Abstract
Background:
Medications are limited for patients with nonalcoholic fatty liver disease (NAFLD). It has been reported that aerobic exercise is effective in reducing the characteristics of NAFLD, although unclear data have ascertained the effects of high-intensity interval aerobic exercise on health-related quality of life (HRQoL) in diabetic obese patients with NAFLD.
Objectives:
This a randomized controlled trial aimed to ascertain the effectiveness of 8-week high-intensity interval exercise on intrahepatic triglycerides (IHTG), visceral lipids and HRQoL in diabetic obese patients with NAFLD.
Study design:
Between August and December 2017, 32 diabetic obese patients with NAFLD aged 45 to 60 years (21 men and 11 women) were enrolled in this study. They were randomly assigned to 2 groups, 16 patients in each group, high-intensity interval (HII) exercise and control groups. The HII group received a program of HII aerobic exercise for 8 weeks with medications of NAFLD and the control group received only medications without any type of exercise intervention. The test of IHTG, visceral lipids, and HRQoL were recorded at the initial assessment and at the end of the program after 8 weeks.
Results:
There were significant differences between the 2 groups at the end of the study. These study findings exhibited significant improvements in IHTG, VO2peak, visceral lipids, glycohemoglobin, plasma glucose, and all dimensions of HRQoL in the HII group (P <.05), But there was non-significant improvement in any measure in the control group (P >.05) after the 8-week intervention.
Conclusion:
Eight-week high-intensity interval aerobic exercise has a beneficial effect on IHTG, visceral lipids, and HRQoL in diabetic obese patients with NAFLD. Effort and awareness should be dedicated to encouraging the active lifestyle among different population, especially diabetic obese patients with NAFLD.
Keywords: diabetes mellitus, high-intensity exercise, HRQoL, NAFLD, obesity
1. Introduction
Obesity, type 2 diabetes mellitus, and fatty liver disease combining with low levels of physical activity are prominent health risks for mortality and morbidity.[1,2] More than 1/3 of the current population is suffering from obesity with a significant proportion of medical complications which can negatively influence their quality of life.[3] These complications comprise alterations of the metabolism of glucose and fat, insulin resistance (HOMA-IR) and diabetes mellitus.[4] Also, there is a strong positive correlation between obesity with type 2 diabetes and nonalcoholic fatty liver disease (NAFLD).[5] In addition, fatty liver disease and other metabolic syndrome manifestations may result in higher risk of hepatic and cardiac-related mortality.[5–7]
The previous study has reported the health risks of obesity and the body fat concentration, especially visceral adipose tissue which was progressively identified as the existence of higher significance to ascertain the cardiovascular and metabolic outcomes of more fatness.[8] Moreover, it was reported that excessive intrahepatic triglycerides (IHTGs) is a common characteristic of obesity and mostly raises cardiovascular disorders, metabolic syndrome, and the HOMA-IR risk.[9,10]
Treatment programs which decrease hepatic triglyceride (TG) content are usually associated with a consequential improvement of metabolic function, comprising reconstruction of normal blood glucose level in type 2 diabetes mellitus.[11] Still, attributes to the intrusive universe of conventional measurement of IHTG through histological classification and liver biopsy, there are few studies respecting the impacts of lifestyle modifications, including (exercise training and diet control) on IHTG no decisive medications survive to decrease hepatic fats.[12]
NAFLD is a frequent elaboration of obesity and related to metabolic alterations in hepatic very low-density lipoprotein (VLDL), the high concentration of serum TGs[13,14] and significant lowering in health-related quality of life (HRQoL).[15] Lifestyle interventions, including regular exercise training and diet control, decrease IHTG and enhance the metabolic abnormalities related to NAFLD.[15–17]
Exercise intervention is considered as a potential constituent of NAFLD treatment and it was approved by the American Association for the Study of Liver Diseases and Association of American Gastroenterology.[18,19] The recommendations of the American Gastroenterological Association are established upon the association of NAFLD, with HOMA-IR and obesity but, there is limited published articles illustrate the influences of exercise training in the management of NAFLD. A cross-sectional study has ascertained the relationship between physical activity levels and hepatic histological changes in patients with NAFLD and has approved that there is a non-significant correlation between physical activity levels and histological changes in NAFLD and found a higher VO2peak in patients with mild NAFLD, reflecting the important role of exercise intensity in the treatment of NAFLD.[20] Another study assessed the influences of 3 months of exercise training combining with diet control on patients with NAFLD and this study exhibited a positive reduction in steatosis and a non-significant decrease in fibrosis.[21] The previous studies that have evaluated the effects of physical activity and exercise training in patients with NAFLD have been restricted by using of substitute markers of NAFLD (steatosis image, or liver biochemistry) and by the limited hepatic histology.[22–25] Previous data on the association between the physical activity levels and hepatic histological changes in patients with NAFLD are still limited.
An early study by Oh et al approved that exercise training alone decreased liver dysfunction as the exercise training lead to few reductions in visceral fat and weight. The results of this study showed that moderate to vigorous exercise for 4 hours and 10 minutes weekly had the best reduction in hepatic fat and improvement in the underlying pathophysiology.[26] Also, it was observed that resistance exercise has a beneficial effect on hepatic fat concentration in patients with NAFLD.[27] While, resistance exercise was approved to improve energy expenditure by improving muscle size[28] and decrease HOMA-IR,[28,29] a parallel disorder status of obesity.
Recently, high-intensity interval (HII) exercise has been demonstrated as a new modality of exercise therapy intervention.[30,31] This type of exercise training has a beneficial effect on metabolic syndrome[32] and cardiovascular disease.[33] Very poor data demonstrated the effect of HII in patients with NAFLD and limited studies evaluated the effect of exercise therapy on HRQoL in hepatic patients. The hypothesis of this study was that HII may decrease IHTG, visceral lipids and improving HRQoL in diabetic obese patients with hepatic disease.
2. Objectives
The purpose of this study was to ascertain the effect of 8-week HII exercise on IHTG, visceral lipids, and HRQoL in diabetic obese patients with NAFLD.
3. Patients and methods
3.1. Patients
Between August and December 2017, 32 diabetic obese patients with NAFLD aged 45 to 60 years (21 men and 11 women) were enrolled in this study according to the study criteria. All patients were following in the outpatient clinic for diabetes, endocrine, and endemic diseases and referred by a physician of tropical medicine and endemic disease based on the approval of the department of endemic diseases to the outpatient clinic of physical therapy, Cairo University Hospitals. All patients were diagnosed with NAFLD, type 2 diabetes mellitus and class II and III of obesity (body mass index [BMI] ≥35 kg/m2). They were receiving pentoxifylline, omega-3 fatty acids, and metformin (Table 1). The diagnostic criteria of NAFLD were based on the diagnostic guidelines for NAFLD in the Asia-Pacific region.[34] To nullify a type II error, a preliminary power analysis (power, 0.80; α = 0.05; effect size, 0.5) decided a sample size of 32 patients for the study. The 32 patients were randomly classified into 2 groups. Group I consisted of 16 patients (10 males and 6 females), received medical treatment with a program of HII aerobic exercise 3 times/week for 8 weeks (HII group) and group II consisted of 16 patients (9 males and 7 females), received only medical treatment without any exercise program (control group). Any patient had a severe life-limiting illness (cancer, renal failure), uncontrolled heart disease, neuromuscular limitations, orthopedic problems, and endocrine disorders that could affect physical exercise were excluded from the study. This study was approved by the ethical committee of Physical Therapy Department [No.: PT/2017/00-018]. Written informed consent was obtained from all patients participated in this study.
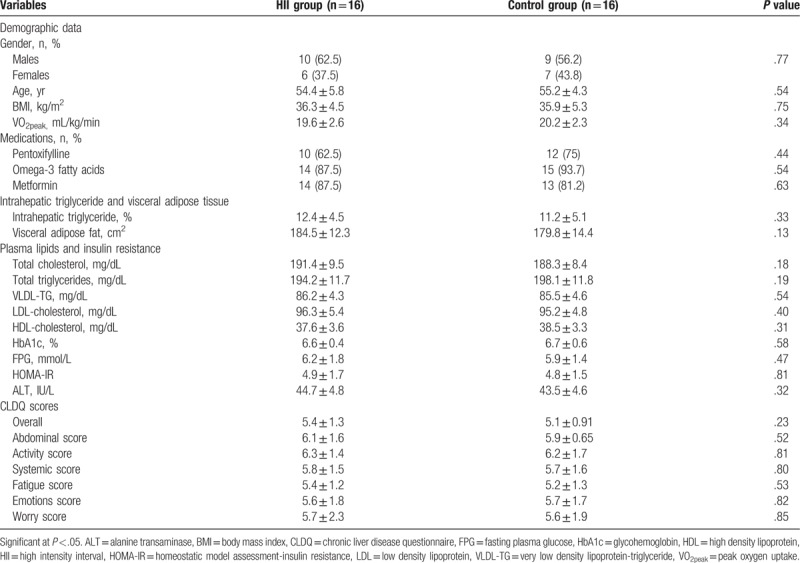
Table 1.
Demographic and baseline characteristics of all patients who participated in this study before intervention.
3.2. Sample size
The sample size for this study was calculated using the IHTG measure. A previous study has approved that the aerobic exercise showed a significant mean difference in IHTG measure (1.76) with standard deviation (2.2).[35] According to this difference and ability to achieve an 80% power (α=0.05), our study required 13 patients in each group. The dropout rate of 20% is assumed in the study; therefore 16 patients were recruited in each group to assure that 13 patients would complete the study.
3.3. Randomization
From 37 participants, 32 patients were eligible to enroll in this study. Three patients did not meet the study criteria and 2 patients declined to participate in the study without any informed reason. Randomization was applied using secured envelopes. The examiner arranged the secured envelopes, which contained a piece of colored paper indicating HII group and a piece of uncolored paper indicating control group. This randomization was performed before study intervention. The flow diagram showing the patients participating in the study is shown in Figure 1.
Figure 1.
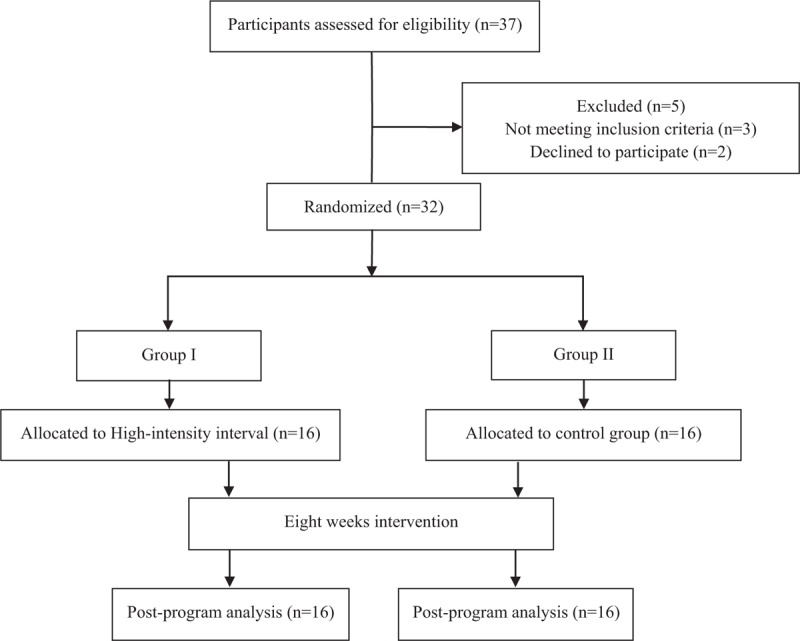
Flow diagram showing the patients who participated in the study.
3.4. Instruments
Body weight, height, and BMI were measured for all patients using weight and height scale, Cycle Ergometer (Monark RC6 Novo, Langley) was used for warming up, cool down exercises and VO2peak test, and HRQoL of patients with NAFLD were assessed using the Chronic Liver Disease Questionnaire (CLDQ). Magnetic resonance imaging (MRI) with a 3T scanner (General electric, WI) was used to assess the hepatic fat content.
4. Procedure
4.1. Baseline assessment
All participants were evaluated for the IHTG, visceral lipids, and CLDQ before the intervention (pre-program) and at the end of 8 weeks of intervention (post-program) by the previously examiner who was blinded concerning the group to which each patient was appointed.
At the initial assessment, all patients were informed about the nature, procedure, and benefits of the study. Randomly, the sample was assigned to 2 groups equal in number, each group consisted of 16 patients; HII group (Group I), received a program of HII aerobic exercise 3 times/week for 8 weeks in addition of medical treatment and control group (Group II), received only medications without any exercise program. The test of IHTG, visceral lipids, VO2peak, plasma glucose, and CLDQ were recorded at the initial assessment and at the end of the program after 8 weeks of intervention.
4.2. Radiological assessment
Assessment of hepatic fat was performed using MRI with a 3T scanner (General electric, WI) through imaging of chemical shift. Each patient was assessed for imaging in the supine position using the body coil. The patient was instructed to make a single breath hold during imaging, the liver was imaged 3 separate images slice pairs. IHTG proportionate to water was estimated as 100 × (signal amplitude of TG)/(signal amplitude of water). MRI was employed in this study because it was recognized and validated and was considered the most accurate noninvasive approach assessing the hepatic fat levels in patients with type 2 diabetes.[36,37]
4.3. Biochemical measurements
The venous blood sample was taken at the morning after fasting at least 10 hrs for biochemical analysis, including alanine-transaminase (ALT), total cholesterol, total TGs, high-density lipoproteins (HDLs), low-density lipoproteins (LDLs), VLDLs, glycohemoglobin (HbA1c), fasting plasma glucose (FPG), and HOMA-IR.
4.4. Intervention
In the HII group, each patient in this group participated in a program of high-intensity aerobic exercise for 8 weeks, 3 times per week with each exercise session lasting for nearly 40 minutes morning. Each patient was instructed to not eat for 2 hours before the exercise session to avoid exercise-induced airway obstruction.
The HII exercise program was performed on a cycle Ergometer with firmly grasping the rails to maintain balance after the patient has accustomed to starting the training program. The exercise session was started with a 5-minute warm-up which involved cycling exercise without resistance of the Ergometer followed by 3 sets of 4-min cycling sessions at 80% to 85% of the VO2max with 2-minute interval at 50% of the VO2max between sets. The session ended with 5 minutes of cool-down exercise.
During the study period, all patients were instructed to abide by the physician's recommendations including; not change their diets and lifestyle not perform any exercise activities outside the treatment program for the 8 weeks of intervention.
4.5. Post-intervention assessment
After 8-week intervention, peak oxygen uptake (VO2peak), IHTG, visceral lipids, plasma lipids, HbA1c, HOMA-IR, and CLDQ were reassessed in the HII and control groups.
4.6. Statistical analysis
Descriptive statistics were performed in the form of means and standard deviations. The Kolmogorov–Smirnov test was used to assess the normality of data. Inferential statistics evaluated changes of all measurements (aerobic capacity, IHTGs, visceral lipids, plasma lipids, HOMA-IR, and CLDQ) using unpaired t test between HII and control groups and paired t test was performed to measure changes within group, SPSS version 22.0 (SPSS, Chicago, IL) was used for statistical analysis with a significance level of P <.05 for all statistical measurements.
5. Results
5.1. Baseline demographic and clinical characteristics
As demonstrated in Table 1, there were non-significant differences between the HII and control groups at the beginning of the study regarding their gender, ages, BMI, VO2peak, IHTG, ALT, visceral adipose fat, plasma lipids, insulin sensitivity, and all dimensions of CLDQ.
5.2. Aerobic capacity and anthropometry
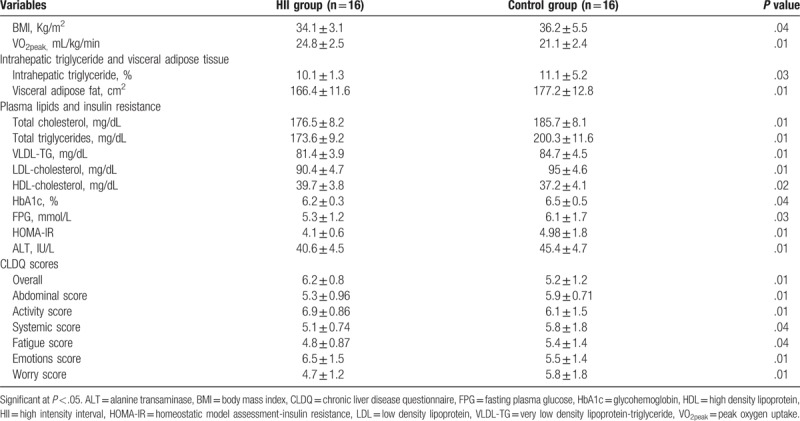
VO2peak was significantly improved in the HII group versus the control group. The BMI was significantly decreased in the HII group but non-significant changes in the control groups. The post-intervention outcomes showed significant differences in VO2peak and BMI in favor of the HII group (Tables 2 and 3).
Table 2.
Pre- and post- treatment mean values of all measures for HII and control groups.
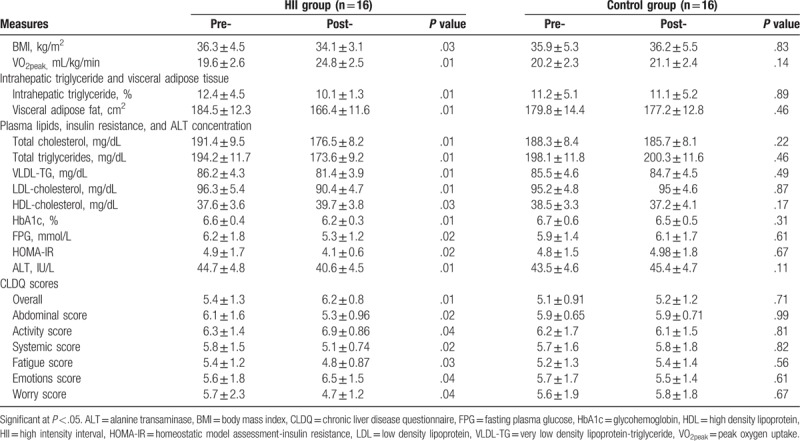
Table 3.
Differences between HII and control groups after intervention at the end of the study.
5.3. Intrahepatic triglyceride and visceral adipose tissue
There was a non-significant difference between the 2 groups before the intervention in IHTG % and visceral adipose fat (Table 1). Comparison of the pre- and post-intervention mean values of the IHTG % and visceral adipose fat in the HII group reported a statistically significant reduction while reported a non-significant reduction in the control group (Table 2). Therefore, the post-intervention outcomes showed significant differences in favor of the HII group (Table 3).
5.4. Plasma lipids, HOMA-IR, and ALT concentration
As demonstrated in Table 1, comparison of the pre-intervention mean values of total cholesterol, TGs, HDLs, LDLs, VLDLs, HbA1c, FPG, HOMA-IR, and ALT between the HII and control groups indicated non-significant differences. At the end of the study, the HII group showed significant improvement in all these measures with non-significant changes in the control group (Table 2). Comparing the pre- and post-treatment mean values of the mentioned measures recorded significant differences between the HII and control groups in favor of the HII group (Table 3).
5.5. Chronic liver disease questionnaire
As demonstrated in Table 1, Comparison of the pre-intervention mean values of all dimensions of CLDQ, including overall, abdominal, activities, systemic, fatigue, emotions, and worry scores between both groups indicated non-significant differences. While, post-intervention comparison indicated significant differences in all dimensions, finding measures in favor of the HII group (Table 3). As presented in Table 2, comparison of the pre and post-intervention mean values of all dimensions of CLDQ indicated significant differences in the HII group. But, the control group showed non-significant changes at the end of the study.
6. Discussion
This randomized controlled trial evaluated the effectiveness of 8-week HII exercise (40 minutes cycling exercise 3 times per week) on IHTGs, visceral lipids, and HRQoL in diabetic obese patients with NAFLD. The main outcomes of the present study showed that the adherence to HII aerobic exercise training reduced the BMI, IHTG, visceral adipose fat, plasma lipids, HbA1c, and HOMA-IR and improved HRQoL and aerobic capacity in diabetic obese patients with NAFLD. The examiners confirmed that HII aerobic exercise was set appeasement while patients were comfortable to rule; they verified equitable contest and used satisfactions, and also included in the aerobic exercise program.
According to the present study findings, 8-week HII exercise in form of cycling exercise at 80% to 85% of the VO2max with interval at 50% of the VO2max for 40 minutes 3 times per week showed a significant reduction in IHTG, visceral adipose fat, and BMI in patients with NAFLD. This reported reductions in our study results were documented in previous studies of the aerobic training program in patients with NAFLD.[27,35]
The underlying mechanism of reduction in IHTG with HII aerobic exercise is indicating a reduction of plasma lipids and an increase of insulin sensitivity. This study emphasizes the vital role of aerobic exercise training in patients with extreme hepatic lipids. Although, the accumulation of visceral adipose fat resulted from the inward flow of fatty acids and synthesized of adipokines and cytokines, which decrease insulin sensitivity and increase liver lipids[38,39] while the exact mechanism of the relation between the visceral lipids and liver metabolism is still unclear. The obvious permanence of the metabolic control in our study is startling given the decrease of IHTG and visceral adipose fat and the strong correlation between liver lipids and hepatic HOMA-IR.[40,41] The IHTG reduction is significantly needed for decreasing HOMA-IR and improving plasma glucose control, as it is lately reported in recent studies.[40,42,43]
In agreement with our study results, the previous study explained that 20 minutes running or cycling at submaximal heart rate pursued with 20 minutes warming up/cooling down 3 times weekly for 4 weeks have led to an improvement of insulin sensitivity and reduction of fasting plasma glucose in patients with type 2 diabetes mellitus.[44]
Many studies investigated the required intensity of exercise to improve metabolic profiles. One study assessed the clinical effects of 24-week moderate intensity at 60% VO2max and high-intensity exercise at 80% VO2max in form of cycling 3 sessions per week on glucose concentration, TGs, and insulin sensitivity. Exercise at 60% and 80% VO2max 3 times per week was effective to reduce HOMA-IR and improve insulin sensitivity.[45] Two recent studies affirmed this result when evaluated in obese subjects.[46,47].
Twelve-week aerobic exercise on the treadmill at 70% VO2max (80% maximum heart rate) has led to the definitive loss of body weight which was related to a decrease in abdominal obesity, visceral fat, and improvement of insulin sensitivity.[46] Moreover, this study explained that exercise training also reduced abdominal and visceral fat without weight loss.
Also, another study provided that aerobic exercise 50 to 60 minutes daily for 4 weeks starting at 60% to 65% Max HR and increasing to 80% to 85% Max HR reduced visceral fat, increased glucose oxidation, and reduced HOMA-IR with only 3% weight loss.[47]
Our study results approved that the IHTG reduction following 8-week HII aerobic exercise associated with a reduction of plasma alanine-transaminase (ALT). In spite of increasing of ALT is a common predictor of hepatic disorder,[48] alterations of ALT are not associated with liver histology changes[49] and many patients with NAFLD have normal values of plasma ALT.[50] Few patients had normal values of ALT at baseline assessment in the present study, although 2 patients had a normal value of plasma ALT when the recommended limit measures of nineteen IU/L for females and 30 IU/L for males were applied.[51] However, a recent review approved that the incidence rate for diabetic patients was 1.83/1 unit raise plasma ALT.[52] the present study showed a significant reduction in plasma ALT in the HII group and approved positive medical implications of the HII aerobic exercise in diabetic obese patients with NAFLD.
Tropical physicians, especially who treat NAFLD patients are looking for improving their clinical results. In addition to preventing the complications of the hepatic disease, a vital outcome measure is the HRQoL. Comprehending the impacts of weight reduction on HRQoL in patients with NAFLD is obligatory to advise hepatic patients, inspire clinical trials with patient-consulted measures, and design healthy cost- impressiveness templates. Previous studies regarding the characteristics of HRQoL in patients with NAFLD have been recognized in 2 healthy groups.[53,54] while limited data studied the effectiveness of aerobic exercise on HRQoL in hepatic patients. The present study showed that reduction of BMI is significantly correlated with improvement of the HRQoL.
Moreover, our study provides and gives evidence on NAFLD and HRQoL in the following potential points.
-
(1)
This study exhibited that BMI reduction improves HRQoL. In contrast to the present study findings, a systematic review explaining that weight control is not correlated with HRQoL measures.[55] Particularly, their assessment for HRQoL didn’t include CLDQ. Also, previous data approved that 5 percent weight reduction resulted in the enhancement of the HRQoL.[56,57] Our study showed significant improvement of all dimensions of CLDQ, especially diabetic obese patients with NAFLD.
-
(2)
Our findings verify that the mean value of the initial CLDQ of diabetic obese patients with NAFLD was similar to the HRQoL measure in the previous study by Dan et al.[15]
-
(3)
Moreover, the findings of our study exhibit that the patients who reduced BMI in the HII group improved their daily exercise obligations and HRQoL while patients who did not reduce BMI did not improve HRQoL.
The present study approved that 8-week HII aerobic exercise (40 minutes 3 times/week at 80% to 85% of the VO2max with interval at 50% of the VO2max) improve VO2peak, IHTG, visceral lipids, plasma lipids, HOMA-IR, and HRQoL in diabetic obese patients with NAFLD. Ideal management of NAFLD begins with exercise intervention and the optimum consequences as the following; the metabolic process by which aerobic exercise, particularly HII improves insulin sensitivity through increasing the oxidation of the free fatty acids in the skeletal muscles. Therefore, insulin sensitivity and oxidation of the free fatty acids lead to glucose-lipid metabolism. Also, Regular HII exercise showed significant reduction of liver fat deposition through improving energy expenditure, skeletal fat oxidation, and reduction in visceral adipose fat.
6.1. Limitations
The main limitation of our study was the absence of intermediate (4 weeks) and long-term (1 year) follow-up assessment. The second limitation, dietary intake and physical activity of the study subjects were not supervised. Future studies should increase the number of patients to include different intensities of aerobic exercise and have intermediate and long-term follow-up assessment.
7. Conclusions
According to the results of the study, 8-week HII exercise has a beneficial effect on IHTGs, visceral lipids, and HRQoL in diabetic obese patients with NAFLD. Additional implications for clinical practice should be devoted to encouraging the HII exercise among the different population, especially diabetic obese patients with NAFLD.
Acknowledgments
The authors would like to thank all patients who participated in the study.
Author contributions
Conceptualization: Walid Kamal Abdelbasset, Gaber S. Soliman.
Data curation: Walid Kamal Abdelbasset, Sayed A. Tantawy, Dalia M. Kamel, Gaber S. Soliman.
Formal analysis: Walid Kamal Abdelbasset, Sayed A. Tantawy, Dalia M. Kamel, Gaber S. Soliman.
Funding acquisition: Dalia M. Kamel, Bader A. Alqahtani.
Investigation: Sayed A. Tantawy.
Methodology: Walid Kamal Abdelbasset, Sayed A. Tantawy.
Project administration: Walid Kamal Abdelbasset.
Resources: Dalia M. Kamel, Bader A. Alqahtani.
Software: Bader A. Alqahtani, Gaber S. Soliman.
Supervision: Walid Kamal Abdelbasset, Dalia M. Kamel.
Validation: Walid Kamal Abdelbasset, Bader A. Alqahtani.
Visualization: Walid Kamal Abdelbasset, Dalia M. Kamel, Gaber S. Soliman.
Writing – original draft: Walid Kamal Abdelbasset, Sayed A. Tantawy, Gaber S. Soliman.
Writing – review & editing: Walid Kamal Abdelbasset, Sayed A. Tantawy, Dalia M. Kamel, Bader A. Alqahtani.
Walid Kamal Abdelbasset orcid: 0000-0003-4703-661X.
Footnotes
Abbreviations: ALT = alanine-transaminase, BMI = body mass index, CLDQ = Chronic Liver Disease Questionnaire, HbA1c = glycohemoglobin, HDLs = high-density lipoproteins, HII = high-intensity interval, HOMA-IR = insulin resistance, HRQoL = health-related quality of life, IHTG = intrahepatic triglyceride, LDLs = low-density lipoproteins, MRI = magnetic resonance imaging, NAFLD = nonalcoholic fatty liver disease, TGs = triglycerides, VLDLs = very low-density lipoproteins, VO2peak = peak oxygen uptake.
The research did not secure any specific grant or source of funding from any specific organization in either the private or public sector.
All the study procedures which carried out including human participants were based on the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
The authors declare that they do not have any conflicts of interest.
References
- [1].Dunn W, Xu R, Wingard DL, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol 2008;103:2263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356–9. [DOI] [PubMed] [Google Scholar]
- [3].Pi-Sunyer FX. The obesity epidemic: pathophysiology and consequences of obesity. Obes Res 2002;10suppl 2:97S–104S. [DOI] [PubMed] [Google Scholar]
- [4].McMillan KP, Kuk JL, Church TS, et al. Independent associations between liver fat, visceral adipose tissue, and metabolic risk factors in men. Appl Physiol Nutr Metab 2007;32:265–72. [DOI] [PubMed] [Google Scholar]
- [5].Younossi ZM, Gramlich T, Matteoni CA, et al. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol 2004;2:262–5. [DOI] [PubMed] [Google Scholar]
- [6].Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007;30:1212–8. [DOI] [PubMed] [Google Scholar]
- [7].Church TS, Kuk JL, Ross R, et al. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology 2006;130:2023–30. [DOI] [PubMed] [Google Scholar]
- [8].Bays H, Dujovne CA. Adiposopathy is a more rational treatment target for metabolic disease than obesity alone. Curr Atheroscler Rep 2006;8:144–56. [DOI] [PubMed] [Google Scholar]
- [9].Hwang JH, Stein DT, Barzilai N, et al. Increased intrahepatic triglyceride is associated with peripheral insulin resistance: in vivo MR imaging and spectroscopy studies. Am J Physiol Endocrinol Metab 2007;293:E1663–9. [DOI] [PubMed] [Google Scholar]
- [10].Targher G, Bertolini L, Poli F, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 2005;54:3541–6. [DOI] [PubMed] [Google Scholar]
- [11].Petersen KF, Dufour S, Befroy D, et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005;54:603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sass DA, Chang P, Chopra KB. Nonalcoholic fatty liver disease: a clinical review. Dig Dis Sci 2005;50:171–80. [DOI] [PubMed] [Google Scholar]
- [13].Adiels M, Taskinen MR, Packard C, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia 2006;49:755–65. [DOI] [PubMed] [Google Scholar]
- [14].Fabbrini E, Mohammed BS, Magkos F, et al. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology 2008;134:424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dan A, Kallman J, Wheeler A, et al. Health-related quality of life in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2007;26:815–20. [DOI] [PubMed] [Google Scholar]
- [16].Lazo M, Solga SF, Horska A, et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care 2010;33:2156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kantartzis K, Machann J, Schick F, et al. Effects of a lifestyle intervention in metabolically benign and malign obesity. Diabetologia 2011;54:864–8. [DOI] [PubMed] [Google Scholar]
- [18].Koot BG, van der Baan-Slootweg OH, Tamminga-Smeulders CL, et al. Lifestyle intervention for non-alcoholic fatty liver disease: prospective cohort study of its efficacy and factors related to improvement. Arch Dis Child 2011;96:669–74. [DOI] [PubMed] [Google Scholar]
- [19].American Gastroenterological Association medical position statement: nonalcoholic fatty liver disease. Gastroenterology. 2002; 123:1702–4. [DOI] [PubMed] [Google Scholar]
- [20].Krasnoff JB, Painter PL, Wallace JP, et al. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology 2008;47:1158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ueno T, Sugawara H, Sujaku K, et al. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol 1997;27:103–7. [DOI] [PubMed] [Google Scholar]
- [22].Kim HK, Park JY, Lee KU, et al. Effect of body weight and lifestyle changes on long-term course of nonalcoholic fatty liver disease in Koreans. Am J Med Sci 2009;337:98–102. [DOI] [PubMed] [Google Scholar]
- [23].St George A, Bauman A, Johnston A, et al. The independent effects of physical activity in patients with non-alcoholic fatty liver disease. Hepatology 2009;50:68–76. [DOI] [PubMed] [Google Scholar]
- [24].Chen SM, Liu CY, Li SR, et al. Effects of therapeutic lifestyle program on ultrasound-diagnosed nonalcoholic fatty liver disease. J Chin Med Assoc 2008;71:551–8. [DOI] [PubMed] [Google Scholar]
- [25].Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, et al. Role of leisure-time physical activity in nonalcoholic fatty liver disease: a population-based study. Hepatology 2008;48:1791–8. [DOI] [PubMed] [Google Scholar]
- [26].Oh S, Shida T, Yamagishi K, et al. Moderate to vigorous physical activity volume is an important factor for managing nonalcoholic fatty liver disease: a retrospective study. Hepatology 2015;61:1205–15. [DOI] [PubMed] [Google Scholar]
- [27].Hallsworth K, Fattakhova G, Hollingsworth KG, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011;60:1278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ibanez J, Izquierdo M, Arguelles I, et al. Twice-weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care 2005;28:662–7. [DOI] [PubMed] [Google Scholar]
- [29].Vilar Gomez E, Rodriguez De Miranda A, Gra Oramas B, et al. Clinical trial: a nutritional supplement Viusid, in combination with diet and exercise, in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther 2009;30:999–1009. [DOI] [PubMed] [Google Scholar]
- [30].DiPietro L, Dziura J, Yeckel CW, et al. Exercise and improved insulin sensitivity in older women: evidence of the enduring benefits of higher intensity training. J Appl Physiol 2006;100:142–9. [DOI] [PubMed] [Google Scholar]
- [31].Matsuo T, Saotome K, Seino S, et al. Effects of a low-volume aerobic-type interval exercise on VO2max and cardiac mass. Med Sci Sports Exerc 2014;46:42–50. [DOI] [PubMed] [Google Scholar]
- [32].Matsuo T, So R, Shimojo N, et al. Effect of aerobic exercise training followed by a low-calorie diet on metabolic syndrome risk factors in men. Nutr Metab Cardiovasc Dis 2015;25:832–8. [DOI] [PubMed] [Google Scholar]
- [33].Wisloff U, Stoylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 2007;115:3086–94. [DOI] [PubMed] [Google Scholar]
- [34].Farrell GC, Chitturi S, Lau GK, et al. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol 2007;22:775–7. [DOI] [PubMed] [Google Scholar]
- [35].Johnson NA, Sachinwalla T, Walton DW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009;50:1105–12. [DOI] [PubMed] [Google Scholar]
- [36].Tang A, Loomba R, Lavine JE, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 2013;267:422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hamilton G, Middleton MS, Bydder M, et al. Effect of PRESS and STEAM sequences on magnetic resonance spectroscopic liver fat quantification. J Magn Reson Imaging 2009;30:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bergman RN, Kim SP, Catalano KJ, et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006;14Suppl 1:16S–9S. [DOI] [PubMed] [Google Scholar]
- [39].Van der Poorten D, Milner KL, Hui J, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008;48:449–57. [DOI] [PubMed] [Google Scholar]
- [40].Finelli C, Tarantino G. Have guidelines addressing physical activity been established in nonalcoholic fatty liver disease. World J Gastroenterol 2012;18:6790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Samuel VT, Liu ZX, Qu X, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 2004;279:32345–53. [DOI] [PubMed] [Google Scholar]
- [42].Lim EL, Hollingsworth KG, Aribisala BS, et al. Reversal of type 2 diabetes: normalization of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011;54:2506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cuthbertson DJ, Shojaee-Moradie F, Sprung VS, et al. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 2016;130:93–104. [DOI] [PubMed] [Google Scholar]
- [44].Aoi W, Naito Y, Yoshikawa T. Dietary exercise as a novel strategy for the prevention and treatment of metabolic syndrome: effects on skeletal muscle function. J Nutr Metab 2011;2011:676208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ryan AS. Exercise in aging: its important role in mortality, obesity and insulin resistance. Aging health 2010;6:551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Benatti FB, Lira FS, Oyama LM, et al. Strategies for reducing body fat mass: effects of liposuction and exercise on cardiovascular risk factors and adiposity. Diabetes Metab Syndr Obes 2011;4:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ray L, Lipton RB, Zimmerman ME, et al. Mechanisms of association between obesity and chronic pain in the elderly. Pain 2011;152:53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology 2001;121:91–100. [DOI] [PubMed] [Google Scholar]
- [49].Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obesity Surg 2006;16:1278–86. [DOI] [PubMed] [Google Scholar]
- [50].Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–95. [DOI] [PubMed] [Google Scholar]
- [51].Omagari K, Takamura R, Matsutake S, et al. Serum alanine aminotransferase concentration as a predictive factor for the development or regression of fatty liver. J Clin Biochem Nutr 2011;49:200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fraser A, Harris R, Sattar N, et al. Alanine aminotransferase, c-glutamyl transferase, and incident diabetes. Diabetes Care 2009;32:741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kistler K, Molleston J, Unalp A, et al. Symptoms and quality of life in obese children and adolescents with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2010;31:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Afendy A, Kallman J, Stepanova M, et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther 2009;30:469–76. [DOI] [PubMed] [Google Scholar]
- [55].Warkentin L, Das D, Majumdar S, et al. The effect of weight loss on health-related quality of life: systematic review and meta-analysis of randomized trials. Obes Rev 2014;15:169–82. [DOI] [PubMed] [Google Scholar]
- [56].Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ackermann RT, Edelstein SL, Narayan K, et al. Changes in health state utilities with changes in body mass in the Diabetes Prevention Program. Obesity 2009;17:2176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


