TO THE EDITOR
Delayed and chronic wounds, including diabetic wounds, pose an enormous burden on the healthcare system (Centers for Disease Control and Prevention 2014). Skin resident γδ T cells promote wound closure by producing FGF7 and FGF10, which promote keratinocyte proliferation and wound re-epithelialization (Jameson et al. 2002). Resident γδ T cells are reported to promote repair by stimulating stem cells residing in the hair follicle to multiply and traffic to the wound (Lee et al. 2017). Recently it was reported that CCR6+ γδ T cells are recruited to a corneal epithelial abrasion via CCL20 where they promote healing (Li et al. 2011). Delayed wound healing in diabetic mice correlates with reduced numbers of recruited Vγ4 T cells and reduced IL-17A production (Liu et al. 2016). The precise mechanism by which γδ T cells are recruited to the site of a wound and their importance in dermal skin healing in wild-type (WT) mice has yet to be determined.
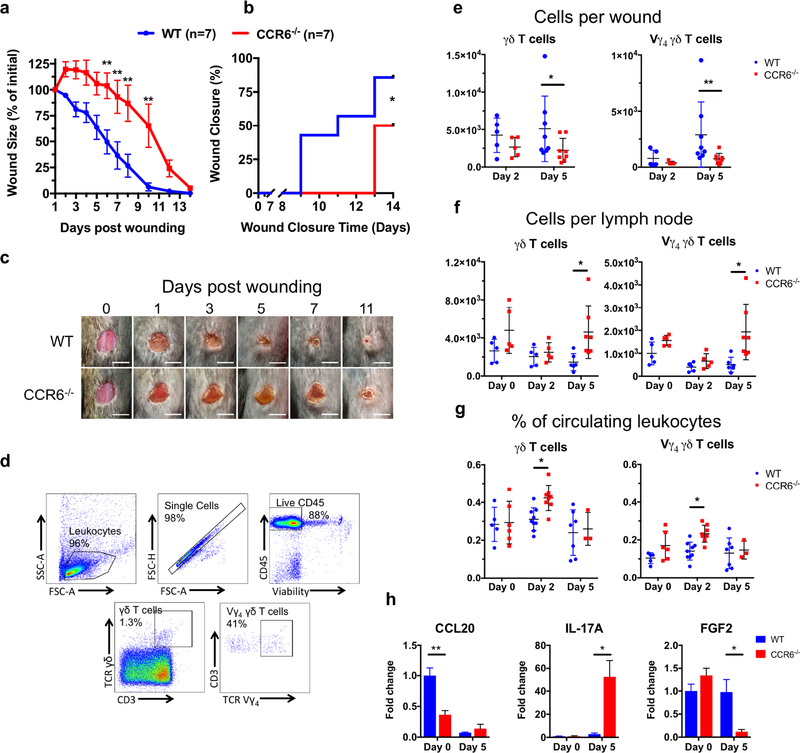
To investigate the role CCR6 plays in dermal wound healing, we administered a 6 mm full thickness skin wound on the dorsum of WT and CCR6−/− mice and measured wound area over 2 weeks. CCR6−/− mice had significant larger wound areas beginning at day 6 and a 4-day delay in wound closure compared to WT mice (Fig. 1a–c). All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of California, Davis and performed following the guidelines of Animal Welfare Act and Health Resource Extension Act.
Figure 1: CCR6−/− mice have hindered dermal wound healing, defective γδ T cell trafficking to a wound, and dysregulated IL-17A and FGF2 expression in the wound.
WT and CCR6−/− mice were administered a 6mm full thickness skin wound on the dorsum. (a-c) Wound size was measured daily. γδ T cells and Vγ4 T cells were enumerated from (e) wounds, (f) lymph nodes, and (g) blood by flow cytometry (d). Unwounded skin (day 0) or day 5 wounds were collected from animals and expression of cytokines was determined by RT-qPCR (h). Animals per group are presented in each graph and 4–5 animals per group for (h). Data is presented as Mean ± SEM.
*P ≤ 0.05 and **P ≤ 0.01 between CCR6−/− and WT. Scale bar = 5 mm.
To compare the numbers of γδ T cells in the wounds of WT and CCR6−/− mice, wounds were excised on days 2 and 5 and CD45+ leukocytes were enumerated by flow cytometry. On day 5, CCR6−/− mice contained significantly fewer γδ T cells than WT mice, and additional phenotyping revealed fewer Vγ4 T cells in CCR6−/− mice (Fig. 1e). Vγ4 T cells are the predominant peripheral γδ T cell subset and play a central role in infection (Huber et al. 2001) and skin graft rejection (Li et al. 2017). CCR6−/− mice do not exhibit altered intraepithelial lymphocyte subpopulations (Varona et al. 2001; Mabuchi et al. 2013), so we expected that the decreased numbers of γδ T cells in CCR6−/− wounds was due to impaired trafficking to the wound bed. To test this, lymph nodes and blood samples from unwounded and day 2 and 5 post-wounded mice were evaluated. In both CCR6−/− and WT mice, fewer γδ and Vγ4 T cells were present in the lymph nodes on day 2 compared to day 0, indicating that γδ T cells egress from the lymph nodes into the circulation after wounding. However, on day 5 significantly more γδ T cells, including the Vγ4 subset, were observed in CCR6−/− mice compared with WT mice (Fig. 1f). In blood, the frequency of circulating γδ and Vγ4 T cells peaked on day 2 in CCR6−/− mice before returning to levels observed in WT mice on day 5 (Fig. 1g). Taken together, following wounding in WT mice, γδ T cells mobilized from the lymph nodes and utilized CCR6 to traffic from the circulation to the wounded skin. In contrast, following wounding in CCR6−/− mice, circulating γδ T cells were unable to traffic to the wound, likely due to the inability of CCR6 to interact with its chemokine ligand CCL20 (Fig. 1h).
We next sought to compare cytokine mRNA expression from day 5 wounds by RT-qPCR (Fig. 1h). CCR6−/− mice had increased expression of IL-17A compared with WT mice, which is surprising given that IL-17A is produced by γδ T cells in the skin of normal and diabetic wounds (Liu et al. 2016; Li et al. 2017; 2018). Other cells may be compensating for the absence of γδ T cells in the wound by producing IL-17A, which can impede wound closure by inhibiting IGF-1 production by dendritic epidermal T cells (Li et al. 2018) and promote neutrophil infiltration (Takagi et al. 2017). Most remarkable was the 8-fold decrease in FGF2 expression in the wounds of CCR6−/− mice compared with WT mice. FGF2 is a potent inducer of wound healing by acting on keratinocytes and fibroblasts to increase proliferation and migration at the epidermal leading edge (Kurita et al. 1992). Moreover, a subset of γδ T cells exhibit the capacity to produce FGF2 (Laggner et al. 2011), raising the possibility that trafficking of CCR6+ γδ T cells themselves might be the source of FGF2 in wounded skin. These data indicate that expression of IL-17A and FGF2 in healing skin is in part regulated by trafficking γδ T cells.
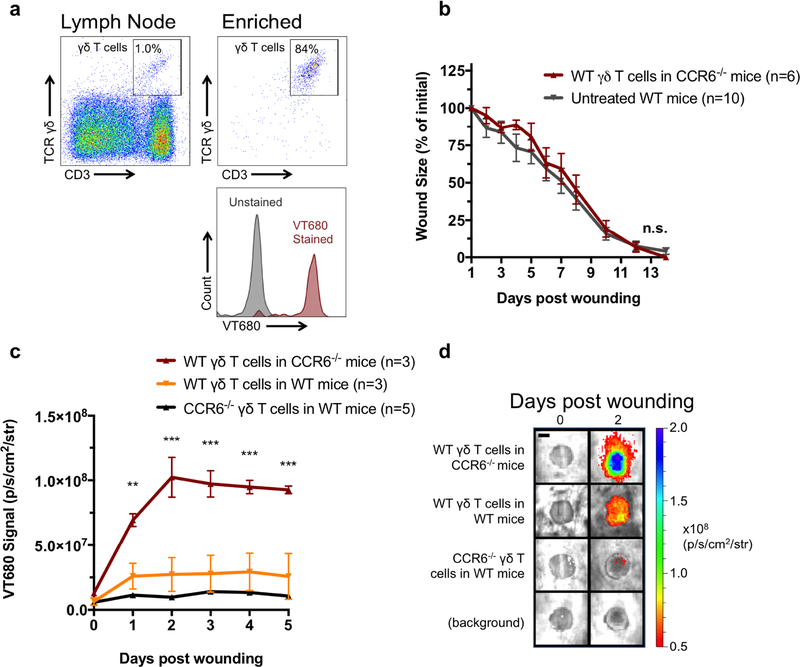
We next evaluated whether normal wound healing could be restored in CCR6−/− mice by adoptive transfer of WT γδ T cells from WT mice. Lymph node cell suspensions from WT mice were bead-enriched for γδ T cells, increasing the frequency of γδ T cells from ~1% to ~85% (Fig 2a). Enriched cells were labeled with VT680 (a far-red fluorescent membrane dye) to track their trafficking by whole animal in vivo florescence imaging and adoptively transferred (100,000 cells/100 L) via tail vein injection into CCR6−/− mice that were wounded the following day. Transferred γδ T cells effectively normalized wound-healing rates in CCR6−/− mice to that of the WT mice (Fig. 2b and Supplemental Fig. 1), and both conditions showed markedly faster closure than the previously wounded CCR6−/− mice (Fig. 1A).
Figure 2: Adoptive transfer of γδ T cells restores wound healing in CCR6−/− mice.
(a) γδ T cells were enriched from the lymph nodes of WT and CCR6−/− mice and stained with the membrane dye VT680. 100,000 γδ T cells were transferred into mice via the tail vein. Mice were wounded the subsequent day, and (b) wound size was measured daily. (c and d) VT680 signal was measured for 5 days to measure γδ T cell trafficking. Data is presented as Mean ± SEM. * P **P ≤ 0.01 and ***P ≤ 0.001 comparing CCR6−/− mice receiving WT γδ T cells to other groups using a repeated measures two-way analysis of variance followed by Bonferroni’s post hoc test (b and c) Scale bar = 3 mm.
In vivo fluorescence imaging for the VT680-labeled γδ T cells revealed that the exogenous γδ T cells used CCR6 to traffic to wounds, which reached a maximum by 2 days and persisted through day 5 (Fig. 2c and 2d). To confirm that CCR6 was necessary for trafficking, lymph node-enriched γδ T cells from WT mice were transferred into WT and CCR6−/− mice and γδ T cells from CCR6−/− mice were transferred to WT mice. Notably, transfer of CCR6−/− γδ T cells to WT mice resulted in ~70% less efficient trafficking to the wound than transfer of WT γδ T cells to CCR6−/− mice. Furthermore, transfer of CCR6−/− γδ T cells to WT mice resulted in 50% less recruitment to the wound than transfer of WT γδ T cells in WT mice, suggesting limited recruitment of γδ T cells independent of CCR6 (Fig. 2c and 2d). Taken together, these data illustrate a critical role for CCR6+ γδ T cells in the homeostatic process of normal wound healing.
Mobilizing CCR6+ γδ T cells from lymph nodes into the circulation or administration of CCL20 into wounds are two potential immunological based therapies to enhance their recruitment to impaired wounds. On the other hand, blocking CCR6 signaling is under investigation as an anti-inflammatory therapeutic in preclinical models of psoriasis and rheumatoid arthritis (Hirota et al. 2007; Mabuchi et al. 2013). We conclude that targeting CCR6/CCL20 may provide an effective strategy to maintain optimal host immune cell function and homeostasis in damaged and diseased skin.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grants R01AI129302 (to S.I.S.), 1R01AR063091-01A1 (to S.T.H), and R01AR069502 and R01AR073665 (to L.S.M), California institute of regenerative cures PC1–08118 (to R.R.I.), and the Training Program in Pharmacology: From Bench to Bedside at UC Davis (NIH T32 GM099608 to L.S.A). The Molecular and Genomic Imaging (CMGI) at the University of California Davis provided superb technological support.
CONFLICTS OF INTEREST
L.S.M. has received grant support from AstraZeneca, Pfizer, Boerhinger Ingelheim, Regeneron Pharmaceuticals, Moderna Therapeutics, is a shareholder of Noveome Biotherapeutics and is on the scientific advisory board of Integrated Biotherapeutics, which are developing therapeutics and vaccines against Staphylococcus aureus and other pathogens and these associations are unrelated to the work reported in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY
Datasets related to this article can be found at doi:10.17632/n5tvt5hn5p.1, hosted at Mendeley.com.
REFERENCES
- Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. 2014.
- Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007. November 26;204(12):2803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SA, Graveline D, Born WK, O’Brien RL. Cytokine production by Vgamma(+)-T-cell subsets is an important factor determining CD4(+)-Th-cell phenotype and susceptibility of BALB/c mice to coxsackievirus B3-induced myocarditis. J Virol. 2001. July;75(13):5860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, et al. A Role for Skin γδ T Cells in Wound Repair. Science. American Association for the Advancement of Science; 2002. April 26;296(5568):747–9. [DOI] [PubMed] [Google Scholar]
- Kurita Y, Tsuboi R, Ueki R, Rifkin DB, Ogawa H. Immunohistochemical localization of basic fibroblast growth factor in wound healing sites of mouse skin. Arch Dermatol Res. Springer-Verlag; 1992. August;284(4):193–7. [DOI] [PubMed] [Google Scholar]
- Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N, et al. Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J Immunol. American Association of Immunologists; 2011. September 1;187(5):2783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Gund R, Dutta A, Pincha N, Rana I, Elife SG, et al. Stimulation of hair follicle stem cell proliferation through an IL-1 dependent activation of γδT-cells. cdnelifesciencesorg. 2017. December 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Huang Z, Yan R, Liu M, Bai Y, Liang G, et al. Vγ4 γδ T Cells Provide an Early Source of IL-17A and Accelerate Skin Graft Rejection. The Journal of investigative dermatology. 2017. December;137(12):2513–22. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang Y, Zhou L, Liu M, Liang G, Yan R, et al. Vγ4 T Cells Inhibit the Pro-healing Functions of Dendritic Epidermal T Cells to Delay Skin Wound Closure Through IL-17A. Front Immunol. 2018;9:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Burns AR, Miller SB, Smith CW. CCL20, γδ T cells, and IL-22 in corneal epithelial healing. FASEB J. Federation of American Societies for Experimental Biology; 2011. August 1;25(8):2659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Xu Y, Zhang X, Liang G, Chen L, Xie J, et al. Defects in dermal Vγ4 γ δ T cells result in delayed wound healing in diabetic mice. Am J Transl Res. 2016;8(6):2667–80. [PMC free article] [PubMed] [Google Scholar]
- Mabuchi T, Singh TP, Takekoshi T, Jia G-F, Wu X, Kao MC, et al. CCR6 Is Required for Epidermal Trafficking of γδ-T Cells in an IL-23-Induced Model of Psoriasiform Dermatitis. Journal of Investigative Dermatology. Elsevier Masson SAS; 2013. January 1;133(1):164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi N, Kawakami K, Kanno E, Tanno H, Takeda A, Ishii K, et al. IL-17A promotes neutrophilic inflammation and disturbs acute wound healing in skin. Eming SA, Tomic-Canic M, editors. Exp Dermatol. Wiley/Blackwell (10.1111); 2017. February;26(2):137–44. [DOI] [PubMed] [Google Scholar]
- Varona R, Villares R, Carramolino L, Goya I, Zaballos A, Gutiérrez J, et al. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J Clin Invest. American Society for Clinical Investigation; 2001. March;107(6):R37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.