Abstract
Objectives:
Many patients who undergo endovascular aortic aneurysm repair (EVR) will receive repeat procedures, or reinterventions, to address suboptimal device performance and prevent aneurysm rupture. Quality improvement initiatives measuring reintervention after EVR has focused on fee-for-service Medicare patients. However, as patients aged <65 years and those with Medicare Advantage represent an important growing subgroup, we used a novel approach leveraging a state data source which captures all ages and types of insurance.
Methods:
We identified patients who underwent EVR (2011–2015) within the Vascular Quality Initiative (VQI) registry and were also listed in the Statewide Planning and Research Cooperative System (SPARCS) all-payer claims database of New York. We linked patients in the VQI to their SPARCS claims file at the patient level with 96% match rate. We compared outcomes between fee-for-service Medicare eligible, defined as age ≥65 or on dialysis, versus non-eligible patients, defined as those younger than 65 and not on dialysis. Our primary outcome was reintervention. We used Cox-proportional hazards regression and propensity-score matching for risk adjustment.
Results:
We studied 1,285 patients with a median follow up of 16 months (range 1–57 months). Mean age was 74 years, 79% were male, and 84% of procedures were elective. Nearly one in six patients were not Medicare eligible (14%), while the remainder (86%) were Medicare eligible. Medicare eligible patients were less likely to be male (77% versus 91%, p<.001), have a history of smoking (79% versus 93%, p<.001), and have a non-elective procedure (15% versus 23%, p=.013). The 3-year Kaplan-Meier rate of reintervention was 21%. We found similar rates of reintervention between Medicare eligible patients versus those who were not (19% versus 20%, log-rank p=.199; unadjusted hazard ratio (HR): 0.75, 95% confidence interval (CI): 0.49–1.16). This finding persisted in both the adjusted and propensity-matched analyses (adjusted HR: 0.82 (CI: 0.50–1.34); propensity-matched HR: 0.70; CI: 0.36–1.37).
Conclusions:
Reintervention can be monitored using administrative claims from both Medicare and non-Medicare payers, and serve as an important outcome metric after EVR in patients of all ages. The rate of reintervention appears to be similar between older, Medicare eligible individuals, and those who are not yet eligible.
Keywords: Reintervention after EVR, All-payer claims, Device performance measurement
Here is the edited TOC summary:
VQI data is linked to all payer claims from the state of New York to study reintervention among 1285 patients. The rate of reintervention was similar between Medicare eligible and non eligible patients, indicating that reintervention can be monitored using claims data and serve as a quality metric for EVR performance in patients of all ages and insurance types.
Introduction:
More than 50,000 Americans undergo endovascular aortic aneurysm repair (EVR) each year.1, 2 Studies suggest that up to 30% of patients who undergo EVR may require additional procedures, termed reinterventions, to address suboptimal device performance, type 2 endoleak, or other procedure-related problems and ensure their aneurysm remains free from rupture over time.3–8 The rate at which reinterventions occur represents an important quality indicator for these implanted devices, monitoring of which is endorsed by both the Food and Drug Administration and the Society for Vascular Surgery.9, 10
Despite these recommendations, accurately measuring reintervention in contemporary practice is difficult. In “real world” settings, patients often receive postoperative care at institutions other than the hospital where the operation was performed.11, 12 In addition, compliance with manual entry of patient follow-up into quality improvement registries is variable.12 These two factors in aggregate make assessing the true rate of reintervention after EVR problematic and likely leads to an incomplete assessment of postoperative outcomes. Therefore, developing a mechanism to accurately assess the rate of reintervention after EVR in the “real world” setting is integral to the objective measurement of device performance.
To address this problem, prior investigators have utilized Medicare claims data to improve follow-up for patients undergoing EVR.3, 11, 13 Linking Medicare claims to existing clinical registries such as the Vascular Quality Initiative (VQI) has allowed an accurate assessment of the rate of reintervention after EVR.14 However, because these studies are limited to Medicare fee-for-service patients, they fail to capture individuals who are under 65 years of age and therefore have not yet reached Medicare eligibility, and those who subscribe to Medicare Advantage plans. Whether such a method of reintervention assessment is possible for this important and growing subgroup of patients is unknown.
Therefore, the objective of this study was to create a combined registry-claims system capable of capturing patients of all ages and insurance types and then to assess the rate of reintervention after EVR for both Medicare eligible patients (over 65 years and/or dialysis dependent) and younger patients who had not yet reached Medicare eligibility. Our hypothesis was that reintervention could be assessed for all patients using this method, and that reintervention rates would be similar across the groups.
Methods:
Data sources and cohort creation
We used data from the VQI to identify patients who underwent EVR in New York.15, 16 Patients were eligible for inclusion if they had undergone EVR at a VQI participating center in New York between January 2011 and September 2015. Patients who had more than one aortic procedure in the same day were assigned according to the first procedure (open or endovascular repair) they underwent. We then obtained corresponding discharge records from the New York Statewide Planning and Research Cooperative System (SPARCS) all-payer claims database. The SPARCS system captures discharge data from all in-hospital and emergency department encounters for the state of New York.16 Next, we linked patients in the VQI with their respective SPARCS claims file. A validated sequential linkage algorithm was used for indirect linkage.17 Indirect identifiers were the facility identification number and patients’ year and month of birth, sex, and procedure date. All variables and exact procedure date were used in the first step. In later steps, flexibility was allowed by omitting patients’ sex or month of birth and having a 3-day window before or after the procedure date. At each step, flexibility in only one of these aspects was permitted while matching to VQI data. This algorithm has been validated internally to have >90% sensitivity and >99% accuracy.17 We obtained a successful match in 96% of patients (1,285 / 1,336). The most common reason for non-match was that VQI patients could not be identified in SPARCS. This occurred at random. This combined VQI-SPARCS database formed our cohort for analysis. SPARCS data was available from January 2011 to September 2015. SPARCS data after October 1st, 2015 was not analyzed because at that time billing encounters transitioned from the international classification of diseases ninth revision (ICD-9) to the tenth revision (ICD-10) and therefore coding algorithms used to identify clinical events in ICD-9 could no longer be used. VQI follow-up information was available through December 2017.
Primary exposure, outcomes, and definitions
Our primary exposure was Medicare eligibility. Medicare eligibility was defined at the time of the patients index operation as those aged ≥65 years or on dialysis.18 To examine the utility of all-payer claims datasets in evaluating reintervention in non-Medicare eligible patients, we performed an analysis on patients who were Medicare eligible versus those who were not.
Our primary outcome of interest was reintervention after EVR. We defined reintervention after EVR consistent with prior work, as any repeat procedure related to the aneurysm or for the aneurysm repair after discharge from the index hospitalization.14 We examined reintervention from both the VQI and SPARCS data. Reinterventions found in both sources were counted only once. We measured reintervention events in the SPARCS database with ICD-9 with a validated algorithm used in our research as part of longitudinal work examining outcomes after EVR.3, 14, 19 Patients were censored at death or at the end of their known VQI-SPARCS follow-up. Vital status was determined using the Social Security Death Index. All SPARCS data prior to September 2015 consisted of ICD-9 billing encounters. Therefore, no ICD-10 codes were used.
Statistical analysis
Patient characteristics were compared between those who were Medicare eligible at the time of the index EVR procedure and those who were not Medicare eligible. We report absolute numbers and percentages where appropriate. Continuous variables are represented as means with standard deviations or medians with interquartile ranges, and categorical variables are listed as percentages. We used Student’s t-test to compare continuous variables and Chi-square analysis to compare categorical variables.
We first examined the rate of reintervention after EVR using Kaplan-Meier survival estimation. We used the log-rank test to compare survival curves stratified by Medicare eligibility. We next compared the unadjusted hazard ratio (HR) of reintervention between patients who were Medicare eligible at the time of their index operation versus those who were not using a Cox-proportional hazard regression. The difference between the unadjusted hazard ratio and the Kaplan-Meier analysis is that the Cox model enforces the proportional hazards assumption.20
Two different methods were used to account for differences in patients’ characteristics between groups. We first used a multivariable Cox-proportional hazard regression model to adjust for confounding variables, including patients sex, race, clinical characteristics, pre-operative medication, and operative characteristics. As age and dialysis are closely related to Medicare eligibility, we created two Cox-regression models, one including age and dialysis, and one without. We noted no meaningful difference between the two models and report the model that does not include age and dialysis.
We next created a nearest neighbor propensity-matched cohort balanced in baseline covariates, similar to previous work.21 We used the known clinical and procedure characteristics in Table I to create a logistic regression model where the dependent variable was Medicare eligibility. We calculated the probability of Medicare eligibility (propensity score) for each patient.22 We matched patients who were not Medicare eligible to similar patients who were. Patients were not matched on age or dialysis as these characteristics determine Medicare eligibility.18 We then calculated the HR of reintervention between the two groups using Cox-proportional hazards regression with robust variance estimation to account for censoring.
Table 1:
Clinical and Operative Characteristics of the Cohort
| Entire Cohort Medicare Eligibility | Propensity-Matched Cohort Medicare Eligibility | |||||
|---|---|---|---|---|---|---|
| Variable |
No n=175 |
Yes n=1,110 |
p |
No n=167 |
Yes n=167 |
p |
| Demographics | ||||||
| Age mean (SD), y | 59.7 (4.0) |
76.6 (7.0) |
<.001 | 59.7 (4.1) |
75.9 (7.2) |
<.001 |
| Male, % | 90.9 | 76.5 | <.001 | 90.4 | 95.8 | .052 |
| White race | 78.7 | 86.1 | .095 | 80.2 | 82.6 | .573 |
| Clinical Characteristics | ||||||
| Coronary Disease | 27.4 | 28.0 | .872 | 27.0 | 30.5 | .468 |
| Coronary Revascularization | 14.9 | 12.3 | .327 | 14.6 | 13.2 | .751 |
| Heart Failure | 8.6 | 11.5 | .265 | 7.2 | 10.2 | .331 |
| COPD | 21.4 | 31.7 | .006 | 21.1 | 28.3 | .127 |
| Smoking History | 93.1 | 78.7 | <.001 | 92.8 | 88.0 | .137 |
| Prior Aortic Surgery | 2.3 | 2.8 | .694 | 2.4 | 3.6 | .521 |
| Positive preoperative stress test | 8.5 | 7.5 | .807 | 8.4 | 4.8 | .186 |
| BMI >35 | 18.9 | 8.4 | <.001 | 18 | 18.0 | 1 |
| Hypertension | 81.0 | 87.0 | .033 | 80.8 | 84.4 | .386 |
| Diabetes | 23.1 | 21.4 | .607 | 22.3 | 22.8 | .919 |
| Chronic Kidney Disease* | 8.6 | 8.2 | .850 | 7.8 | 12.8 | .137 |
| Preoperative Medications | ||||||
| Aspirin | 61.5 | 61.7 | .959 | 61.7 | 63.5 | .734 |
| P2y12 inhibitor | 15.5 | 19.0 | .276 | 16.2 | 21.6 | .208 |
| Statin | 59.2 | 66.4 | .065 | 59.9 | 67.7 | .139 |
| Operative Characteristics | ||||||
| Urgency | ||||||
| Elective | 77.1 | 84.8 | .013 | 79 | 78.4 | 524 |
| Urgent | 14.3 | 11.0 | 14.4 | 12.0 | ||
| Emergent (ruptured) | 8.6 | 4.2 | 6.6 | 9.6 | ||
| AAA Diameter | ||||||
| <5.5 cm | 49.7 | 47.1 | .628 | 50.9 | 47.5 | .703 |
| 5.5–6.4 cm | 28.6 | 32.2 | 28.7 | 28.4 | ||
| >=6.5 cm | 21.7 | 20.7 | 20.4 | 24.1 | ||
| Iliac aneurysm | 23.8 | 17.6 | .048 | 22.8 | 24.6 | .699 |
| Conversion to open | 0 | 0.1 | .693 | 0 | 0.6 | .324 |
Creatinine >1.7 milligrams per deciliter.
SD, standard deviation; COPD, chronic obstructive pulmonary disease; BMI, body mass index in kilograms / meter squared; AAA, abdominal aortic aneurysm.
We performed all statistical analyses using Stata version 12 software (College Station, Texas).
Human subjects protection
VQI data is collected under the auspices of an Agency for Healthcare Research and Quality designated Patient Safety Organization. This study was approved by the Center for the Protection of Human Subjects at Dartmouth and the Weill Cornell Institutional Review Board. All patient personal health information was protected, records and outcomes were de-identified, and no testing or procedures were required for this study in accordance with SPARCS data use agreements. Thus, a HIPAA waiver and a waiver of consent were obtained.
Results:
Cohort characteristics
We studied 1,285 patients who underwent EVR during the study period (Table I). Median follow up was 16 months (range 1–57 months). Patients who were not eligible for Medicare at the time of their index operation made up 13.6% of the cohort (175 / 1,285). Patients who were not Medicare eligible were younger (mean age = 59.7 years versus 76.6 years; p<.001) and more likely to be male (90.9% versus 76.5%; p<.001).
Patients who were not Medicare eligible were similar to Medicare eligible patients in most clinical characteristics with the exception of chronic obstructive pulmonary disease (not Medicare eligible = 21.4% versus Medicare eligible = 31.7%; p=.006), hypertension (not Medicare eligible = 81.0% versus Medicare eligible = 87.0%; p=.033) or having a body mass index of >35 kilograms per meter squared (not Medicare eligible = 18.9% versus Medicare eligible = 8.4%, p<.001). There were important differences in operative characteristics between the two groups. Those who were not Medicare eligible were more likely to have an urgent or emergent procedure (not Medicare eligible = 22.9% versus Medicare eligible = 15.2%, p=.013), or have an iliac artery aneurysm (not Medicare eligible = 23.8% versus Medicare eligible = 17.6%, p=.048).
Given the above differences, we created a propensity-matched cohort to better align baseline characteristics between Medicare eligible and non-Medicare eligible patients. Propensity matching yielded 167 matched pairs of patients. While there was a trend towards more male patients in the group that was not Medicare eligible (not Medicare eligible = 90.4%, versus Medicare eligible = 95.8%, p=.052), the propensity matched cohort was well balanced in all other clinical and operative characteristics (Table I).
Rates of reintervention
The Kaplan-Meier estimated rate of reintervention after EVR for the entire cohort increased over the 3-year follow-up period (Figure 1). At 1 year, the cumulative rate was 5%. This increased to 14% at 2 years, and 20% at 3 years. The shape of the curves indicated that many reintervention events occurred during the initial postoperative period, and then again between one and two years postoperatively. While fewer events occurred after 2 years, the rate did not appear to decline in slope after two years.
Figure 1:
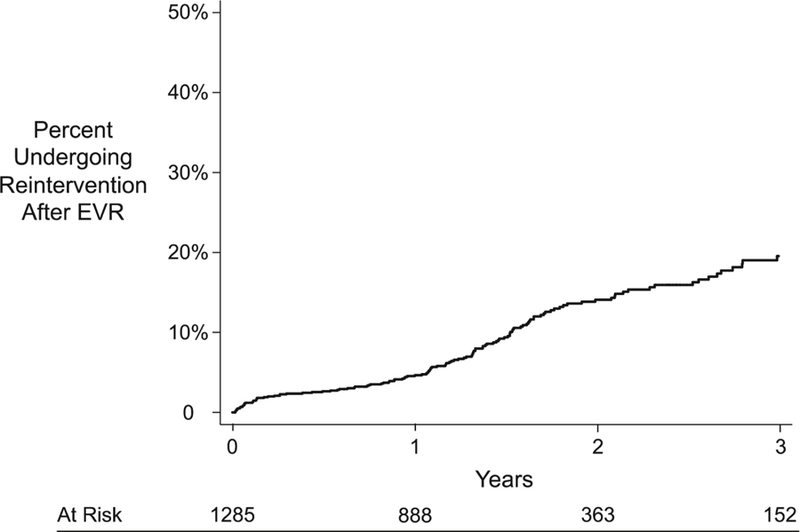
Kaplan-Meier Estimated Cumulative Incidence of Reintervention After EVR Among Participating Centers in New York
EVR, endovascular aneurysm repair
Standard error <10%
We found no statistically significant difference in the Kaplan-Meier estimated rate of reintervention after EVR for patients who were Medicare eligible versus those who were not (Figure 2). The cumulative rate of reintervention for those who were Medicare eligible was 5% at 1 year and rose to 19% at 3 years. Among patients who were not Medicare eligible, the rate of reintervention was 4% at 1 year, and 20% at 3 years (log-rank p=.199). The adjusted regression model revealed no statistically significant difference between the two groups, with an adjusted HR of 0.82 (95% confidence interval (CI): 0.50–1.34). The unadjusted HR was also not statistically significant (0.75, CI: 0.49–1.16).
Figure 2:
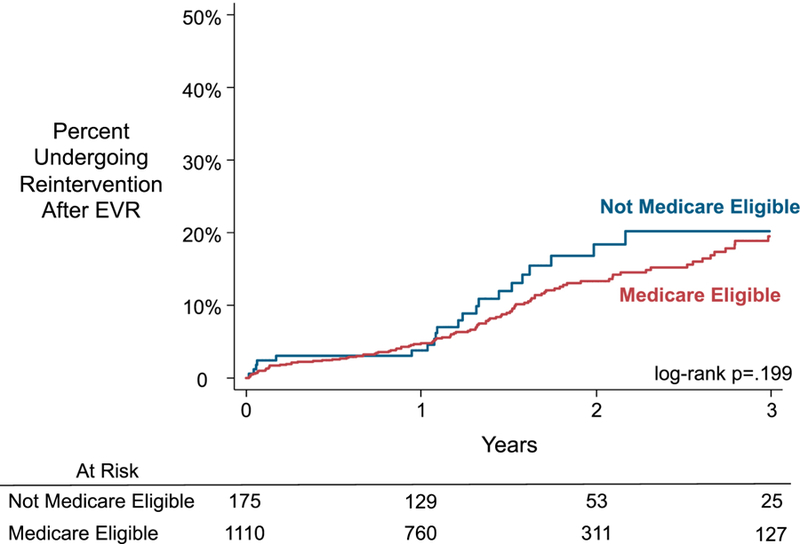
Kaplan-Meier Estimated Cumulative Incidence of Reintervention After EVR Among Participating Centers in New York, Stratified by Medicare Eligibility
EVR, endovascular aneurysm repair
Standard error <10%
The propensity-matched model demonstrated similar findings (Figure 3). Among the propensity-matched cohort, the cumulative rate of reintervention at 1 and 3 years for patients who were not Medicare eligible was 4% and 21% respectively. The cumulative rate for patients who were Medicare eligible at the time of their index operation at 1 and 3 years was 7% and 23% respectively (log-rank p=.276). However, because of the sample size, standard error for the 3-year estimate of those who were Medicare eligible was 10.6%. The HR of reintervention for the propensity-matched cohort was similar to the unadjusted and adjusted models with a propensity-matched HR of 0.70 (CI: 0.36–1.37).
Figure 3:
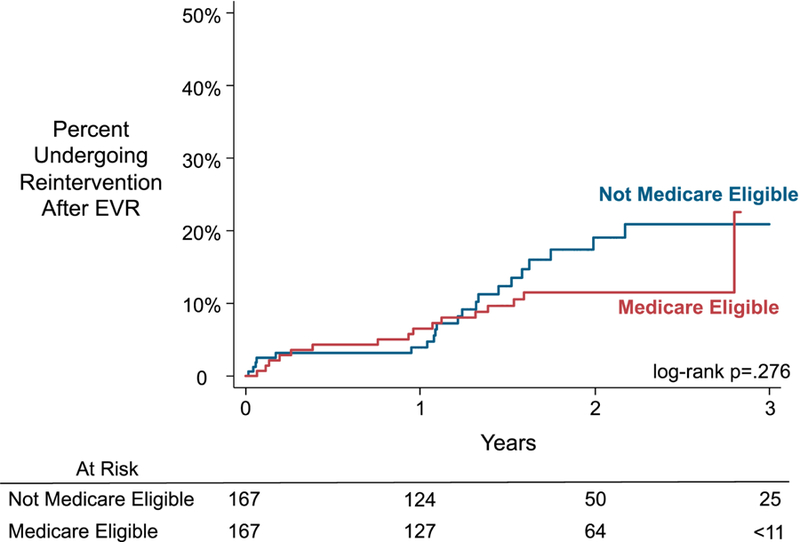
Kaplan-Meier Estimated Cumulative Incidence of Reintervention After EVR Among
EVR, endovascular aneurysm repair
Data censored where standard error >10%
Predictors of reintervention
Our Cox-proportional hazards regression model revealed additional factors which were significantly associated with reintervention after EVR. Patients with a history of any type of aortic surgery prior to their index EVR demonstrated a nearly 3.5-fold higher risk of reintervention (HR = 3.47, CI: 1.71–7.04, p<.001). Patients who underwent their index EVR for a ruptured aortic aneurysm had a HR of reintervention of 3.43 (CI: 1.57–7.50, p=.002). The year in which the operation was performed appeared to correlate with the rate of reintervention, with an increase in the HR of reintervention for each year. When compared to patients who had their operations performed in 2011, those who underwent EVR in 2012 had a HR of reintervention of 0.97 (range 0.36–2.59), those in 2013 had a HR of 1.61 (range 0.56–4.58), those in 2014 had a HR of 2.76 (range 1.01–7.55) and finally those undergoing surgery in 2015 had a HR of 4.28 (range 1.51–12.14). Comorbidities including heart failure, coronary artery disease, chronic obstructive pulmonary disease, diabetes, and chronic kidney disease, did not demonstrate a statistically significant association. In addition, the size of the aortic aneurysm at the index operation was not significantly associated with the likelihood of reintervention, nor was the presence of an iliac artery aneurysm. In multivariable modeling, Medicare eligibility (age ≥65 years or on dialysis) was not associated with reintervention (HR = 0.82, CI: 0.50–1.33, p=.427).
Discussion:
In this study, we successfully linked clinical registry data from the VQI to all-payer claims data from the SPARCS system of New York at the patient level. Using this novel combined data source, we found that approximately one-in-five patients underwent reintervention after within the first 3 years after EVR. While other investigators have used claims data to assess the rate of reintervention after EVR, these analyses are limited to fee-for-service Medicare patients.11, 13 By utilizing all payer claims data, our study was able to capture patients of all age groups and insurance types.
Patients who are not yet eligible for Medicare make up a growing and important subgroup of individuals who undergo EVR, representing nearly one-in-six patients in our cohort. Interestingly, we found that patients who were Medicare eligible underwent reintervention at a similar rate to those who were not Medicare eligible. While our power is limited to detect smaller differences between these groups, our finding of no difference is important, as it indicates that once patients have undergone EVR they are treated with reintervention at the similar rate regardless of whether they are eligible for Medicare coverage.
Our study highlights the need for diligent surveillance after EVR. We found that younger patients – those under the age of 65 at the time of their initial EVR – can expect an approximately 20% chance of undergoing reintervention within the first three years after their procedure, and this risk does not appear to diminish over the first three years. Our findings demonstrate that a novel approach which leverages both clinical registry data and all-payer claims can be used to monitor important quality metrics after EVR in patients of all age groups and insurance types.
Surveillance after EVR is challenging in clinical practice. Adherence to follow-up is important, as up to 30% of patients can expect to undergo reintervention within the first five years after EVR, a finding supported by our observational analysis described herein and by others.5, 11, 13, 14 Despite recommendations for regular surveillance by the Food and Drug Administration, the Society for Vascular Surgery, and other professional cardiovascular organizations, many clinical practices have difficulty with longitudinal patient follow-up after EVR.9–12, 23, 24 This concern is not without merit, as reports have described that nearly half of patients have not had the annual surveillance recommended by the Society for Vascular Surgery by three years after their initial procedure.11 As the proportion of EVR procedures out of the total aortic repairs continues to increase over time, these challenges are likely to worsen.19
Linked registry-claims data sources may provide an efficient option for monitoring reintervention after EVR. Using linkage between clinical registries and administrative data sources for outcome assessment has been successful for a number of clinical specialties, including the American College of Cardiology, the Organ Procurement and Transplantation Network, and the Surveillance Epidemiology and End Results cancer registry.25–27 The VQI has also been linked to fee-for-service Medicare claims, and allowed ascertainment of reintervention EVR at three years with 92% sensitivity and 96% specificity.3, 14 Our work described herein demonstrates the same strategy can also be employed for younger patients who are not Medicare eligible and those on Medicare Advantage plans by leveraging all-payer claims data. Finally, we found rates of reintervention similar to prior validation studies, indicating that the VQI-SPARCS registry-claims system likely accurately reflects the rate of reintervention at VQI participating centers in New York.14
Our study has limitations. This study was limited to the state of New York. All-payer claims systems exist in other states but are often difficult to obtain. The collection and dissemination of claims information for the purpose of surveillance would represent a step forward for clinical research and quality measurement, should stakeholders begin to analyze these data for quality improvement and comparative effectiveness research. The SPARCS database captures in hospital and emergency department encounters within the state of New York, we cannot comment on patients who have moved to another state or have traveled outside of the state for treatment, or patients who were seen in the outpatient clinic. However, as most reintervention procedures happen in a hospital setting, we feel that it is unlikely that this would meaningfully affect our results. Furthermore, because this analysis is limited to New York patients, our statistical power is limited to detect small differences between groups and lack of differences in some cases may represent a type 2 error. However, our reported rates of reintervention are consistent with prior validation efforts, indicating this system likely provides an accurate representation of reinterventions following EVR. We considered patients “Medicare eligible” if they were over 65 or on dialysis. Other factors which may also lead to Medicare eligibility such as a spouse on Medicare, or being on social security disability income, were not captured by our analysis. Not all reinterventions are of equal magnitude. More granular characterization of reintervention events is an area of active investigation for our research group. In addition, the SPARCS claims system does not include data on care performed at Veteran’s Association hospitals. In addition, we did not have data available on surveillance or imaging compliance after EVR. Examining the association between recommended surveillance and reintervention is an area of active investigation for our group. Therefore, patients who receive reintervention at those institutions are not captured. This study was retrospective in nature, the decision to perform a reintervention was ultimately at the discretion of the operating surgeon.
Conclusions:
Among a cohort of patients who underwent EVR in the state of New York, we successfully linked registry data in the VQI to the SPARCS all-payer claims data system at the patient level. We found that approximately one-in-five patients can expect to undergo reintervention after EVR, and this rate does not differ between older, Medicare eligible individuals, and those who have not yet reached eligibility. Our findings demonstrate that reintervention can be monitored using claims from both Medicare and non-Medicare payers. This combined registry-claims data system may offer solutions to some of the challenges posed by post-EVR surveillance by monitoring key outcomes in a way that is less labor intensive than current methods.
JVS-D-18–01040R2, Claims-Based Surveillance for Reintervention after Endovascular Aneurysm Repair Among Non-Medicare Patients
Type of Research:
Retrospective analysis of prospectively collected data from VQI and an all-payer claims database from the state of New York.
Key Findings:
Endovascular aortic aneurysm repair in 1,285 patients resulted in a 3-year reintervention rate of 21%, similar between Medicare eligible and non-eligible patients.
Take Home Message:
Reinterventions following EVAR is an important quality measure and can be determined by claims based databases.
Acknowledgments
Sources of Funding. Financial support was provided by the Food and Drug Administration (U01-FD005478), the National Institute on Aging (PO1-AG19783), and the National Institutes of Health Common Fund (U01-AG046830). The funders had no role in the design or execution of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to report.
References:
- 1.Dua A, Kuy S, Lee CJ, Upchurch GR Jr., Desai SS. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010. Journal of vascular surgery 2014;59(6):1512–7. [DOI] [PubMed] [Google Scholar]
- 2.Suckow BD Goodney PP Columbo JA Kang R Stone DH Sedrakyan A Cronenwett JL Fillinger MF. National Trends in Open Surgical, Endovascular and Branched/Fenestrated Endovascular Aortic Aneurysm Repair in Medicare Patients. Journal of vascular surgery 2017(IN PRESS). [DOI] [PMC free article] [PubMed]
- 3.Hoel AW, Faerber AE, Moore KO, Ramkumar N, Brooke BS, Scali ST, et al. A pilot study for long-term outcome assessment after aortic aneurysm repair using Vascular Quality Initiative data matched to Medicare claims. Journal of vascular surgery 2017;66(3):751–9 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paravastu SC, Jayarajasingam R, Cottam R, Palfreyman SJ, Michaels JA, Thomas SM. Endovascular repair of abdominal aortic aneurysm. The Cochrane database of systematic reviews 2014(1):CD004178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel R, Sweeting MJ, Powell JT, Greenhalgh RM. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. The Lancet 2016;388(10058):2366–74. [DOI] [PubMed] [Google Scholar]
- 6.Stather PW, Sidloff D, Dattani N, Choke E, Bown MJ, Sayers RD. Systematic review and meta-analysis of the early and late outcomes of open and endovascular repair of abdominal aortic aneurysm. The British journal of surgery 2013;100(7):863–72. [DOI] [PubMed] [Google Scholar]
- 7.Al-Jubouri M, Comerota AJ, Thakur S, Aziz F, Wanjiku S, Paolini D, et al. Reintervention after EVAR and open surgical repair of AAA: a 15-year experience. Annals of surgery 2013;258(4):652–7; discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 8.Ohrlander T, Dencker M, Acosta S. Morphological state as a predictor for reintervention and mortality after EVAR for AAA. Cardiovascular and interventional radiology 2012;35(5):1009–15. [DOI] [PubMed] [Google Scholar]
- 9.Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: executive summary. Journal of vascular surgery 2009;50(4):880–96. [DOI] [PubMed] [Google Scholar]
- 10.The United States Food and Drug Administration. Safety Alerts for Human Medical Products 2017. [cited 2018 March 1st]; Available from: https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm611193.htm.
- 11.Garg T, Baker LC, Mell MW. Postoperative Surveillance and Long-term Outcomes After Endovascular Aneurysm Repair Among Medicare Beneficiaries. JAMA Surg 2015;150(10):957–63. [DOI] [PubMed] [Google Scholar]
- 12.Judelson DR, Simons JP, Flahive JM, Patel VI, Healey CT, Nolan BW, et al. Determinants of Follow-Up Failure in Patients Undergoing Vascular Surgery Procedures. Annals of vascular surgery 2017;40:74–84. [DOI] [PubMed] [Google Scholar]
- 13.Schermerhorn ML, Buck DB, O’Malley AJ, Curran T, McCallum JC, Darling J, et al. Long-Term Outcomes of Abdominal Aortic Aneurysm in the Medicare Population. N Engl J Med 2015;373(4):328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Columbo JA, Kang R, Hoel AW, Kang J, Leinweber KA, Tauber KS, et al. A comparison of reintervention rates after endovascular aneurysm repair between the Vascular Quality Initiative registry, Medicare claims, and chart review LID - S0741-5214(18)30916-9 [pii] LID - 10.1016/j.jvs.2018.03.423 [doi]. (1097–6809 (Electronic)) [DOI] [PMC free article] [PubMed]
- 15.The Vascular Quality Initiative. [cited 2017 March 1st]; Available from: http://www.vascularqualityinitiative.org/.
- 16.Statewide Planning an Research Cooperative System. [cited 2016 June 1st]; Available from: https://www.health.ny.gov/statistics/sparcs/.
- 17.Mao J, Etkin C, Lawallen DG, Sedrakyan A. Linkage Between Orthopedic Registry and Administrative Data Using Indirect Identifiers for National Device Infrastructure Development 2018; Available from: https://www.ispor.org/ScientificPresentationsDatabase/Presentation/81182?pdfid=53639. [DOI] [PubMed]
- 18.Center for Medicare and Medicaid Services. [cited 2017 March 17th]; Available from: www.cms.gov.
- 19.Suckow BD, Goodney PP, Columbo JA, Kang R, Stone DH, Sedrakyan A, et al. National trends in open surgical, endovascular, and branched-fenestrated endovascular aortic aneurysm repair in Medicare patients. Journal of vascular surgery 2018;67(6):1690–7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society Series B (Methodological) 1972;34(2):187–220. [Google Scholar]
- 21.Columbo JA, Martinez-Camblor P, MacKenzie TA, Staiger DO, Kang R, Goodney PP, et al. Long-Term Survival After Carotid Endarterectomy and Carotid Stenting: A Propensity-Matched Analysis. Journal of vascular surgery 2018;Article IN PRESS.
- 22.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17(19):2265–81. [DOI] [PubMed] [Google Scholar]
- 23.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics−-2012 update: a report from the American Heart Association. Circulation 2012;125(1):e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hicks CW, Zarkowsky DS, Bostock IC, Stone DH, Black JH 3rd, Eldrup-Jorgensen J, et al. Endovascular aneurysm repair patients who are lost to follow-up have worse outcomes. Journal of vascular surgery 2017;65(6):1625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40(8 Suppl):IV-3–18. [DOI] [PubMed] [Google Scholar]
- 26.Brennan JM, Peterson ED, Messenger JC, Rumsfeld JS, Weintraub WS, Anstrom KJ, et al. Linking the National Cardiovascular Data Registry CathPCI Registry with Medicare claims data: validation of a longitudinal cohort of elderly patients undergoing cardiac catheterization. Circ Cardiovasc Qual Outcomes 2012;5(1):134–40. [DOI] [PubMed] [Google Scholar]
- 27.Massie AB, Kucirka L, Segev DL. Big Data in Organ Transplantation: Registries and Administrative Claims. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2014;14(8):1723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]


