Abstract
Introduction:
In the National Lung Screening Trial (NLST) lung cancer screening trial all cases with a 4-mm nodule (micronodule) and no other findings were classified as a negative study. The prevalence and malignant potential of micronodules in the NLST is evaluated to understand if this classification was appropriate.
Methods and Materials:
In the NLST a total of 53,452 participants were enrolled with 26,722 undergoing low-dose CT screening. To determine whether a micronodule developed into a lung cancer, a list from the NLST database of those participants who developed lung cancer and had a micronodule recorded was selected. The CT images of this subset were reviewed by experienced, fellowship-trained thoracic radiologists (RM, CC, PB, DA), all of whom participated as readers in the NLST.
Results:
There were 26,722 participants who underwent CT in the NLST of which 11,326 (42%) participants had at least one CT with a micronodule. 5,560 (49%) of these participants had at least one positive CT examination of which 409 (3.6%) subsequently were diagnosed with lung cancer. Of the 409 lung cancer cases with a micronodule recorded, there were 13 cases in which a micronodule developed into a lung cancer. Considering the 13 cases, they represent 1.2 % (13/1089) of the lung cancers diagnosed in the CT arm of the NLST and 0.11% (13/11,326) of the total micronodule cases, 0.23% (13/5,560) of the micronodule and at least one positive CT examination cases, and 3.2% (13/409) of the micronodule cases diagnosed with lung cancer. The average size of the nodule at baseline was 3.0 x 2.5 mm (range < 4 x 2) and at the positive CT the nodule was 11.1 x 8.6 mm (range 2 x 14); a difference of average change in size of 8.1 x 6.1mm. The average number of days from first CT with a micronodule recorded to positive CT was 459 days (range 338 - 723), the mean time from first CT with micronodule to lung cancer diagnosis was 617 days (range 380 - 1140) and the mean time from positive CT to lung cancer diagnosis was 160 days (range 18 – 417). Histologically, there was one small cell carcinoma and 12 non-small cell with stages of IA in 8 (62%), Stage IB in 2 (15%) and 1 each Stage IIIA, IIIB, and IV. The overall survival of non-small cell lung cancer cases with a micronodule was not significantly different than the survival of the CT subset diagnosed with NSCL (p = 0.36).
Conclusion:
Micronodules are common among lung cancer screened participants and are capable of developing into lung cancer; however, following micronodules by annual CT screening surveillance is appropriate and does not impact overall survival or outcome.
Keywords: Micronodule, NLST, Lung Cancer CT Screening, Lung Cancer
Introduction:
Imaging of the lungs has been dramatically improved with multidetector CT technology that allows thin section collimation. While these advances have significantly improved detection and characterization of pulmonary diseases, the improvements have not been without challenges. For instance, small (< 4 mm) non-calcified nodules, referred to as micronodules, are now often seen on CT examinations and usually cannot be characterized as benign or malignant.1,2 There is little scientific data regarding the expected evolution or outcome of a micronodule, and thus the increased detection and reporting of micronodule has led to consternation among many radiologists and their clinical colleagues in regards to the appropriate management.3,4 Clinical management of patients with small pulmonary nodules varies and depends on numerous factors including the risk of malignancy and, to some extent, the preferences of the patient and the referring physician.3,5 The adaptation of Fleischner criteria in 2005 for managing nodules aided in management of nodules detected in the general population 6. The threshold of intervention was based on a nodule larger than 4 mm. These criteria were revised in 2017 raising the threshold of intervention to a 6 mm nodule. 7
At the time of development of lung cancer screening trials, opinions were varied in regards to the potential malignant nature of micronodules, and therefore management recommendations were mostly empirical.8 The National Lung Screening Trial (NLST) was a large randomized controlled trial of low dose CT in which all nodules less than 4-mm in maximum diameter were recorded as a micronodule and in the absence of other significant findings, the study was classified as negative study for lung cancer. Any non-calcified nodule 4 mm or larger was considered a positive screen. Secondary analysis of NLST data determined that nodules less than 4 mm in maximum diameter occurred in 20% (15,131 of 75,126) of CT screens, and a diagnosis of lung cancer was made within one year in 13 cases (0.09%). Nodules equal to 4 mm occurred in 3.7% (2,765/75,126) of CT screens, and a diagnosis of lung cancer was made within one year in 6 cases (0.22%). However, this analysis was based on the imaging reports, not from review of the images, and thus it could not be determined if the micronodule itself represented the lung cancer or was simply an incidental finding.9 Another analysis of the NLST data indicated that using a size threshold of 6.0 mm for a positive study would result in a decrease in false positive studies by 34% with only a delay in lung cancer diagnosis in 0.9%.10 A recent publication of the NLST data suggest that the screening interval of a negative baseline screening CT could be longer than an annual CT without impacting the cancer rates. 11 However, because only a small number of negative CTs in the NLST included those with a micronodule, it is not known if longer interval for cases with a micronodule would be safe.
While these recent analyses of the NLST data support the safety of raising the size threshold for a positive screen, there has not been an analysis of the NLST data to assess the malignant potential of micronodules in the lung cancer screening population. To better understand this issue, we reviewed the CT images and clinical data of all participants in the NLST in whom a micronodule and lung cancer diagnosis was recorded.
Methods and Materials:
The NLST enrolled participants who were between 55 and 74 years of age who had a minimum 30 pack year smoking history and if former smokers, had quit within the last 15 years. All participating institutions had IRB approval. A total of 53,452 participants were enrolled with 26,722 randomly assigned to low-dose CT screening; CT examinations were performed according to the NLST standardized protocol. 12 A CT screen in which a pulmonary nodule 4 mm or larger in maximum diameter with no benign characteristics was recorded as a positive study whereas a CT screen with the largest pulmonary nodule < 4mm in maximum diameter was recorded as a negative study. Only the presence of the micronodule was notated on the case report form; the specific lung lobe location was not recorded.
To determine the prevalence of micronodules in the NLST, the NLST database was searched for all CT examinations in which a micronodule was recorded. To determine whether a micronodule developed into a lung cancer, a list from the database of those participants who developed lung cancer and had a micronodule recorded was selected. In order to assess the evolution and outcome of a micronodule, only those participants with follow-up CT examinations were utilized, which limited our review to the T0 and T1 CT examinations (micronodule recorded at T0 with lung cancer diagnosis at T1/T2 or micronodule at T1 with lung cancer diagnosis at T2; if there were discrepant recordings of a micronodule (micronodule at T0, but not at T1 and cancer at T2, for example) then those cases were excluded. The CT images of this subset were reviewed by experienced, fellowship-trained thoracic radiologists (RM, CC, PB, DA), all of whom participated as readers in the NLST.
From the NLST database, the first CT in which a micronodule was recorded was reviewed to confirm the presence of the micronodule and to record its lobar location. As in the NLST, nodules were measured in two dimensions using electronic calipers; sizes were recorded from axial images using the longest diameter and the longest perpendicular diameter. This CT (positive micronodule, but NLST negative study) was compared with the follow up CT (NLST positive studies) to determine if the location of the diagnosed lung cancer was in the same lobe as the micronodule. If the micronodule was located in the same lobe, then a determination as to whether the lung cancer arose from the micronodule or not was done by consensus of two readers. This consisted on comparison of the images using anatomical landmarks such as pulmonary vessels and airways of the lobe in which lung cancer was diagnosed. Only those cases in which it was readily apparent that the micronodule developed into a lung cancer were used for this analysis; if there was any uncertainty, the case was not used. Those cases in which the lung cancer was determined to arise from the micronodule were used for nodule characteristics, clinical analysis and outcomes.
Statistical Analysis:
The analysis addressed two primary questions, first which factors were associated with the occurrence of a micronodule and subsequent lung cancer and second whether time to lung cancer death differed between participants with a micronodule that developed into lung cancer and participants with all other lung cancers. Multivariable logistic regression model was used to investigate factors associated with the presence of micronodules and the association of micronodule presence to lung cancer. The model included variables for participant demographics, smoking history, and geographic region. Multivariable Cox regression was used to compare time to lung cancer death between participants with micronodules that developed into non-small cell lung cancer and all others with non-small cell lung cancer. The model included variables for age, gender, tumor size by pathology, cancer type, cancer stage, and indicator of micronodule that developed into lung cancer and was stratified by stage at diagnosis. Nominal p-values are reported without adjustment for multiplicity of inferences and with 0.05 as the threshold for nominal significance. Computations were performed using SAS/STAT version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results:
There were 26,722 participants who underwent CT in the NLST which constitutes our database.
Prevalence of micronodules: Table 1
Table 1:
MN prevalence, positive study and lung cancer rate in the NLST
| Micronodule Identified on At Least One CT Screen | No Micronodules Identified | Total | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At Least One Positive CT Screening Exam | Never Positive | Total | At Least One Positive CT Screening Exam | Never Positive | Total | At Least One Positive CT Screening Exam | Never Positive | Total | ||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Lung Cancer | 319 | 5.74 | 90 | 1.56 | 409 | 3.61 | 547 | 11.89 | 133 | 1.23 | 680 | 4.42 | 866 | 8.52 | 223 | 1.35 | 1089 | 4.08 |
| No Lung Cancer | 5241 | 94.26 | 5676 | 98.44 | 10917 | 96.39 | 4053 | 88.11 | 10663 | 98.77 | 14716 | 95.58 | 9294 | 91.48 | 16339 | 98.65 | 25633 | 95.92 |
| Total CT Randomized Participants | 5560 | 100.00 | 5766 | 100.00 | 11326 | 100.00 | 4600 | 100.00 | 10796 | 100.00 | 15396 | 100.00 | 10160 | 100.00 | 16562 | 100.00 | 26722 | 100.00 |
Overall, there were 11,326 of 26,722 (42%) participants who had at least one CT examination in which a micronodule was recorded. Of these, 5,560 (49%) had at least one positive CT screen, while the other 5,766 (51%) never had a positive CT screen. Additionally, of those with a micronodule, there were 409 of 11,326 (3.6%) participants subsequently diagnosed with lung cancer.
There were 15,396 (58%) participants who did not have a micronodule recorded. Of this group, 4,600 (30%) had at least one positive CT scan, and 10,796 (70%) never had a positive CT. Of those without a micronodule, there were 680 (4.4%) diagnosed with lung cancer.
Factors associated with micronodule occurrence: Table 2
Table 2.
Multivariable analysis of factors associated with occurrence of micronodule
| Variable | Label | Odds Ratio Estimates | P-value | ||
|---|---|---|---|---|---|
| Estimate | 95% Confidence limits | ||||
| Geographic region | Midwest/West vs. Northeast/South | 1.719 | 1.632 | 1.811 | <.0001 |
| Race | American Indian or Alaskan Native vs white | 0.768 | 0.496 | 1.188 | 0.0020 |
| Asian vs white | 0.831 | 0.698 | 0.989 | ||
| Black or African-American vs white | 1.209 | 1.069 | 1.366 | ||
| More than one race vs white | 1.063 | 0.849 | 1.329 | ||
| Native Hawaiian or Other Pacific Islander vs white | 0.672 | 0.436 | 1.035 | ||
| Ethnicity | Hispanic or Latino vs non-Hispanic or Latino | 0.886 | 0.722 | 1.088 | 0.2479 |
| Smoking status | Current vs. Former | 1.061 | 1.008 | 1.116 | 0.0232 |
| Smoking history | 30 to < 40 vs >= 70 pack years | 0.983 | 0.912 | 1.060 | 0.3454 |
| 40 to < 50 vs >= 70 pack years | 0.958 | 0.891 | 1.030 | ||
| 50 to < 60 vs >= 70 pack years | 0.969 | 0.890 | 1.056 | ||
| 60 to < 70 vs >= 70 pack years | 1.048 | 0.957 | 1.147 | ||
| Age | Age in years at randomization | 1.015 | 1.010 | 1.020 | <.0001 |
| Gender | Female vs. Male | 1.354 | 1.286 | 1.426 | <.0001 |
| Education | Education in years | 1.014 | 1.003 | 1.025 | 0.0112 |
In multivariable regression analysis participant age, gender, education, smoking status (current/former), race and geographic region were associated with the occurrence of micronodules (Table 2). In particular, micronodules were more likely to occur in women than men (OR=1.35, 95% CI: (1.29, 1.43), in older vs younger participants, and in participants with more years of education vs fewer. Micronodules were also more likely to occur in African Americans vs white participants (OR=1.120, 95% CI (1.06, 1.35)) and less likely in Asian vs white participants (OR= 0.831, 95% CI: (0.698, 0.989). Micronodules were more likely to occur in current vs former smokers (OR=1.06, 95% CI: (1.01, 1.12) and in participants from the West/Mid-West vs Northeast or South (OR=1.72, 95% CI: (1.63, 1.81).
Micronodules and lung cancer:
In multivariable logistic regression analysis controlling for geographic region, participants with a micronodule reported on the CT scans were less likely to develop lung cancer (OR=0.81, 95% CI(0.72, 0.93). There was no difference in the frequency of lung cancer diagnosis between those with only a micronodule compared to those with a micronodule and another nodule with benign calcification.
Lung Cancer Cases: Table 3
Table 3.
Nodule Characteristics
| Case | Baseline (mm) | Positive CT (mm) | Texture | Stage | Histology | Interval Days | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Maximum Diameter | Perpendicular Diameter | Maximum diameter | Perpendicular Diameter | MN CT to positive CT | MN CT to CA diagnosis | Positive CT to lung cancer diagnosis | ||||
| 1 | 3 | 2 | 11 | 10 | Solid | IA | Adenocarcinoma | 378 | 432 | 54 |
| 2 | 4 | 2 | 13 | 8 | Solid | IB | Large Cell Carcinoma | 723 | 876 | 153 |
| 3 | 3 | 3 | 20 | 14 | Solid | IV | Adenosquamous Carcinoma | 359 | 450 | 91 |
| 4 | 2 | 2 | 13 | 10 | Solid | IA | Unclassified Carcinoma | 338 | 380 | 42 |
| 5 | 2 | 2 | 6 | 4 | GGO | IIIA | Adenocarcinoma | 371 | 763 | 392 |
| 6 | 2 | 2 | 7 | 6 | Solid | IA | Adenocarcinoma | 385 | 508 | 123 |
| 7 | 2 | 2 | 6 | 5 | Solid | IB | Adenocarcinoma | 386 | 512 | 126 |
| 8 | 4 | 3 | 12 | 8 | Solid | IA | Squamous Cell Carcinoma | 503 | 550 | 47 |
| 9 | 4 | 3 | 15 | 8 | Solid | IIIB | Small Cell Carcinoma | 364 | 400 | 36 |
| 10 | 4 | 4 | 9 | 8 | Part-solid | IA | Adenocarcinoma | 330 | 797 | 467 |
| 11 | 2 | 2 | 10 | 9 | Solid | IA | Squamous Cell Carcinoma | 359 | 476 | 117 |
| 12 | 4 | 2 | 8 | 8 | Solid | IA | Bronchiolo-alveolar Carcinoma | 723 | 1140 | 417 |
| 13 | 3 | 3 | 15 | 14 | Solid | IA | Adenocarcinoma | 721 | 739 | 18 |
| Average | 3 | 2.5 | 11.1 | 8.6 | 459 | 617 | 160 | |||
Of the 409 lung cancer cases with a micronodule recorded, 126 cases had follow-up CT examinations for assessment. The remaining 283 cases did not have sufficient follow-up CT examinations (lung cancer diagnosed at the first NLST CT (n=51), lung cancer diagnosed at same time micronodule recorded (17), discrepant recording of micronodule (n = 22), micronodule recorded at T2 (n = 20), lung cancer diagnosed after CT screenings ended (n = 173). Of the 126 cases, 54 with a micronodule recorded at T0 had lung cancer diagnosed at T1 and 72 with a micronodule at T1 had lung cancer diagnosed at T2.
Of the 126 cases there were 15 cases in which the recorded micronodule developed into a larger nodule considered to represent the lung cancer [Images 1, 2, 3]. When the readers evaluated the cases, there were two cases in which the readers agreed the recorded micronodule was > 4mm and thus would not be considered a micronodule. Therefore for our calculations, we have included only those 13 cases whereby the readers agreed there was a micronodule that developed into a lung cancer (Image 1). Of note, there is no impact in the overall outcome by excluding the 2 cases. These 13 cases represent 1.2 % (13/1089) of the lung cancers diagnosed in the CT arm of the NLST and 0.11% (13/11,326) of the total micronodule cases, 0.23% (13/5,560) of the micronodule and at least one positive CT examination cases, and 3.2% (13/409) of the micronodule cases diagnosed with lung cancer.
Figure 1 (A and B):
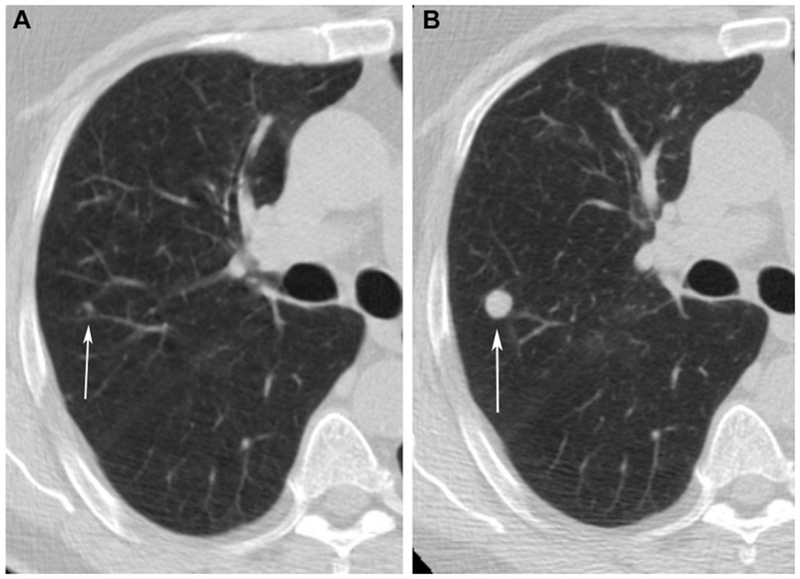
Baseline CT demonstrates growth of a micronodule (arrows) in the right upper lobe between baseline (A) and one year follow up (B).
Figure 2 (A and B):
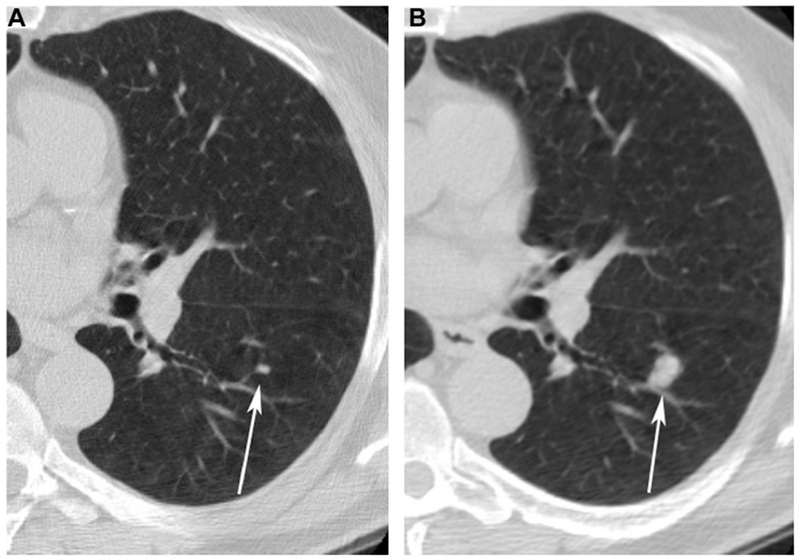
Baseline CT demonstrates growth of a micronodule (arrows) in the left lower lobe between baseline (A) and one year follow up (B).
Figure 3 (A and B):
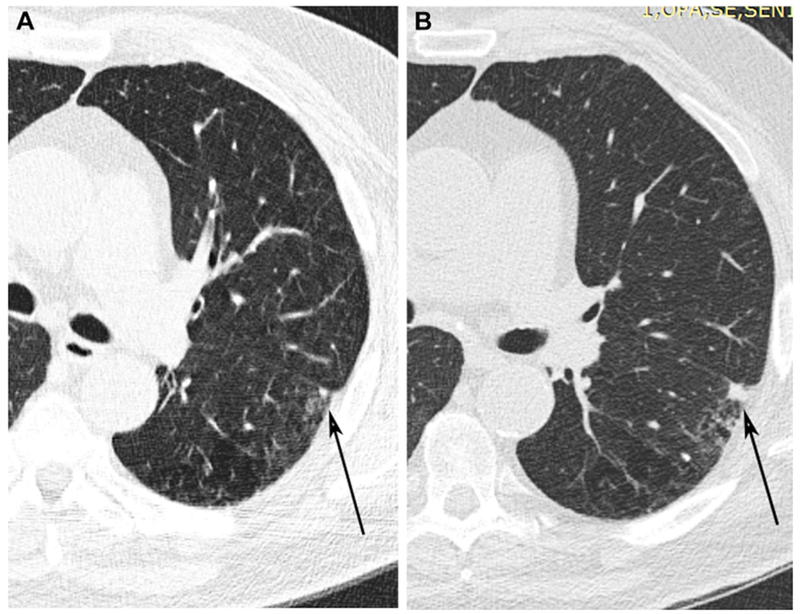
Baseline CT demonstrates growth of a micronodule (arrows) in the left lower lobe adjacent to the major fissure between baseline (A) and one year follow up (B).
In regards to the characteristics of the 13 micronodules, the average size at baseline was 3.0 x 2.5 mm (range < 4 x 2 mm) and at the time of the positive CT screen the nodule was 11.1 x 8.6 mm (range 2 x 14 mm). This results in a difference of average change in size of 8.1 x 6.1 mm. In regards to texture of the nodule, 11 were solid, one part-solid and one ground glass.
The average number of days from first CT with a micronodule recorded to positive CT of the 13 cases was 459 days (range 338 - 723), the mean time from first CT with micronodule to lung cancer diagnosis was 617 days (range 380 - 1140) and the mean time from positive CT screen to lung cancer diagnosis was 160 days (range 18 – 417). One of these 13 cases was a small cell carcinoma and the others were non-small cell lung cancer (NSCLC) (adenocarcinoma (6); squamous cell carcinoma (2); bronchiolo-alveolar cell carcinoma (1); large cell carcinoma (1); adenosquamous cell carcinoma (1); and unclassified (1). The stage at surgery was Stage IA in 8 (62%), Stage IB in 2 (15%) and 1 each Stage IIIA, IIIB, and IV. The average years of follow-up or death after lung cancer diagnosis in these NSCLC cases was 3.6 years (std = 1.6; range 0.14, 5.5 years; the small cell carcinoma follow up was 1.8 years).
In multivariable Cox regression analysis comparing overall survival of the subset of participants with lung cancer arising from a micronodule to all other NLST participants with lung cancer, there was not a significant difference in survival (HR= 1.73, CI:(0.54, 5.52), p=0.36). Participant gender, age, tumor type, and tumor lesion size, and lung cancer stage were included in the regression model as potential confounders.
Discussion:
There has been a significant increase in the detection rate of pulmonary micronodules on CT, but unfortunately in the majority of cases the micronodule cannot be further characterized as benign or malignant.1,2 It is known that small nodules (< 1-cm) detected on thoracic CT and resected with video-assisted thoracoscopic surgery have a relatively high likelihood of malignancy (48%-58%), which is even higher in those patients with a pre-existing malignancy (62%-81%).14,15 In contrast, Midthun et al.16 reported from their experience in the Mayo Clinic CT Screening Trial that the likelihood of malignancy was 0.2% for nodules smaller than 3 mm and 0.9% for nodules 4-7 mm. There is much consternation and debate on clinical management of < 4 mm nodules, which varies widely depending on multiple factors.3,4 In regards to the lung cancer screening population, there is insufficient scientific data regarding the malignant potential of micronodules and the impact on the lung screening process. For this reason, we evaluated the malignant potential of the micronodules recorded in NLST participants.
In the NLST, micronodules were very common (42%), and it was also common (49%) for participants with micronodules to have at least one CT examination that included a nodule or other non-specific finding suspicious for lung cancer. Lung cancer was diagnosed in 3.6% (409/11,326) of all participants with micronodules. Thirteen of those lung cancers could be identified as arising from a micronodule. Although the 13 cases constitute a small percentage (0.11%) of all participants with micronodules reported, they do constitute 1.2% of the lung cancers in the CT arm of the NLST. Thus, it is important to recognize the malignant potential of micronodules in a lung cancer screening population. Although micronodules are below the currently accepted diameter threshold for defining a positive screen, it is helpful to record the specific location of a micronodule within the lung (lobe, image slice number) to ensure accurate follow up.
The micronodules that were subsequently diagnosed as malignant grew at various rates over the approximate one year interval with an average increase in size of 8.6 x 6.1 mm, which is essentially an 8 fold increase in volumetric size. The average size of the lung cancer arising from a micronodule at diagnosis was approximately 10 mm. The large majority of micronodules that developed into a lung cancer were solid at presentation, which may be explained by the fact that sub-solid nodules of < 4mm may be easily missed or even undetectable on larger slice thicknesses (e.g. 2.5 mm). There were discrepancies in the recording of micronodules in the data which may be secondary to the nodule not being present due to imaging parameters, lack of detection, or other factors. Unfortunately, in the NLST, the location of micronodules was not recorded.
Perhaps most importantly, there was no apparent impact on the patient’s survival compared to the overall NLST lung cancer population by observing the micronodule through annual screening follow up. While our study thus supports the general practice of classifying screening CT scans with one or more micronodules as a negative screening result, we believe that the malignant potential of micronodules warrants recording their location on screening reports to facilitate tracking on future annual screening CT scans.
The American College of Radiology Lung-RADS™ is a standardized reporting and management system for lung cancer screening examinations.17 This document was developed by a consensus panel using the most recent data and publications from lung cancer screening trials which led to the recommendation that nodules ≤ 6 mm do not require active follow up, in part because screening patients are expected to return for annual screening. While our study is not designed to assess this recommendation, in part our findings support this initiative and indicate annual follow-up of small nodules can be performed safely.
A limitation of our study is that, of the 1089 lung cancers in the CT screening arm, 369 were diagnosed after CT screening was completed and exclusion of this large group could adversely affect our overall patient analysis. In addition, assessments of nodule diameter and attenuation are not precise when the nodule diameter approximates the CT slice thickness.
Conclusion:
Micronodules are common among lung cancer screened participants and the prevalence increases with increasing age and with geographic locale. Although micronodules are capable of developing into lung cancer, our review did not demonstrate a difference in survival of this group compared to the overall NLST lung cancer group. Therefore, following micronodules with annual CT screening surveillance is appropriate.
Acknowledgments
Funding:
This research was supported by contracts from the Division of Cancer Prevention, National Cancer Institute (NCI), National Institutes of Health, Department of Health and Human Services, and by grants (U01 CA80098 and CA79778) to support the American College of Radiology Imaging Network (ACRIN) under a cooperative agreement with Cancer Imaging Program, Division of Cancer Treatment and Diagnosis, NCI. The NCI and ACRIN were actively involved in the design of the study and collection of data, but the authors had full responsibility for the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
DISCLOSURES
Ms. Sicks reports grants from National Cancer Institute, during the conduct of the study.
Dr. Munden reports grants from National Cancer Institute, during the conduct of the study; non-financial support from Optellum, outside the submitted work.
Dr. Gatsonis reports grants from National Cancer Institute, during the conduct of the study; personal fees from Zionexa/Cyclopharma, personal fees from EBG, outside the submitted work.
Dr. Chiles reports grants from National Cancer Institute, during the conduct of the study.
Dr. Boiselle has nothing to disclose.
Dr. Aberle reports grants from National Cancer Institute, during the conduct of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
RF Munden, Interim Vice President Clinical Operations and Cancer Services, Wake Forest Baptist Health and School of Medicine, One Medical Center Blvd Winston-Salem, NC 27157, Phone: 336-716-7095, Fax: 336-713-4267.
C Chiles, Wake Forest University Health Sciences Center Medical Center Boulevard, Winston-Salem, NC 27157, Office: 336-716-9250, Fax: 336-713-42676285.
PM Boiselle, Charles E. Schmidt College of Medicine, Florida Atlantic University, 777 Glades Road BC 71 Room 239, Boca Raton, FL 33431, tel: 561.297.4341, fax: 561.297.0914.
JD Sicks, Brown University, Box G-S121-7, 121 S. Main Street, Providence, RI 02912, Phone: 401-863-9987, Fax: 401-863-9182.
DR Aberle, Department of Radiological Sciences/MII, David Geffen School of Medicine at UCLA, 924 Westwood Blvd Suite 420, Los Angeles, CA 90024, Office: 310-794-8996.
CA Gatsonis, Department of Biostatistics, School of Public Health, Brown University, Box G-S121, 121 S Main Street, 7th Floor, Providence, RI 02912, Office: 401-863-9183, Fax: 401-863-9182.
References:
- 1.Costello P Spiral CT of the thorax. Seminars in ultrasound, CT, and MR 1994;15:90–106. [DOI] [PubMed] [Google Scholar]
- 2.Kalender WA, Polacin A, Suss C. A comparison of conventional and spiral CT: an experimental study on the detection of spherical lesions. J Comput Assist Tomogr 1994;18:167–76. [DOI] [PubMed] [Google Scholar]
- 3.Jeudy J, White CS, Munden RF, Boiselle PM. Management of small (3-5-mm) pulmonary nodules at chest CT: global survey of thoracic radiologists. Radiology 2008;247:847–53. [DOI] [PubMed] [Google Scholar]
- 4.Munden RF, Hess KR. “Ditzels” on chest CT: survey of members of the Society of Thoracic Radiology. AJR Am J Roentgenol 2001;176:1363–9. [DOI] [PubMed] [Google Scholar]
- 5.Prosch H, Strasser G, Oschatz E, Schober E, Schneider B, Mostbeck GH. Management of patients with small pulmonary nodules: a survey of radiologists, pulmonologists, and thoracic surgeons. AJR Am J Roentgenol 2006;187:143–8. [DOI] [PubMed] [Google Scholar]
- 6.MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395–400. [DOI] [PubMed] [Google Scholar]
- 7.MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228–43. [DOI] [PubMed] [Google Scholar]
- 8.Munden RF, Erasmus JJ, Wahba H, Fineberg NS. Follow-up of small (4 mm or less) incidentally detected nodules by computed tomography in oncology patients: a retrospective review. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2010;5:1958–62. [DOI] [PubMed] [Google Scholar]
- 9.Gierada DS, Pinsky P, Nath H, Chiles C, Duan F, Aberle DR. Projected outcomes using different nodule sizes to define a positive CT lung cancer screening examination. J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yip R, Henschke CI, Yankelevitz DF, Smith JP. CT screening for lung cancer: alternative definitions of positive test result based on the national lung screening trial and international early lung cancer action program databases. Radiology 2014;273:591–6. [DOI] [PubMed] [Google Scholar]
- 11.Patz EF Jr., Greco E, Gatsonis C, Pinsky P, Kramer BS, Aberle DR. Lung cancer incidence and mortality in National Lung Screening Trial participants who underwent low-dose CT prevalence screening: a retrospective cohort analysis of a randomised, multicentre, diagnostic screening trial. The Lancet Oncology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Lung Screening Trial Research T, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Lung Screening Trial Research T, Aberle DR, Adams AM, et al. Baseline characteristics of participants in the randomized national lung screening trial. Journal of the National Cancer Institute 2010;102:1771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginsberg MS, Griff SK, Go BD, Yoo HH, Schwartz LH, Panicek DM. Pulmonary nodules resected at video-assisted thoracoscopic surgery: etiology in 426 patients. Radiology 1999;213:277–82. [DOI] [PubMed] [Google Scholar]
- 15.Munden RF, Pugatch RD, Liptay MJ, Sugarbaker DJ, Le LU. Small pulmonary lesions detected at CT: clinical importance. Radiology 1997;202:105–10. [DOI] [PubMed] [Google Scholar]
- 16.Midthun DE. Evaluation of nodules detected by screening for lung cancer with low dose spiral computed tomography. Lung Cancer-J Iaslc 2003;41(suppl 2):S40. [Google Scholar]
- 17.Lung CT Screening Reporting and Data Systen (Lung-RADS). 2014. (Accessed March 27, 2015, at www.acr.org/Quality-Safety/Resources/LungRADS)


