchi-Yimagou E, Sobngwi E, Matsha TE, & Kengne AP (2013). Diabetes mellitus and inflammation. Curr Diab Rep, 13, 435–444. [DOI] [PubMed] [Google Scholar]
- Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A, Laheru D, Wolfgang CL, Wang J, Hruban RH, Anders RA, Jaffee EM, & Zheng L (2014). Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res, 2, 616–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, & Balkwill F (2008). Cancer-related inflammation. Nature, 454, 436–444. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Furukawa T, Yachida S, Nishimura M, Seki A, Nonaka K, Aida J, Takubo K, Ishiwata T, Kimura W, Arai T, & Mino-Kenudson M (2017). The Prevalence and Clinicopathological Characteristics of High-Grade Pancreatic Intraepithelial Neoplasia: Autopsy Study Evaluating the Entire Pancreatic Parenchyma. Pancreas, 46, 658–664. [DOI] [PubMed] [Google Scholar]
- McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, Rattigan Y, Roeser JC, Lankapalli RH, Zhang H, Jaffee EM, Drake CG, Housseau F, Maitra A, Kolls JK, Sears CL, Pardoll DM, & Leach SD (2014). Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell, 25, 621–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels AD, Newhook TE, Adair SJ, Morioka S, Goudreau BJ, Nagdas S, Mullen MG, Persily JB, Bullock TNJ, Slingluff CL Jr., Ravichandran KS, Parsons JT, & Bauer TW (2018). CD47 Blockade as an Adjuvant Immunotherapy for Resectable Pancreatic Cancer. Clin Cancer Res, 24, 1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midha S, Chawla S, & Garg PK (2016). Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett, 381, 269–277. [DOI] [PubMed] [Google Scholar]
- Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I, Ben-Baruch NE, Marth C, Madry R, Christensen RD, Berek JS, Dorum A, Tinker AV, du Bois A, Gonzalez-Martin A, Follana P, Benigno B, Rosenberg P, Gilbert L, Rimel BJ, Buscema J, Balser JP, Agarwal S, & Matulonis UA (2016). Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med, 375, 2154–2164. [DOI] [PubMed] [Google Scholar]
- Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, & DeNardo DG (2013). Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res, 73, 1128–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, Rashid NU, Williams LA, Eaton SC, Chung AH, Smyla JK, Anderson JM, Kim HJ, Bentrem DJ, Talamonti MS, Iacobuzio-Donahue CA, Hollingsworth MA, & Yeh JJ (2015). Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet, 47, 1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, Ireland L, Sakai T, Sakai K, Kim YS, Engle D, Campbell F, Palmer D, Ko JH, Tuveson DA, Hirsch E, Mielgo A, & Schmid MC (2016). Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol, 18, 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nywening TM, Belt BA, Cullinan DR, Panni RZ, Han BJ, Sanford DE, Jacobs RC, Ye J, Patel AA, Gillanders WE, Fields RC, DeNardo DG, Hawkins WG, Goedegebuure P, & Linehan DC (2018). Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut, 67, 1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, & Kalinski P (2011). PGE(2)induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res, 71, 7463–7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi A, Graffeo CS, Zambirinis CP, Rehman A, Hackman M, Fallon N, Barilla RM, Henning JR, Jamal M, Rao R, Greco S, Deutsch M, Medina-Zea MV, Bin Saeed U, Ego-Osuala MO, Hajdu C, & Miller G (2012). Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans. J Clin Invest, 122, 4118–4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi A, Nguyen AH, Bedrosian AS, Mushlin HM, Zarbakhsh S, Barilla R, Zambirinis CP, Fallon NC, Rehman A, Pylayeva-Gupta Y, Badar S, Hajdu CH, Frey AB, Bar-Sagi D, & Miller G (2012). MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp Med, 209, 1671–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive KP (2017). Fanning the Flames of Cancer Chemoresistance: Inflammation and Anticancer Therapy. J Oncol Pract, 13, 181–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, Rugo HS, Cohen RB, O’Neil BH, Mehnert JM, Lopez J, Doi T, van Brummelen EMJ, Cristescu R, Yang P, Emancipator K, Stein K, Ayers M, Joe AK, & Lunceford JK (2019). T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028. J Clin Oncol, 37, 318–327. [DOI] [PubMed] [Google Scholar]
- Pergamo M, & Miller G (2017). Myeloid-derived suppressor cells and their role in pancreatic cancer. Cancer Gene Ther, 24, 100–105. [DOI] [PubMed] [Google Scholar]
- Puleo F, Nicolle R, Blum Y, Cros J, Marisa L, Demetter P, Quertinmont E, Svrcek M, Elarouci N, Iovanna J, Franchimont D, Verset L, Galdon MG, Deviere J, de Reynies A, Laurent-Puig P, Van Laethem JL, Bachet JB, & Marechal R (2018). Stratification of Pancreatic Ductal Adenocarcinomas Based on Tumor and Microenvironment Features. Gastroenterology, 155, 1999–2013 e1993. [DOI] [PubMed] [Google Scholar]
- Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres LE, Werba G, Zhang K, Guo Y, Li Q, Akkad N, Lall S, Wadowski B, Gutierrez J, Kochen Rossi JA, Herzog JW, Diskin B, Torres-Hernandez A, Leinwand J, Wang W, Taunk PS, Savadkar S, Janal M, Saxena A, Li X, Cohen D, Sartor RB, Saxena D, & Miller G (2018). The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov, 8, 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, & Bar-Sagi D (2012). Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell, 21, 836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, & Joyce JA (2013). CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med, 19, 1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, & Pollard JW (2011). CCL2 recruits inflammatory monocytes to facilitate breasttumour metastasis. Nature, 475, 222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, & Pollard JW (2010). Macrophage diversity enhances tumor progression and metastasis. Cell, 141, 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech AJ, Dada H, Kotzin JJ, Henao-Mejia J, Minn AJ, Twyman-Saint Victor C, & Vonderheide RH (2018). Radiotherapy and CD40 Activation Separately Augment Immunity to Checkpoint Blockade in Cancer. Cancer Res, 78, 4282–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renehan AG, Tyson M, Egger M, Heller RF, & Zwahlen M (2008). Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet, 371, 569–578. [DOI] [PubMed] [Google Scholar]
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, & Stanger BZ (2012). EMT and dissemination precede pancreatic tumor formation. Cell, 148, 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzwajg M, Jourquin F, Tailleux L, & Gluckman JC (2002). CD40 ligation and phagocytosis differently affect the differentiation of monocytes into dendritic cells. J Leukoc Biol, 72, 1180–1189. [PubMed] [Google Scholar]
- Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, & Rosenberg SA (2010). Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother, 33, 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD, Wang-Gillam A, Eberlein TJ, Denardo DG, Goedegebuure SP, & Linehan DC (2013). Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res, 19, 3404–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi V, Kurtom S, Tarique M, Lavania S, Malchiodi Z, Hellmund L, Zhang L, Sharma U, Giri B, Garg B, Ferrantella A, Vickers SM, Banerjee S, Dawra R, Roy S, Ramakrishnan S, Saluja A, & Dudeja V (2018). Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology, 155, 33–37 e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert B, Grunwald B, Kobuch J, Cui H, Schelter F, Schaten S, Siveke JT, Lim NH, Nagase H, Simonavicius N, Heikenwalder M, Reinheckel T, Sleeman JP, Janssen KP, Knolle PA, & Kruger A (2015). Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology, 61, 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, & Jemal A (2019). Cancer statistics, 2019. CA Cancer J Clin, 69, 7–34. [DOI] [PubMed] [Google Scholar]
- Singhal S, Stadanlick J, Annunziata MJ, Rao AS, Bhojnagarwala PS, O’Brien S, Moon EK, Cantu E, Danet-Desnoyers G, Ra HJ, Litzky L, Akimova T, Beier UH, Hancock WW, Albelda SM, & Eruslanov EB (2019). Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci Transl Med, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleightholm RL, Neilsen BK, Li J, Steele MM, Singh RK, Hollingsworth MA, & Oupicky D (2017). Emerging roles of the CXCL12/CXCR4 axis in pancreatic cancer progression and therapy. Pharmacol Ther, 179, 158–170. [DOI] [PubMed] [Google Scholar]
- Spranger S, Bao R, & Gajewski TF (2015). Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature, 523, 231–235. [DOI] [PubMed] [Google Scholar]
- Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K, Wilson Z, Eberlein C, Candido JB, Clarke M, Nixon C, Connelly J, Jamieson N, Carter CR, Balkwill F, Chang DK, Evans TRJ, Strathdee D, Biankin AV, Nibbs RJB, Barry ST, Sansom OJ, & Morton JP (2016). CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell, 29, 832–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromnes IM, Hulbert A, Pierce RH, Greenberg PD, & Hingorani SR (2017). T-cell Localization, Activation, and Clonal Expansion in Human Pancreatic Ductal Adenocarcinoma. Cancer Immunol Res, 5, 978–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy JA, Ziv E, Culhane AC, Paull EO, Sivakumar IKA, Gentles AJ, Malhotra R, Farshidfar F, Colaprico A, Parker JS, Mose LE, Vo NS, Liu J, Liu Y, Rader J, Dhankani V, Reynolds SM, Bowlby R, Califano A, Cherniack AD, Anastassiou D, Bedognetti D, Rao A, Chen K, Krasnitz A, Hu H, Malta TM, Noushmehr H, Pedamallu CS, Bullman S, Ojesina AI, Lamb A, Zhou W, Shen H, Choueiri TK, Weinstein JN, Guinney J, Saltz J, Holt RA, Rabkin CE, Lazar AJ, Serody JS, Demicco EG, Disis ML, Vincent BG, & Shmulevich L (2018). The Immune Landscape of Cancer. Immunity, 48, 812–830 e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tienhoven GV, Versteijne E, Suker M, Groothuis KBC, Busch OR, Bonsing BA, Hingh I. H. J. T. d., Festen S, Patijn GA, Vos-Geelen J. d., Zwinderman AH, Punt CJA, Eijck C. H. J. v., & Group, a. a. o. i. o. t. i. p. i. t. t. o. b. o. t. D. P. C. (2018). Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): A randomized, controlled, multicenter phase III trial. Journal of Clinical Oncology, 36, LBA4002–LBA4002. [Google Scholar]
- Tsujikawa T, Kumar S, Borkar RN, Azimi V, Thibault G, Chang YH, Balter A, Kawashima R, Choe G, Sauer D, El Rassi E, Clayburgh DR, Kulesz-Martin MF, Lutz ER, Zheng L, Jaffee EM, Leyshock P, Margolin AA, Mori M, Gray JW, Flint PW, & Coussens LM (2017). Quantitative Multiplex Immunohistochemistry Reveals Myeloid-Inflamed Tumor-Immune Complexity Associated with Poor Prognosis. Cell Rep, 19, 203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteijne E, Vogel JA, Besselink MG, Busch ORC, Wilmink JW, Daams JG, van Eijck CHJ, Groot Koerkamp B, Rasch CRN, & van Tienhoven G (2018). Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg, 105, 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Marinis JM, Beal AM, Savadkar S, Wu Y, Khan M, Taunk PS, Wu N, Su W, Wu J, Ahsan A, Kurz E, Chen T, Yaboh I, Li F, Gutierrez J, Diskin B, Hundeyin M, Reilly M, Lich JD, Harris PA, Mahajan MK, Thorpe JH, Nassau P, Mosley JE, Leinwand J, Kochen Rossi JA, Mishra A, Aykut B, Glacken M, Ochi A, Verma N, Kim JI, Vasudevaraja V, Adeegbe D, Almonte C, Bagdatlioglu E, Cohen DJ, Wong KK, Bertin J, & Miller G (2018). RIP1 Kinase Drives Macrophage-Mediated Adaptive Immune Tolerance in Pancreatic Cancer. Cancer Cell, 34, 757–774 e757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf K, Ring AM, Ho CC, Volkmer JP, Levin AM, Volkmer AK, Ozkan E, Fernhoff NB, van de Rijn M, Weissman IL, & Garcia KC (2013). Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science, 341, 88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, & Vonderheide RH (2015). Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol Res, 3, 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, & Hruban RH (2013). Recent progress in pancreatic cancer. CA Cancer J Clin, 63, 318–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormann SM, Song L, Ai J, Diakopoulos KN, Kurkowski MU, Gorgulu K, Ruess D, Campbell A, Doglioni C, Jodrell D, Neesse A, Demir IE, Karpathaki AP, Barenboim M, Hagemann T, Rose-John S, Sansom O, Schmid RM, Protti MP, Lesina M, & Algul H (2016). Loss of P53 Function Activates JAK2-STAT3 Signaling to Promote Pancreatic Tumor Growth, Stroma Modification, and Gemcitabine Resistance in Mice and Is Associated With Patient Survival. Gastroenterology, 151, 180–193 e112. [DOI] [PubMed] [Google Scholar]
- Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, Velculescu VE, Kinzler KW, Vogelstein B, & Iacobuzio-Donahue CA (2010). Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature, 467, 1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmin-Karim S, Bruck PT, Moreau M, Kunjachan S, Chen GZ, Kumar R, Grabow S, Dougan SK, & Ngwa W (2018). Radiation and Local Anti-CD40 Generate an Effective in situ Vaccine in Preclinical Models of Pancreatic Cancer. Front Immunol, 9, 2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambirinis CP, Levie E, Nguy S, Avanzi A, Barilla R, Xu Y, Seifert L, Daley D, Greco SH, Deutsch M, Jonnadula S, Torres-Hernandez A, Tippens D, Pushalkar S, Eisenthal A, Saxena D, Ahn J, Hajdu C, Engle DD, Tuveson D, & Miller G (2015). TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J Exp Med, 212, 2077–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambirinis CP, & Miller G (2013). Signaling via MYD88 in the pancreatic tumor microenvironment: A double-edged sword. Oncoimmunology, 2, e22567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Li L, Jiang H, Knolhoff BL, Lockhart AC, Wang-Gillam A, DeNardo DG, Ruzinova MB, & Lim KH (2017). Constitutive IRAK4 Activation Underlies Poor Prognosis and Chemoresistance in Pancreatic Ductal Adenocarcinoma. Clin Cancer Res, 23, 1748–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yan W, Mathew E, Bednar F, Wan S, Collins MA, Evans RA, Welling TH, Vonderheide RH, & di Magliano MP (2014). CD4+ T lymphocyte ablation prevents pancreatic carcinogenesis in mice. Cancer Immunol Res, 2, 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yan W, Mathew E, Kane KT, Brannon A 3rd, Adoumie M, Vinta A, Crawford HC, & Pasca di Magliano M (2017). Epithelial-Myeloid cell crosstalk regulates acinar cell plasticity and pancreatic remodeling in mice. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, Cullinan DR, Luo J, Bearden AR, Lavine KJ, Yokoyama WM, Hawkins WG, Fields RC, Randolph GJ, & DeNardo DG (2017). Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression. Immunity, 47, 323–338 e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, & DeNardo DG (2014). CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res, 74, 5057–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]