Abstract
Understanding variability in smoking patterns may inform smoking cessation interventions. Retrospective reports of cigarettes smoked per day may be biased and typically do not provide temporal precision regarding when cigarettes are smoked. However, real-time, user-initiated tracking, such as logging each time a cigarette is smoked, can be burdensome over long time frames. In this study, adult, non-treatment seeking daily smokers (N=22) used an electronic, smart lighter to light and timestamp cigarettes for 14 days. Participants reported number of cigarettes smoked per day (CPD) via a mobile device (daily diary) and retrospectively reported CPD at the end of the study using the Timeline Followback (TLFB). Self-reported lighter satisfaction and adherence varied with 68% of participants reporting that they liked using the lighter and participants reporting using the lighter for 92% of cigarettes smoked, on average. Lighter-estimated CPD did not differ from daily diary-estimated CPD, but was significantly lower than TLFB estimates. The lighter resulted in greater day-to-day variability relative to other methods and fewer rounded cigarette counts (digit bias) relative to the TLFB. The lighter appears to be feasible for capturing data on smoking patterns in daily smokers. Though false positive cigarettes are likely low, additional technologies that augment data captured from the lighter may be necessary to reduce false negatives (missed cigarettes) and alternative lighter designs may appeal more to certain smokers.
Keywords: Timeline Followback, Daily Diary, Methodology, Ambulatory Assessment, Technology, Cigarettes
Among cigarette smokers, variability in cigarettes smoked from day-to-day and within-day can provide meaningful information. For example, efforts to stop smoking are often characterized by variable smoking patterns (Hughes et al., 2013) and reductions in cigarettes smoked may lead to future cessation attempts (Hughes & Carpenter, 2006). Additionally, clustering of cigarettes at certain times per day (Chandra et al., 2007), around alcohol use (Shiffman et al., 2009), caffeine use (Treloar et al., 2014), while socializing (Shiffman et al., 2009), or near in time to medication administration (Winhusen, Theobald, & Lewis, 2016; Richter et al., 2007) can provide valuable information about factors that may increase or decrease smoking and the risk of relapse. To better understand these proximal risk factors, smoking measures must accurately reflect precise timing and variability in smoking behavior, while still providing accurate estimations of overall quantity of cigarettes smoked. The goal of this report is to present feasibility of a novel method for tracking cigarettes smoked via an electronic, smart lighter, the Quitbit. Initial convergent validity is also presented by examining agreement between the Quitbit electronic lighter and other methods for assessing cigarettes per day (CPD).
CPD are often assessed via retrospective self-report using an aggregate method (i.e., how many cigarettes do you smoke per day?; Gariti et al., 1998) or a more valid and reliable calendar-based method. In the latter, participants report the number of CPD in a given time period on a calendar with the use of techniques to enhance historical reporting (Timeline Follow-back; Sobell & Sobell, 1992; Lewis-Esquerre et al., 2005; Harris et al., 2009; Collins et al., 2009). These methods are simple and brief to administer, but are limited by poor recall (Shiffman, 2009). One indication that participants are providing a rough estimate of their CPD is the extent to which they round to the nearest multiple of 5, known as digit bias (Shiffman, 2009; Schwarz & Oyserman, 2001). Both aggregate methods and calendar-based methods show digit bias (Shiffman, 2009).
Methods that reduce retrospective recall, such as daily or more frequent reports of cigarettes smoked, have been shown to reduce digit bias (Shiffman, 2009; Perkins, Jao, & Karelitz, 2013) and may capture more variability in cigarette smoking from day-to-day (Perkins et al., 2013; Hughes et al., 2017). Some research utilizes real-time reports of cigarettes smoked via ecological momentary assessment (EMA; Stone & Shiffman, 1994). In these designs, smokers report or “log” a cigarette on a mobile or handheld device as they smoke or immediately after finishing a cigarette. End of day reports will sometimes allow for participants to record cigarettes that they forgot to log in real-time. In addition to allowing for calculation of daily cigarette counts, this method provides precise timing of cigarettes smoked. Thus, researchers can study contextual factors (e.g., day vs. evening, setting) that influence smoking rates. However, this method relies on user-initiated input and requires smokers to self-initiate a report on their mobile device, which increases burden and may lead to missed cigarette reports (Shiffman & Scholl, 2018).
Methods that lower the burden for (and presumably increase the compliance or accuracy of) reporting cigarette smoking in real-time have been pursued. Examples include counts of cigarette butts that the smoker collects throughout the day (Shiffman & Scholl, 2018; Blank et al., 2016; Saddleson et al., 2017), sophisticated algorithms to detect cigarette smoking from wrist and/or arm accelerometer readings and/or respiration data (Skinner et al., 2019; Imtiaz et al, 2017; Sazonov, Lopez-Meyer, & Tiffany, 2013; Saleheen et al., 2015; Raiff et al., 2014), electronic cigarette packs which record the time when the pack lid is opened and closed (Richter et al., 2007), and instrumented lighters that record a timestamp when activated (Imtiaz et al., 2017). An ongoing trial provides smokers with an electronic, smart lighter (Quitbit) that records a timestamp when activated to light a cigarette (Winhusen, Theobald, & Lewis, 2016). Winhusen and colleagues conducted field-testing with the Quitbit and found that timestamps recorded by the lighter were accurate within the minute (Winhusen, Theobald, & Lewis, 2016). A recent small-scale study combining an instrumented lighter (unique from Quitbit) with accelerometer readings suggested that use of a lighter may improve accuracy of a comprehensive smoking detection system during a 24-hour period (Senyurek et al., 2019). However, their data suggested accidental recordings and missed cigarettes. Aside from this initial field-testing, we are unaware of feasibility or validity studies on the use of the Quitbit electronic lighter to measure cigarette smoking in naturalistic settings. The potential of this method relies on smokers’ willingness to use the lighter over the course of several days or weeks.
The aim of this report was to assess feasibility and initial validity of using an electronic lighter (Quitbit) to characterize smoking patterns among daily smokers not attempting to quit or reduce. First, we present self-reported adherence with the lighter. Second, we provide descriptive data on participant satisfaction with the lighter. Finally, we compare three methods for assessing daily cigarette counts and variability in CPD (electronic lighter, Timeline Followback, daily diaries).
2. Method
As part of a 15-day pilot study examining sex differences in responses to smoking, stressful, and neutral cues, non-treatment-seeking adult smokers were asked to exclusively use the Quitbit lighter when smoking cigarettes. By design, an equal number of males (n= 11) and females (n=11) were recruited. Sample demographics and smoking characteristics are shown in Table 1. Participants were eligible if they were between the ages of 18-45, reported smoking at least 5 cigarettes per day, on average, during the last 6 months, and provided an expired breath carbon monoxide (CO) sample of ≥5 parts per million at study entry. Participants were excluded if they were unable to abstain from other nicotine products during the study (e.g., cigars, e-cigarettes), if they were currently seeking treatment for smoking cessation or engaged in a quit attempt, or if they met criteria for a moderate or severe substance use disorder (unless in remission). Participants were also excluded if they participated in a study with a similar protocol within the past 6 months (to avoid familiarity with the protocol and cues). If eligible, participants were provided instruction on how to complete daily diaries (morning reports) on a mobile device, loaned a lighter (described in more detail below), and scheduled for follow-up visits at Day 7 and study completion (Day 15). On Day 7, participants returned to the clinic and data from the lighter were manually downloaded to a computer by research staff. Participants returned to the clinic for their final study visit on Day 15. They returned study equipment and completed questionnaires, including a retrospective account of CPD during their study participation. Study procedures were approved by the Institutional Review Board at the Medical University of South Carolina.
Table 1.
Sample Demographic Information (N=22)
| M (SD) or % | |
|---|---|
| Age - M (SD) | 31.0 (8.9) |
| Gender (%) | |
| Female | 50.0 |
| Race (%) | |
| White/Caucasian | 81.8 |
| Black/African American | 13.6 |
| More Than One Race | 4.5 |
| Ethnicity (%) | |
| Not Hispanic or Latino | 100.0 |
| Marital Status (%) | |
| Single, Divorced, Separated, Widowed | 81.8 |
| Married | 18.2 |
| Highest Level of Education (%) | |
| High School Diploma or GED | 68.2 |
| Associate’s Degree or Equivalent | 13.6 |
| Bachelor’s Degree or Equivalent | 13.6 |
| Graduate or Professional Degree | 4.5 |
| Employment/Income (%) | |
| Currently unemployed | 9.1 |
| Student Only | 13.6 |
| Income less than $25,001 | 40.9 |
| Cigarette Use (At Screening) - M (SD) | |
| Carbon Monoxide Level (ppm) | 26.0 (16.1) |
| Average CPD (30-Day TLFB) | 15.3 (8.2) |
| Nicotine Dependence (FTND) | 4.2 (2.3) |
2.1. Quitbit Electronic Lighter
Participants were instructed to use the Quitbit electronic lighter to light all cigarettes smoked during the study. The device automatically recorded the time of each lighting event. To use the lighter, participants first opened the cap of the lighter and held in a button for several seconds, which activated a heating element (coil)—replacing the flame used in traditional lighters. Participants then held their cigarette to the heating element. The time was recorded for every instance that the button was held. If a participant attempted to light a cigarette within two minutes of lighting a prior cigarette, the lighter marked this attempt as “too soon” and it did not count toward the daily cigarette tally. If the button was only quickly pressed (less than 2 seconds), this was considered “too short” to light a cigarette and was not counted. Though a mobile app is available with cessation guidance and feedback from the company that produces the lighter, participants in this study used the lighter independent of the app. However, participants were able to see their daily cigarette count on the lighter, which reset at midnight each night. The heating element on the lighter was replaced at Day 7, if necessary, as they lost their effectiveness with repeated use.
2.2. Self-Report Measures
Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991).
The FTND was used to assess nicotine dependence.
Timeline Follow-Back (TLFB).
The TLFB was used to assess CPD for the 30 days prior to study participation and on Day 15 (to cover study period). In addition to reporting CPD via TLFB on Day 15, participants reported the number of times each day when someone else used their lighter and the number of times when they forgot to use the lighter (but smoked a cigarette), though participants were reminded to keep the lighter with them at all times and not share with other smokers.
Satisfaction with Lighter.
On Day 15, participants completed an internally-created survey to rate satisfaction with various aspects of the study protocol, including the use of the lighter. On a 5 point Likert scale ranging from 1 (Strongly Disagree) to 5 (Strongly Agree), participants answered five items regarding their use of the lighter (shown in Table 2). They also were given the opportunity to provide open-ended feedback regarding the lighter.
Table 2.
Subjective Satisfaction with Lighter (N=22)
| Question | Disagree or Strongly Disagree |
Neutral | Agree or Strongly Agree |
|---|---|---|---|
| I received adequate training on how to use the lighter. | 4.5% | 0.0% | 95.5% |
| It was easy to keep the iPhone and lighter with me at all times. | 22.7% | 4.5% | 72.8% |
| I always used the study lighter when smoking a cigarette. | 13.6% | 13.6% | 72.8% |
| I liked using the lighter. | 18.2% | 13.6% | 68.1% |
| I felt like using the lighter changed my daily routine.a | 47.6% | 19.0% | 33.3% |
Note: a Based on n=21, as one participant skipped this question.
Ambulatory.
Participants completed Daily Diaries via an iPhone (provided to them if needed) which included self-reports of cigarettes smoked in the past 24 hours. The daily diary was sent via an app notification daily at a time chosen by the participant (different weekday and weekend delivery times, if preferred). In addition to a daily diary, participants received four semirandom prompts (one administered randomly within four consecutive three-hour periods). As part of these sessions, participants answered questions about their current situation (not presented here). They also were shown a stressful, smoking, or neutral image (cue).
2.3. Data Analytic Procedure
Data Management.
Data from the lighter were extracted via an executable file provided by the Quitbit company. All other study data were entered directly into REDCap electronic data capture (Harris et al., 2009) by the participant or automatically uploaded from mobile devices to the REDCap platform.
Adherence.
Adherence with the lighter was calculated by dividing the sum of cigarettes logged by the sum of logged and unlogged cigarettes. This was multiplied by 100 to obtain percent adherence. The number of unlogged cigarettes was based on participants’ reported number of cigarettes smoked without using the lighter as collected via TLFB at Day 15. Adherence with the daily diaries was calculated by dividing the number of completed diaries by the number of expected diaries (~14 expected; one diary per day in study) and multiplying by 100.
Cigarettes Per Day.
CPD from the lighter were calculated by tallying all logged cigarettes from 4:00 AM to 3:59 AM the next day. Data from each individual were inspected and it was confirmed that the 4:00 AM cut-off accurately represented participants’ wake-sleep cycles (i.e., this time occurred during a gap in recorded cigarettes each night). Daily diaries assessed cigarettes smoked in the past 24 hours (as reported each morning) and should therefore also roughly correspond to waking hours. Though specific instruction regarding how to define a “day” for the TLFB was not provided, previous research (Schwarz & Oyserman, 2001) has suggested memories are constructed by personal reference periods (i.e., wake-sleep schedules) rather than external reference periods (i.e., midnight to midnight). In analyses comparing daily diary-assessed CPD to other measures of CPD, only days with available data were used. Wilcoxon signed rank tests were used to compare global CPD estimates across methods.
Variability in CPD.
Day-to-day variability in cigarettes smoked was computed for each individual by computing their median successive difference (MSD), similar to an approach used in previous studies (Hughes et al., 2017).
Digit Bias.
Whipple’s index (Denic, Saadi, & Khatib, 2004; Shiffman, 2009; Shiffman & Scholl, 2018) was used as a measure of biased reporting due to rounding cigarettes per day to numbers ending in 0 or 5 (not including zero itself).
Agreement Between Measures.
Agreement between CPD measures (lighter, lighter adjusted for non-adherence, daily diary, TLFB) at the daily level was determined using regression-based Bland-Altman analysis (Bland & Altman, 1999). Instead, mean bias between CPD estimation methods are compared to an a priori level of clinical significance (± 20% of the average CPD across the two methods; Griffith, Shiffman, & Heitjan, 2009) to determine agreement. If the mean bias in estimating CPD falls within the limits of clinical significance, then the measures show adequate levels of agreement. See supplementary materials for more details on the Bland-Altman analysis, median successive difference, and Whipple’s Index.
Results
3.1. Adherence with Study Protocol
Quitbit Lighter.
One participant returned the lighter without any recorded cigarettes. Thus, adherence and validity data are provided for only the n=21 who recorded any smoking via the lighter. Demographic and satisfaction data is reported for all participants.
The remaining 21 participants stated that they forgot or otherwise failed to use the lighter at least once on 11.4% of days (Range=0-14 days; Mean=1.6 days; SD=3.0). The average number of reported cigarettes smoked during the two-week study period, but not recorded on the Quitbit lighter, was 12.7 per person (Range=0-107; SD=27.9). This suggests that participants used the lighter for 92.2% of all smoked cigarettes, on average (Range=41.8-100.0%; SD=14.3), based on self-reported non-adherence. Only three participants had adherence lower than 85%, suggesting that most individuals reported high adherence to lighter use. All participants reported that they were the sole user of the lighter.
Daily Diaries.
Participants completed an average of 84.8% of daily diaries (Range=57.1-100.0%; SD=10.6).
3.2. Device Reliability and Participant Satisfaction with Lighter
One participant required an early Day 7 visit to replace a heating element that was no longer operational. There were no reported issues with the lighter’s battery life for any participants.
See Table 2 for subjective ratings of the lighter. At the end of the study, 15 out of 22 (68.1%) participants “moderately” or “strongly” agreed with the statement, “I liked using the lighter.” Three participants (13.6%) strongly disliked using the lighter. Of note, the three participants who strongly disliked using the lighter were heavier smokers on average (Baseline TLFB CPD=29.0; SD=7.2), as compared to the remaining participants (Baseline TLFB CPD=13.2; SD=6.1; p<0.01). One-third of participants (33.3%) reported that use of the lighter affected their daily routine. Both positive and negative comments about the lighter were provided when asked for qualitative, open-ended feedback. Among participants who provided positive comments (n=7), common themes emerged. Participants stated that they liked the “sleek design”, the fact that the lighter counted their cigarettes, and one participant stated, “I wish I could keep it.” Several participants also suggested that using the lighter and simultaneously monitoring stress and craving states increased their awareness of their smoking behavior, and in some cases, their motivation to reduce smoking. For example, comments included, “helped me to see just how much I smoked depending on stressful situations”, “device was helpful in tracking the amount of cigarettes and tracking daily behaviors which cause smoking”, “the lighter gave me accountability”, and “helped me see how much I was smoking and how long I waited to have another, tried to wait longer as the study progressed.” Among participants who provided negative comments (n=3), it was reported that it “takes too long to light cigarettes [and it] would be much easier with an open flame”, it was “not the easiest lighter to use”, and that it was difficult to light cigarettes while driving, “where many cigarettes are consumed.”
3.3. Cigarettes Per Day
When adjusting the recorded number of CPD according to self-reported missed cigarettes, CPD according to the Quitbit lighter increased by 0.9 CPD, which represented a significant increase based on a Wilcoxon signed rank test (SD=2.0, p<0.01). As denoted in Table 3, Wilcoxon signed rank tests suggested that daily diary-assessed CPD did not differ from the adjusted-lighter measure of CPD (diff= 1.0, SD=3.5, p=0.12), but did differ from the unadjusted-lighter measure (diff= 1.9; SD=4.4, p=0.03). TLFB-assessed CPD were significantly higher than all other measures of CPD: unadjusted lighter (diff=3.3, SD=4.5, p<0.01), adjusted lighter (diff=2.4, SD=3.6, p<0.01), and daily diary-assessed CPD (diff= 1.3, SD=1.9, p<0.01). To rule out the possibility that daily diary-assessed CPD differed from TLFB-assessed CPD due to different patterns of smoking when daily diaries were completed versus missing, we ran sensitivity analyses. TLFB-assessed CPD did not differ depending on whether daily diaries were completed (diff=0.1, SE=0.5, p=0.77).
Table 3.
Sample Descriptive Cigarette Data (N=21) During Study Period
| CPD M (SD) |
MSD M (SD) |
Whipple’s Index |
|
|---|---|---|---|
| Lighter | 10.9 (7.4)a | 3.1 (1.4)a | 92.2a |
| Lighter-adjusted* | 11.8 (7.3)b | 3.5 (1.8)a | 105.1a |
| Daily Diary | 12.8 (9.3)b | 1.9 (1.5)b | 150.3a |
| TLFB | 14.2 (9.7)c | 1.0 (1.3)c | 295.9b |
Note: CPD=Cigarettes Per Day; MSD= Median Successive Difference in Cigarettes Per Day; TLFB=Timeline Followback;
Adjusted for participant reported non-adherence with the lighter; Values in the same column with same superscript do not differ within-person based on Wilcoxon signed rank tests (p<0.05).
3.4. Variability in CPD and Digit Bias
As shown in Table 3, the lighter (adjusted and unadjusted) showed greater day-to-day variability than other methods with MSDs ranging from 3.1 to 3.5. Only lighter-assessed CPD fell within “highly accurate” (unadjusted) and “fairly accurate” (adjusted) ranges for Whipple’s Index, suggesting minimal digit bias toward cigarette counts ending in 0 or 5. Daily diaries and TLFB both showed substantial digit bias with Whipple’s Indices of 150.3 and 295.9, respectively.
3.5. Agreement Between Assessment Methods
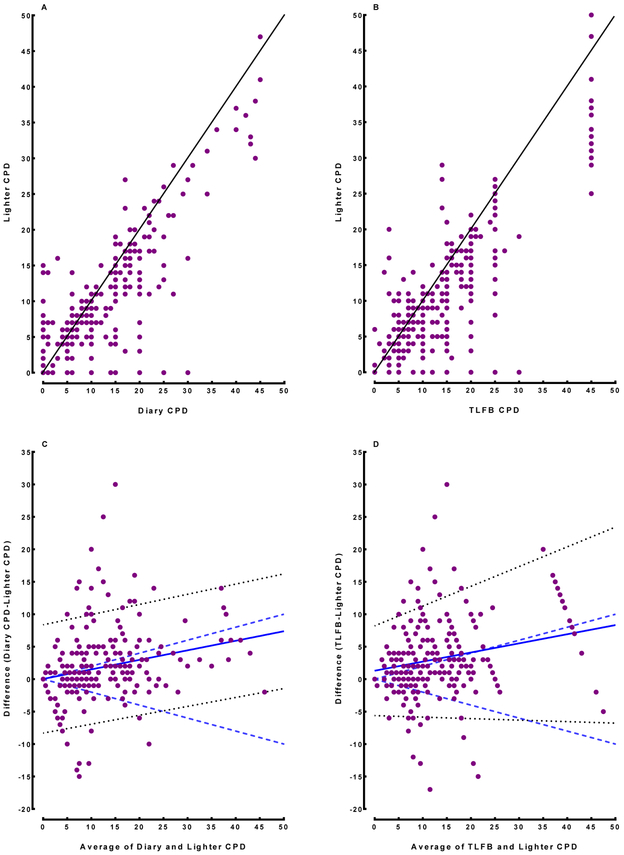
Scatterplots are shown in Figure 1 (Panels A and B), with points that fall on the line showing perfect agreement between methods. CPD estimations varied between the lighter and both daily diary and TLFB methods. Bland-Altman analyses comparing lighter-assessed CPD to diary-assessed CPD are shown in Figure 1, Panel C. The mean bias falls within the a priori limits of clinical significance (+/− 20% of the average CPD across the two methods) which suggests that these methods produce comparable estimates of CPD. Bland-Altman analyses comparing lighter-assessed CPD to TLFB are shown in Figure 1, Panel D and demonstrate poor agreement between these two methods (linear mixed model-derived mean bias falls outside of the a priori limits of clinical significance). This suggests that these measures are not interchangeable.
Figure 1.
Scatter and Bland-Altman Plots Comparing Methods
Note. Panels A (Daily Diary) and B (TLFB) show scatterplots of CPD (X-axis) versus Lighter CPD (Y-axis). Line denotes perfect agreement. Panels C (Daily Diary) and D (TLFB) show Bland-Altman plots of average between Lighter CPD and indicated method plotted against the difference between methods. Solid line indicates linear mixed model-derived mean bias. Dotted lines are the 95% confidence intervals. Dashed lines indicate limits of clinical significance (± 20% of mean CPD).
4. Discussion
In this pilot study examining the feasibility and initial validity of an electronic lighter to detect cigarette smoking, we found variable adherence and satisfaction with the lighter. One participant failed to use the lighter at all during the two-week study period. Among the remaining 21 participants, over 90% of cigarettes were recorded via the lighter, according to participant self-report. However, relying on participants to accurately recall and be truthful about unlogged cigarettes likely means that this is an over-estimate of adherence. Correspondence with other measures of CPD may provide a more accurate marker for adherence. Though the lighter may be a low-burden mechanism for improving self-monitoring, it cannot be used alone to objectively verify smoking, reduction, or abstinence. Participants may simply opt not to use the lighter to light cigarettes, particularly if cessation is incentivized. It is also possible that some logged cigarettes via the lighter were not truly cigarettes smoked. For example, others could use the lighter, the individual could press the button to light a cigarette without actually doing so, or the lighter could be used to light something else. We are reasonably confident that this contributed a negligible amount of error in the current study. All participants reported that they alone used the lighter and lighter CPD was lower than other measures of CPD. In addition, the size of the heating element made it difficult to light other products, such as blunts and other larger cannabis products. Finally, the lighter had built in features to prevent accidental recording of cigarettes, and it did not record immediately sequential button presses or those that were too short for a cigarette to have been lit. Regardless, data collected with wearable devices that detect smoking-specific gestures or puffing through respiration-detecting devices may help provide additional information that, along with the lighter, may better characterize the spatial and temporal characteristics of naturalistic smoking, similar to comprehensive systems in development (Senyurek et al., 2019).
In this small pilot study, satisfaction with the lighter was in the moderate range. A subset of participants disliked the lighter, which may affect utilization. Satisfaction may be enhanced, particularly among heavier smokers, by future product designs that increase ease of lighting a cigarette and decrease lighting time. An open-flame lighter that allows cigarettes to be time-stamped may be preferred by some smokers, increasing ease of use while multi-tasking (i.e., walking, driving). This is feasible, given that an open-flame lighter has been used in prior research (Senyurek et al., 2019). However, it is possible that this design results in increased accidental “logged” cigarettes. To our knowledge, this study is the first to examine real-world smoking behavior using an electronic lighter across multiple weeks. We experienced no problems with battery life on the devices and for most participants, the heating element lasted until their next visit at which time it could be replaced.
Comparing three methods for assessing CPD among daily smokers, we found that an electronic lighter produced greater day-to-day variability in CPD relative to daily diaries and TLFB. The lighter also produced lower estimates of CPD and digit bias relative to TLFB. This is consistent with prior research showing that 1) TLFB CPD estimates tend to be slightly higher than CPD estimates derived from real-time cigarette logs (Griffith et al., 2009) and 2) reduced retrospective recall is associated with reduced digit bias (Shiffman, 2009; Perkins et al., 2013).
Lighter-assessed CPD, particularly when adjusted for reported unlogged cigarettes, did not significantly differ from daily diary-assessed CPD. TLFB produced significantly higher estimates of CPD than all other measures. Though daily diary-assessed and lighter-assessed CPD estimates were comparable, lighter-assessed CPD showed more day-to-day variability and less digit bias than daily diary assessments. It is noteworthy that significant differences in CPD, variability, and digit bias were observed across methods, as we anticipate that the accuracy of TLFB and daily diary assessments may be enhanced by extra monitoring of smoking behavior. In addition, the lighter displayed the cigarette count for the day (though it reset at midnight) which should presumably improve participants’ accuracy in reporting, especially for the daily diaries.
Additional study limitations should be considered. First, the current sample size was limited and larger, diverse samples of smokers are necessary to provide validation of the lighter for measurement of cigarette smoking. Though we piloted an electronic lighter with daily smokers, nondaily smokers represent a growing proportion of total smokers in the United States, where this study took place (Schauer, Malarcher, & Mowery, 2016; Centers for Disease Control, 2016). This pattern may be more difficult to track, but readily captured via a device like the Quitbit. Additional studies employing alternative designs, subgroups of smokers, and larger samples are warranted. Second, it is possible that real-time monitoring of behavior changed smoking behavior. Data from this study suggest this is possible, since participants reported higher CPD at baseline (15.6, excluding the participant who did not use the lighter) as compared to all other indices captured during the monitoring period, particularly those that were captured in real-time (CPD 10.9 – 12.8). However, an alternative explanation is that participants overestimated their CPD (via TLFB) at study entry. Other studies have shown that “monitored” TLFB CPD counts (when additional self-monitoring is occurring such as via daily diaries or EMA) produce lower CPD estimates than unmonitored TLFB estimates (Shiffman, 2009). There are a number of potential reasons for this effect including (as previously mentioned) greater accuracy of reported CPD, reductions in smoking due to awareness of being monitored (McCambridge, Witton, & Elbourne, 2014), and reductions in smoking due to greater awareness of smoking patterns. However, open-ended feedback from participants suggested that both increases in awareness of smoking patterns and increases in motivation to reduce smoking are potential explanations for the observed reduction in cigarettes smoked. Finally, studies with cue exposure may affect substance use (DeSantis et al., 2009), suggesting reactivity could be caused by other study-related tasks. Future research should examine whether a similar decline in CPD is observed when using the lighter without additional concurrent monitoring, a displayed cigarette count on the lighter, and cue exposure.
In sum, these data suggest that an electronic lighter captures the majority of cigarettes smoked for most smokers, although accuracy improves when accounting for unlogged cigarettes. Additional modifications to the lighter or offering variable lighter designs may improve uptake. This has important implications for studies in which the precise counting and timing of cigarettes smoked is desirable. Electronic lighters may be useful in research studies characterizing patterns in smoking, identifying contexts which reduce or exacerbate smoking (e.g., acute medication effects, stress, GPS location), and as a validation tool for other passive, objective measures of smoking in naturalistic settings (Senyurek et al., 2019). The present data represent an initial empirical step in testing smart lighters to track smoking patterns over several weeks in a naturalistic setting.
Supplementary Material
HIGHLIGHTS.
An electronic, smart lighter can be feasibly used to track smoking for 14 days
Smokers reported moderate to high satisfaction with the electronic lighter
Smokers reported using the lighter for 92% of cigarettes smoked
Lighter-assessed cigarettes per day were similar to daily diary reported estimates
The lighter estimated greater variability in cigarettes per day and less digit bias
Footnotes
Conflict of Interest: Rachel L. Tomko, Erin A. McClure, Patrick A. Cato, Julie B. Wang, Joshua L. Karelitz, and Michael E. Saladin declare that they have no conflict of interest. Kevin M. Gray and Matthew J. Carpenter have provided consultation to Pfizer, Inc. Brett E. Froeliger provides consultation to Promentis Pharmaceuticals, Inc. for work unrelated to the content of this manuscript. The lighter company (Quitbit) did not have any involvement in this study or the interpretation and presentation of results. It should be noted that this study is not an endorsement of the product tested here and no authors have any financial or intellectual stake in this company or its products.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bland JM, & Altman DG (1995). Comparing methods of measurement: Why plotting difference against standard method is misleading. Lancet, 346, 1085–1087. [DOI] [PubMed] [Google Scholar]
- 2.Bland JM, & Altman DG (1999). Measuring agreement in method comparison studies. Statistical Methods in Medical Research, 8, 135–160. [DOI] [PubMed] [Google Scholar]
- 3.Bland JM, & Altman DG (2007). Agreement between methods of measurement with multiple observations per individual. Journal of Biopharmaceutical Statistics, 17, 571–582. [DOI] [PubMed] [Google Scholar]
- 4.Blank MD, Breland AB, Enlow PT, Duncan C, Metzger A, & Cobb CO (2016). Measurement of smoking behavior: Comparison of self-reports, returned cigarette butts, and toxicant levels. Experimental and Clinical Psychopharmacology, 24, 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). (2016) Cigarette Smoking Among Adults—United States, 2005–2015. Morbidity and Mortality Weekly Report, 65, 1205–1211. Retrieved from https://www.cdc.gov/mmwr/volumes/65/wr/mm6544a2.htm [DOI] [PubMed] [Google Scholar]
- 6.Chandra S, Shiffman S, Scharf DM, Dang Q, & Shadel WG (2007). Daily smoking patterns, their determinants, and implications for quitting. Experimental and Clinical Psychopharmacology, 15, 67–80. [DOI] [PubMed] [Google Scholar]
- 7.Collins SE, Eck S, Torchalla I, Schroter M, & Batra A (2009). Validity of the timeline followback among treatment-seeking smokers in Germany. Drug and Alcohol Dependence, 105,164–167. [DOI] [PubMed] [Google Scholar]
- 8.Denic S, Saadi H, & Khatib F (2004). Quality of age data in patients from developing countries. Journal of Public Health, 26, 168–171. [DOI] [PubMed] [Google Scholar]
- 9.DeSantis SM, Bandyopadhyay D, Back SE, & Brady KT (2009). Non-treatment laboratory stress- and cue-reactivity studies are associated with decreased substance use among drug-dependent individuals. Drug and Alcohol Dependence, 105, 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gariti PW, Alterman AI, Ehrman RN, & Pettinati HM (1998). Reliability and validity of the aggregate method of determining number of cigarettes smoked per day. American Journal on Addictions, 7, 283–287. [PubMed] [Google Scholar]
- 11.Griffith SD, Shiffman S, & Heitjan DF (2009). A method comparison study of timeline followback and ecological momentary assessment of daily cigarette consumption. Nicotine & Tobacco Research, 11, 1368–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris KJ, Golbeck AL, Cronk NJ, Catley D, Conway K, & Williams KB (2009). Timeline follow-back versus global self-reports of tobacco smoking: A comparison of findings with nondaily smokers. Psychology of Addictive Behaviors, 23, 368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstrom KO (1991). The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addictions, 86, 1119–1127. [DOI] [PubMed] [Google Scholar]
- 15.Hughes JR, & Carpenter MJ (2006). Does smoking reduction increase future cessation and decrease disease risk? A qualitative review. Nicotine & Tobacco Research, 8, 739–749. [DOI] [PubMed] [Google Scholar]
- 16.Hughes JR, Shiffman S, Naud S, & Peters EN (2017). Day-to-Day Variability in Self-Reported Cigarettes Per Day. Nicotine & Tobacco Research, 19, 1107–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes JR, Solomon LJ, Fingar JR, Naud S, Helzer JE, & Callas PW (2013). The natural history of efforts to stop smoking: A prospective cohort study. Drug and Alcohol Dependence, 128, 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imtiaz MH, Romas-Garcia RI, Senurek VY, Tiffany S, & Sazonov E (2017). Development of a multisensory wearable system for monitoring cigarette smoking behavior in free-living conditions. Electronics, 6, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis-Esquerre JM, Colby SM, Tevyaw TO, Eaton CA, Kahler CW, & Monti PM (2005). Validation of the timeline follow-back in the assessment of adolescent smoking. Drug and Alcohol Dependence, 79, 33–43. [DOI] [PubMed] [Google Scholar]
- 20.McCambridge J, Witton J, & Elbourne DR (2014). Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. Journal of Clinical Epidemiology, 67, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perkins KA, Jao NC, & Karelitz JL (2013). Consistency of daily cigarette smoking amount in dependent adults. Psychology of Addictive Behaviors, 27, 723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raiff BR, Karataş Ç, McClure EA, Pompili D, & Walls TA (2014). Laboratory validation of inertial body sensors to detect cigarette smoking arm movements. Electronics, 3, 87–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richter KP, Hamilton AK, Hall S, Catley D, Cox LS, & Grobe J (2007). Patterns of smoking and methadone dose in drug treatment patients. Experimental and Clinical Psychopharmacology, 15, 144–153. [DOI] [PubMed] [Google Scholar]
- 24.Saddleson ML, Wileyto EP, Darwar R, Ware S, & Strasser AA (2017). The importance of filter collection for accurate measurement of cigarette smoking. Tobacco Regulatory Science, 3, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saleheen N, Ali AA, Hossain SM, Sarker H, Chatterjee S, Marlin B, …, & Kumar S (2015). PuffMarker: A multi-sensor approach for pinpointing the timing of first lapse in smoking cessation. InProceedings of the 2015 ACM International Joint Conference on Pervasive and Ubiquitous Computing, 999–1010. [PMC free article] [PubMed] [Google Scholar]
- 26.Sazonov E, Lopez-Meyer P, & Tiffany S (2013). A wearable sensor system for monitoring cigarette smoking. Journal of Studies on Alcohol and Drugs, 74, 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schauer GL, Malarcher AM, & Mowery P (2016). National trends in frequency and amount of nondaily smoking, and relation to quit attempts, 2000-2012. Nicotine & Tobacco Research, 18, 1539–1544. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz N, & Oyserman D (2001). Asking questions about behavior: Cognition, communication, and questionnaire construction. American Journal of Evaluation, 22, 127–160. [Google Scholar]
- 29.Senyurek V, Imtiaz M, Belsare P, Tiffany S, & Sazonov E (2019). Cigarette smoking detection with an inertial sensor and a smart lighter. Sensors, 19(3), 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiffman S (2009). How many cigarettes did you smoke? Assessing cigarette consumption by global report, time-line follow-back, and ecological momentary assessment. Health Psychology, 28, 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiffman S, Kirchner TR, Ferguson SG, & Scharf DM (2009). Patterns of intermittent smoking: An analysis using Ecological Momentary Assessment. Addictive Behaviors, 34, 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiffman S, & Scholl SM (2018). Three approaches to quantifying cigarette consumption. Data from nondaily smokers. Psychology of Addictive Behaviors, 32, 249–254. [DOI] [PubMed] [Google Scholar]
- 33.Skinner AL, Stone CJ, Doughty H, & Munafo MR (2019). StopWatch: The preliminary evaluation of a smartwatch-based system for passive detection of cigarette smoking. Nicotine & Tobacco Research, 21, 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sobell LC, & Sobell MB (1992). Timeline Follow-back: A technique for assessing self-reported ethanol consumption In Allen J & Litten RZ (Eds.), Measuring Alcohol Consumption: Psychosocial and Biological Methods (pp. 41–72). Totowa, NJ: Humana Press. [Google Scholar]
- 35.Stone AA, & Shiffman S (1994). Ecological momentary assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine, 16, 199–202. [Google Scholar]
- 36.Treloar HR, Piasecki TM, McCarthy DE, & Baker TB (2014). Relations among caffeine consumption, smoking, smoking urge, and subjective smoking reinforcement in daily life. Journal of Caffeine Research, 4, 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winhusen T, Theobald J, & Lewis D (2016). Design considerations for a pilot trial using a novel approach for evaluating smoking-cessation medication in methadone-maintained smokers. Contemporary Clinical Trials, 47, 334–339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.