Abstract
Evidence has shown that angiotensin II type 1 receptor antagonists have lower blood pressure and have target organ protective effects, but this is not the case for the drug allisartan isoproxil. The aim of this study was to evaluate the effects of allisartan isoproxil on blood pressure and target organ injury in patients with mild to moderate essential hypertension.
In total, 80 essential hypertensive participants were randomly divided into an allisartan group and a nifedipine group (n = 40 per group), and their blood pressure was measured once per month for 6 months. A 2-dimensional echocardiogram was performed at baseline and at the end of the study. The serum levels of renal injury indexes, endothelial function markers, inflammatory factors, blood biochemical assays and urinary measurements were determined at baseline and at 6 months.
At the end of the study, both systolic and diastolic blood pressure were significantly decreased in the allisartan group compared with baseline and showed the same antihypertensive effect as the nifedipine group. Meanwhile, the left ventricular remodeling, 24-hours levels of urinary microalbumin, endothelial dysfunction, and arterial stiffness were all significantly improved compared with that of the baseline and the nifedipine group (all P < .05).
The present study showed that allisartan isoproxil had favorable blood pressure lowering and heart, renal, and endothelial protective effects in patients with mild to moderate essential hypertension.
Keywords: allisartan isoproxil, blood pressure, cardiovascular disease, essential hypertension, target organ
1. Introduction
Hypertension is recognized as a major risk factor for cardiovascular events, such as stroke, myocardial infarction, heart failure, and renal disease.[1–4] The prevalence of hypertension among United States adults≥20 years of age was estimated at 34.0% in NHANES from 2011 to 2014, and approximately 85.7 million adults≥20 years of age have hypertension; under the new definition of hypertension in 2017, this prevalence would reach 46%.[5,6] It is well known that activation of the renin–angiotensin system (RAS) plays a key role in the development of hypertension and related cardiovascular disease.[7–9] Angiotensin II, the key effector of RAS, can bind to angiotensin II type 1 receptor (AT1R) to mediate cardiovascular disease and can also bind to angiotensin II type 2 receptor (AT2R) to play a cardiovascular protective role.[10,11] Strong evidence has shown that angiotensin II type 1 (AT1) receptor antagonists (ARBs), as first line antihypertensive drugs, have significant blood pressure (BP) lowering effects and also play a target organ protective role in the development of hypertension.[12,13]
Losartan, a representative ARB, is widely used in the treatment of hypertensive patients because of its well-established efficacy and safety profile. Losartan can be quickly absorbed and catalyzed by 2 cytochrome P450 subfamilies, CYP2C9 and CYP3A4, into many metabolites,[14] although only the carboxylic acid derivative (EXP3174) is the active metabolite with an antihypertensive effect. The efficacy of EXP3174 is approximately 15-fold more potent than its parent drug after oral administration. In vitro, EXP3174 is 30 times more potent than losartan for blocking the AT1 receptor.[15] Therefore, EXP3174 is considered the major ingredient of losartan's antihypertensive effects.[16,17]
Allisartan isoproxil is a new selective nonpeptide ARB developed in China. Unlike losartan, allisartan isoproxil can be hydrolyzed by esterases and directly converted into the active substance EXP3174 after absorption by the gastrointestinal tract; there is no need for catalysis by CYP450 metabolism; thus the incidence of interaction between different drugs and adverse drug reactions is low, and have obvious advantages in safety and tolerability. A previous study showed that allisartan isoproxil is highly effective for BP reduction and organ protection, with low toxicity in animal models.[14] However, until now, there has been little evidence in clinical studies. In the present study, we enrolled 80 essential hypertensive participants who were divided into an allisartan group and a nifedipine group to investigate the effects of allisartan isoproxil on BP and target organ injury among individuals with mild to moderate essential hypertension.
2. Methods
2.1. Subjects
This study was a 6-month prospective, double-blinded, randomized, controlled trial. Written informed consent for the collection and use of data was obtained from all the participants. The research protocol was approved by the Ethical Committee of Pingjin Hospital in accordance with the principles of the Declaration of Helsinki.
In total, 90 male and female Han participants, 49 to 80 years of age, and who were first diagnosed with mild to moderate essential hypertension (meeting the following criteria: mean systolic BP (SBP) ≥140 mm Hg and < 180 mm Hg and/or diastolic (DBP) ≥90 mm Hg and <110 mm Hg) were enrolled in the present study. The exclusion criteria were as follows: history of a heart attack or stroke within the preceding 6 months, current angina pectoris, congestive heart failure, diabetes mellitus, serious liver and kidney dysfunction, serious mental or physical illness, infectious disease, malignancy, and definite secondary hypertension at the end of the run-in period.
Randomization of the present study was performed by random number method to minimize the risk of selection bias and avoid any other factors that would influence the researcher or participant in group selection or medication. The participants were randomly divided into an allisartan group and a nifedipine group (n = 40 per group) after a 2-week run-in period. Participants of the allisartan group were administered 240 mg allisartan isoproxil per day, while participants of the nifedipine group were given 30 mg nifedipine gastrointestinal therapeutic system (GITS) per day, and both groups were observed for 6 months. A flowchart is displayed in Figure 1.
Figure 1.

Flowchart of the study.
2.2. Measurements
All the participants were asked to provide information regarding age, education, marital status, physical activity, history of smoking and alcohol consumption, and family history. Baseline body weights, height, and BP were recorded. Routine blood tests and biochemical assays were performed using an automated hematology analyzer (XE-5000, Sysmex, Kobe, Japan) and a Hitachi 7180 Clinical Analyzer (Hitachi, Tokyo, Japan), respectively.[18]
BP was measured by an electronic sphygmomanometer (Omron, HEM-1020, Kyoto, Japan) once a month. The BP value was measured twice after a test measurement. The value was considered valid if the difference between the 2 measurements was <10 mm Hg; otherwise, a third measurement was taken, and the average value was used for analysis.[19]
The echocardiogram was performed by a Philips iE33 system (Phillips, Andover, MA) at baseline and at the end of 6 months. Two-dimensional guided M-mode echocardiogram was performed to evaluate the left ventricular posterior wall thickness (LVPWT), interventricular septal thickness (IVST), left ventricular end-diastolic diameter (LVEDD), and left ventricular ejection fraction (LVEF). The Devereux correction formula was used to calculate the left ventricular mass index (LVMI) as previously described.[20] The carotid intima-media thickness (IMT) was measured using the echo-tracking method by a 12-MHz linear transducer. The carotid intima-media cross-sectional area (IMCSA) was calculated per the following formula: IMCSA = π (the diameter of carotid artery/2+IMT)2-π(the diameter of carotid artery/2)2, as previously described.[21] The brachial-ankle pulse wave velocity (ba-PWV) and ankle-brachial index (ABI) were measured using an automatic arteriosclerosis analyzer (Colin, VP-1000, Tokyo, Japan) as previously described.[22,23] Each measurement was repeated three times, and the average value was used for analysis.
At baseline and at the end of 6 months, 24-hours urine and blood samples were collected. The 24-hours urinary microalbumin (MA) and serum levels of nitric oxide (NO), endothelin (ET), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were measured using enzyme-linked immunosorbent assays (Invitrogen, Carlsbad) as previously described.[24–26]
2.3. Follow-up
All the hypertensive participants were followed-up once a month. Safety and drug tolerability assessments were performed throughout the study by recording adverse events and monitoring vital signs at each visit. Physical examinations and electrocardiograph measurements were performed at regular intervals. Echocardiogram and clinical laboratory parameters from blood and urine were also evaluated at baseline and at the end of 6 months. Our primary outcomes were changes of SBP and DBP in the hypertensive participants, and secondary outcomes were changes of the target organ injury indexes and the serum levels of inflammatory factors.
2.4. Statistical analysis
The major end point in the present study is under noninferiority design.[27] The sample size calculation was based on our pretest (unpublished data): α = 0.05, β = 0.10, standard deviation = 8.5 mm Hg. The patient distribution proportion was 1:1 in the 2 groups, and the minimum sample size was 72. Considering a 10% drop out rate, the target sample size was set to 80 (40 per group). The normal distribution of the data was estimated using the Kolmogorov–Smirnov test. For estimating the overall effects of antihypertensive drugs (time×treatment) on BP over time, 2-way analysis of variance repeated measurements was used. Within-group differences were assessed by paired t-test. Categorical data were compared using χ2-test and Fisher's exact test when expected cell values were <5. The relationships between the serum levels of inflammatory factors and target organ injury indexes were assessed with Pearson's correlation (if the data passed the normality test) or Spearman's rank correlation (if the data failed the normality test). A 2-tailed P < .05 was considered statistically significant. All analyses were performed using SPSS, version 18.0 (SPSS, Chicago, IL).
3. Results
3.1. Baseline characteristics
As illustrated in Table 1, 80 essential hypertensive participants were enrolled in the study. No adverse cardiovascular events occurred during the follow-up period. No apparent differences in baseline characteristics were observed between the 2 groups (all P > .05).
Table 1.
Baseline characteristics of all participants.

3.2. Changes in BP levels in the 2 groups
As shown in Figure 2, there was a continuous decrease of SBP and DBP over time both in the allisartan group and the nifedipine group after the 6-month intervention. However, at the same time point, there were no significant differences in SBP or DBP between the 2 groups (all P > .05). In the allisartan group, the SBP was significantly decreased by 19.88 mm Hg (95% confidence interval (CI): 12.54 to 27.2, P < .001) and the DBP was decreased by 9.69 mm Hg (95% CI: 6.48 to 12.9, P < .001) at the end of the study. In the nifedipine group, the SBP was significantly decreased by 17.96 mm Hg (95% CI: 11.32 to 24.6, P < .001), and the DBP decreased by 10.86 mm Hg (95% CI: 7.23 to 14.5, P < .001) at the end of the study.
Figure 2.
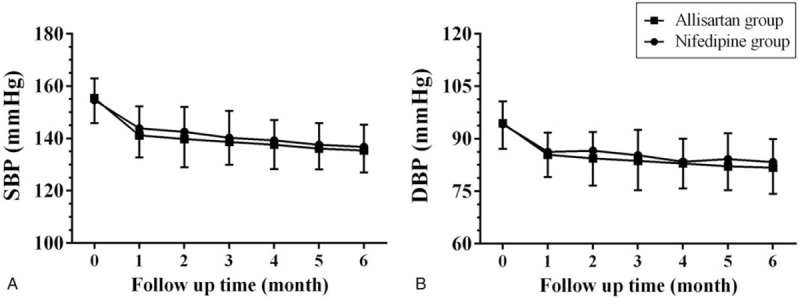
Systolic and diastolic blood pressure changes in the allisartan group and the nifedipine group after the 6-month follow-up. Data are reported as mean ± SD (n = 40 per group). DBP = diastolic blood pressure, SBP = systolic blood pressure.
3.3. Changes in left ventricular remodeling indexes in the 2 groups
As shown in Figure 3, after the 6-month intervention, the LVEDD, LVST, LVPWT and LVMI in the allisartan group were all significantly decreased compared with the baseline levels and the nifedipine group (all P < .05). Meanwhile, in the nifedipine group, only the LVMI was apparently decreased compared with the baseline levels (P < .05). No significant differences in LVEF were found between groups at the end of the study (all P > .05) (Fig. 4).
Figure 3.

Left ventricular remodeling index changes in the allisartan group and the nifedipine group after the 6-month follow-up. Data are reported as mean ± SD (n = 40 per group). IVST = interventricular septal thickness, LVEDD = left ventricular end-diastolic diameter, LVEF = left ventricular ejection fraction, LVMI = left ventricular mass index, LVPWT = left ventricular posterior wall thickness. ∗P < .05 vs the baseline level, #P < .05 vs the nifedipine group of the same time point.
Figure 4.

Renal function index changes in the allisartan group and the nifedipine group after the 6-month follow-up. Data are reported as mean ± SD (n = 40 per group). BUN = blood urea nitrogen, Cr = creatinine, MA = microalbumin. ∗P < .05 vs the baseline level, #P < .05 vs the nifedipine group of the same time point.
3.4. Changes in renal injury indexes in the 2 groups
As displayed in Figure 5, the 24-hour urinary MA in the allisartan group was significantly decreased compared with the baseline levels and the nifedipine group (all P < .05), but there were no apparent differences in the serum levels of creatinine (Cr) or blood urea nitrogen (BUN) found between the 2 groups after the 6-month follow-up (all P < .05).
Figure 5.

Endothelial function index changes in the allisartan group and the nifedipine group the 6-month follow-up. Data are reported as mean ± SD (n = 40 per group). ABI = ankle-brachial index, ba-PWV = brachial-ankle pulse wave velocity, IMCSA = carotid intima-media cross-sectional area, IMT = carotid intima-media thickness.
3.5. Changes in endothelial function indexes in the 2 groups
As illustrated in Figure 5, the levels of endothelial dysfunction in both groups were significantly improved at the end of the study. The serum level of NO was apparently increased, and the serum level of ET was significantly decreased in the allisartan group compared with the baseline levels and the nifedipine group (all P < .05). The same changes in serum levels of NO and ET were observed in the nifedipine group as in the allisartan group compared with baseline levels at the end of the 6-month follow-up (all P < .05).
3.6. Changes in arterial stiffness indexes in the 2 groups
As shown in Figure 6, the carotid IMT and IMCSA and the ba-PWV of the allisartan group were all significantly decreased compared with the baseline levels at the end of the study (all P < .05), but no apparent differences in these indexes were observed in the nifedipine group (all P > .05). Furthermore, no significant differences in ABI were found in either group at the end of the 6 months (all P > .05).
Figure 6.
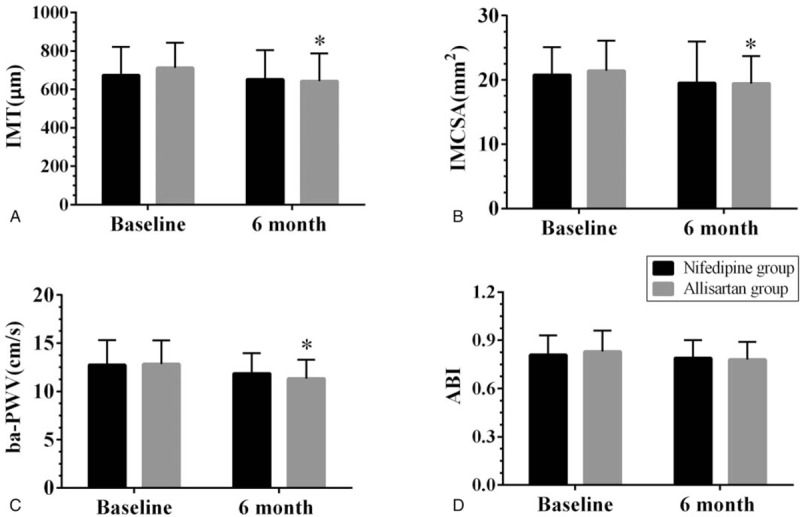
Arterial stiffness index changes in the allisartan group and the nifedipine group after the 6-month follow-up. Data are reported as mean ± SD (n = 40 per group). NO = nitric oxide, ET = endothelin. ∗P < .05 vs the baseline level, #P < .05 vs the nifedipine group of the same time point.
3.7. Changes in serum levels of inflammatory factors in the 2 groups
As displayed in Figure 7, the serum levels of TNF-α and IL-6 in the allisartan group were all significantly decreased compared with the baseline levels at the end of the study (all P < .05). The same changes in serum levels of TNF-α and IL-6 were observed in the nifedipine group as in the allisartan group compared with baseline levels at the end of the 6-month follow-up (all P < .05).
Figure 7.
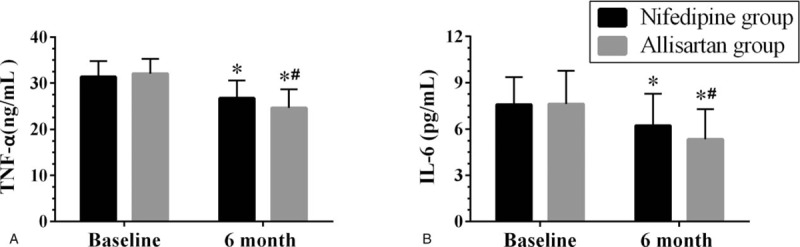
The serum levels of inflammatory factor changes in the allisartan group and the nifedipine group after the 6-month follow-up. Data are reported as mean ± SD (n = 40 per group). IL-6 = interleukin-6, TNF-α = tumor necrosis factor-α. ∗P < .05 vs the baseline level, #P < .05 vs the nifedipine group of the same time point.
3.8. Correlation analysis between the serum levels of inflammatory factors and target organ injury indexes
Correlation analyses were then performed to evaluate the relationships between the serum levels of inflammatory factors and target organ injury indexes. The results showed that both serum levels of TNF-α and IL-6 were positively correlated with the LVEDD, LVMI, 24-hour urinary MA, serum level of Cr, serum level of ET, carotid IMT, and carotid IMCSA and were negatively correlated with the serum level of NO (Figs. 8 and 9).
Figure 8.
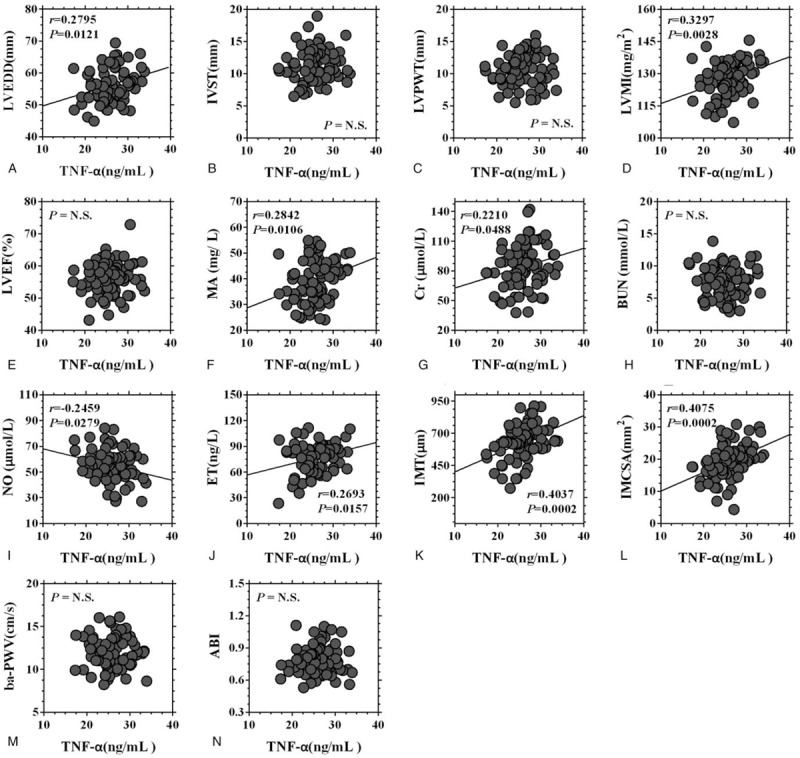
Correlation analysis between the serum level of TNF-α and the target organ injury indexes. ABI = ankle-brachial index, ba-PWV = brachial-ankle pulse wave velocity, BUN = blood urea nitrogen, Cr = creatinine, ET = endothelin, IMCSA = carotid intima-media cross-sectional area, IMT = carotid intima-media thickness, IVST = interventricular septal thickness, LVPWT = left ventricular posterior wall thickness, LVMI = left ventricular mass index, LVEF = left ventricular ejection fraction, LVEDD = left ventricular end-diastolic diameter, MA = microalbumin, NO = nitric oxide, TNF-α = tumor necrosis factor-α.
Figure 9.

Correlation analysis between the serum level of IL-6 and the target organ injury indexes. ABI = ankle-brachial index, ba-PWV = brachial-ankle pulse wave velocity, BUN = blood urea nitrogen, Cr = creatinine, ET = endothelin, IMCSA = carotid intima-media cross-sectional area, IMT = carotid intima-media thickness, IL-6 = interleukin-6, IVST = interventricular septal thickness, LVEDD = left ventricular end-diastolic diameter, LVEF = left ventricular ejection fraction, LVPWT = left ventricular posterior wall thickness, LVMI = left ventricular mass index, MA = microalbumin, NO = nitric oxide.
4. Discussion
The present study investigated the effects of a new ARB, allisartan isoproxil, on lowering BP and protecting target organs in patients with mild to moderate essential hypertension. The results showed that allisartan isoproxil has as powerful an effect on lowering BP as nifedipine. Moreover, after the 6-month intervention, left ventricular remodeling, renal injury, vascular endothelial dysfunction, and artery stiffness were all significantly improved, indicating that allisartan isoproxil can decrease BP and has heart, renal and vascular protecting effects.
ARBs are some of the major first line antihypertensive drugs due to their BP lowering effect, and they are widely used in hypertensive comorbidities such as atherosclerosis, diabetes mellitus and heart failure, due to their target organ protective role.[28–30] As a new nonpeptide ARB, allisartan isoproxil has displayed good safety and antihypertensive effects.[14,31] One recent study assessed the effects of allisartan isoproxil on essential hypertensive patients at low-medium risk; after the 8-week follow-up, the SBP and DBP of the allisartan isoproxil and placebo groups were decreased 14.5/10.4 and 8.3/7.7 mm Hg, respectively, and no deaths or serious adverse events were observed.[31] In the present study, with nifedipine as the control, the SBP and DBP were decreased 19.88/17.96 and 9.69/10.86 mm Hg after the 6-month study, indicating that allisartan isoproxil has a powerful BP lowering effect with a good safety profile for essential hypertensive patients.
Compared with other traditional antihypertensive drugs, the major advantage of ARBs is their heart protecting effects.[32] Left ventricular hypertrophy (LVH) is an independent risk factor for cardiovascular disease,[33,34] and a growing body of evidence has confirmed the role of ARBs in reversing LVH in hypertensive patients.[33,34] Yasunari et al[33] compared valsartan and amlodipine on LVH and found that, after the 8-month intervention, LVMI was reduced 16% vs 1.2%, respectively, which indicated the apparent LVH-reversing effects of ARBs on hypertensive patients. A reanalysis of Val-HeFT study demonstrated that valsartan can both improve the LVEF and LVEDD in heart failure patients and can gain the most antiremodeling effect and clinical benefit.[35] In the present study, we found that allisartan isoproxil significantly reduced the LVEDD, IMST, LVPWT, and LVMI after the 6-month intervention, which was consistent with the previous study.[33–35]
Another advantage of ARBs is their renal protecting effects.[36] Strong evidence has shown that ARBs can reduce the 24-hour MA in patients with hypertension and diabetes mellitus.[37,38] Menne's study observed the effect of olmesartan on the prevention of microalbuminuria in patients with type 2 diabetes and hypertension and found that olmesartan delayed the time to onset of microalbuminuria by 25% (hazard ratio (HR): 0.75; 95% CI: 0.61–0.92, P = .007). Moreover, this protecting effect was independent of the baseline BP and the degree of BP reduction.[37] In our study, we found that allisartan isoproxil significantly reduced the 24-h urinary MA after the 6-month intervention, consistent with the previous study.[37,38]
Endothelial dysfunction is one of the most important initiating and maintenance risk factors of hypertension.[39,40] In patients with essential hypertension, endothelial dysfunction is characterized by the impaired basal and agonist-dependent release of NO; meanwhile, vasoconstriction to endogenous ET is increased.[39] ARBs can increase NO availability and reduce the synthesis of ET by reducing the production of oxygen free radicals and inhibiting the activation of RAS; then, it can decrease BP and reduce the incidence of related cardiovascular diseases.[39,40] In the present study, we found that allisartan isoproxil could significantly improve endothelial dysfunction by reducing the serum level of ET and increase the serum level of NO, consistent with the the related study.[39,40]
Sustained high BP often leads to stiffness of the large arteries. The increased stiffness, in turn, aggravates hypertension by increasing SBP, inducing arterial lesions, and leading to the formation of atherosclerosis.[41] Epidemiological studies have strongly suggested that artery stiffness is associated with excess morbidity and mortality independent of other cardiovascular risk factors.[42,43] A previous study has demonstrated that losartan improved artery stiffness in essential hypertensive patients compared with atenolol, which may be related to inhibiting RAS.[44] In the present study, after the 6-month follow-up, the parameters of artery stiffness in the allisartan group, including carotid IMT and IMCSA and ba-PWV, were all significantly decreased, which may be a possible mechanism of its heart and renal protecting effects.
The mechanisms of target organ protection of ARBs, besides inhibiting the activation of RAS, reducing oxidative stress and suppressing the proliferation of smooth muscle cells, may involve improving the degree of inflammation in target organs.[45] Studies have shown that left ventricular remodeling, renal injury and atherosclerosis are all associated with local tissue and systemic inflammation.[46–48] In the present study, the serum levels of TNF-α and IL-6 in the allisartan group were both significantly decreased at the end of the study, indicating that allisartan isoproxil plays an important role in suppressing the production of inflammatory factors. The correlation analyses showed that both the serum levels of TNF-α and IL-6 were positively correlated with left ventricular remodeling, renal injury, vascular endothelial dysfunction and artery stiffness, which indicated that inhibiting inflammation may be an important mechanism of target organ protecting effects by allisartan isoproxil.
There were 2 major novel findings in the present study. First, as a new ARB drug, there are not enough clinical studies to demonstrate its BP lowering effect. Although Li's study[31] elucidated the structural features, BP lowering efficacy, safety and tolerability, and adverse drug reaction of allisartan isoproxil, it was a placebo-controlled study, and there were no comparisons with other antihypertensive drugs. Therefore, we further investigated the BP lowering effect using nifedipine as a control and found that allisartan isoproxil not only had as powerful a BP lowering effect as nifedipine, but had target organ protective effects after the 6-month intervention. The second finding was that allisartan isoproxil can ameliorate left ventricular remodeling, decrease 24-hour urinary MA, and improve vascular endothelial dysfunction and artery stiffness, indicating that it is a heart, renal and vascular protective drug.
Several limitations should be acknowledged in the present study. First, this trial was a double-blinded, single-center study, which may have led to systematic bias in BP measurements. Second, the measurements of BP were taken in the office, rather than ambulatory or home BP monitoring data, which may be different from the participants’ usual family BP. Third, in order to better demonstrate the BP lowering effect, we selected nifedipine as the control group, which may be less desirable than using an angiotensin II type 1 receptor antagonist as the control drug. Fourth, in order to enroll participants with good compliance, we recruited a relatively old population and a small sample; thus, to extrapolate the results to a more general population, further large sample, multicenter clinical study are needed.
5. Conclusions
The present study showed that allisartan isoproxil has favorable blood lowering and heart, renal and endothelial protective effects in patients with mild to moderate essential hypertension. As a new ARB antihypertensive drug, allisartan isoproxil may provide more benefits to essential hypertensive patients who simultaneously suffer other diseases, such as heart failure, diabetes mellitus and coronary artery disease.
Author contributions
Conceptualization: Yu-Ming Li.
Data curation: Xin Zhou, Yan Dong.
Formal analysis: Guo-Hong Yang, Xin Zhou.
Funding acquisition: Guo-Hong Yang, Yu-Ming Li.
Investigation: Jian-Qi Zhang, Xin Zhou, Yan Dong, Shao-Bo Chen.
Methodology: Guo-Hong Yang, Xin Zhou, Rui Shi, Yan Dong.
Project administration: Jun-Xiang Liu, Rui Shi, Shao-Bo Chen, Yu-Ming Li.
Resources: Jun-Xiang Liu, Shao-Bo Chen.
Software: Guo-Hong Yang, Rui Shi, Yan Dong.
Supervision: Jun-Xiang Liu, Shao-Bo Chen, Yu-Ming Li.
Validation: Rui Shi.
Visualization: Jun-Xiang Liu.
Writing – original draft: Jian-Qi Zhang, Guo-Hong Yang.
Writing – review & editing: Jian-Qi Zhang.
Yu-Ming Li orcid: 0000-0003-1981-8120.
Footnotes
Abbreviations: ABI = ankle-brachial index, ARBs = angiotensin II type 1 receptor antagonists, AT1 = angiotensin II type 1, AT1R = angiotensin II type-1 receptor, AT2R = angiotensin II type-2 receptor, ba-PWV = brachial-ankle pulse wave velocity, BP = blood pressure, BUN = blood urea nitrogen, CI = confidence interval, Cr = creatinine, DBP = diastolic blood pressure, ET = endothelin, GITS = gastrointestinal therapeutic system, HDLC = high density lipoprotein cholesterol, HR = hazard ratio, IL-6 = interleukin-6, IMCSA = carotid intima-media cross-sectional area, IMT = carotid intima-media thickness, IVST = interventricular septal thickness, LDLC = low density lipoprotein cholesterol, LVEDD = left ventricular end-diastolic diameter, LVEF = left ventricular ejection fraction, LVH = left ventricular hypertrophy, LVMI = left ventricular mass index, LVPWT = left ventricular posterior wall thickness, MA = urinary microalbumin, NO = nitric oxide, RAS = renin-angiotensin system, SBP = systolic blood pressure, SD = standard deviation, TNF-α = tumor necrosis factor-α.
JQZ and GHY contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (grant number 81600328), Tianjin Municipal Science and Technology Committee (grant numbers 16JCQNJC11800, 15ZXJZSY00010, 16ZXMJSY00130), and intramural research program from Logistics University of Chinese People's Armed Police Forces (grant numbers 2015ZXKF11, FYM201533).
The authors have no conflicts of interest to disclose.
References
- [1].Carrick D, Haig C, Maznyczka AM, et al. Hypertension, microvascular pathology, and prognosis after an acute myocardial infarction. Hypertension 2018;72:720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mendy A. Association of urinary nitrate with lower prevalence of hypertension and stroke and with reduced risk of cardiovascular mortality. Circulation 2018;137:2295–7. [DOI] [PubMed] [Google Scholar]
- [3].Nielsen PM, Grimm D, Wehland M, et al. The combination of valsartan and sacubitril in the treatment of hypertension and heart failure—an update. Basic Clin Pharmacol Toxicol 2018;122:9–18. [DOI] [PubMed] [Google Scholar]
- [4].Yan Z, Wang Y, Li S, et al. Hypertension control in adults with CKD in China: baseline results from the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). Am J Hypertens 2018;31:486–94. [DOI] [PubMed] [Google Scholar]
- [5].Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 2018;137:e67–492. [DOI] [PubMed] [Google Scholar]
- [6].Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:2199–269.29146533 [Google Scholar]
- [7].Lu Z, Chen Y, Li L, et al. Combination therapy of renin-angiotensin system inhibitors plus calcium channel blockers versus other two-drug combinations for hypertension: a systematic review and meta-analysis. J Hum Hypertens 2017;31:1–3. [DOI] [PubMed] [Google Scholar]
- [8].Uijl E, Danser AHJ. Renin-angiotensin-aldosterone system parameters as biomarker in heart failure patients with preserved ejection fraction: focus on angiotensinogen. Am J Hypertens 2018;31:175–7. [DOI] [PubMed] [Google Scholar]
- [9].Park H, Kim HK, Jeong MH, et al. Clinical impacts of inhibition of renin-angiotensin system in patients with acute ST-segment elevation myocardial infarction who underwent successful late percutaneous coronary intervention. J Cardiol 2017;69:216–21. [DOI] [PubMed] [Google Scholar]
- [10].Ruiz-Rosado JD, Lee YU, Mahler N, et al. Angiotensin II receptor I blockade prevents stenosis of tissue engineered vascular grafts. FASEB J 2018;32:6822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Eguchi S, Kawai T, Scalia R, et al. Understanding angiotensin II type 1 receptor signaling in vascular pathophysiology. Hypertension 2018;71:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Savina NM. Prevention of cardiovascular complications in patients with arterial hypertension while use of angiotensin II receptor antagonists. Possibilities of candesartan. Kardiologiia 2016;56:63–7. [DOI] [PubMed] [Google Scholar]
- [13].Tadevosyan A, Maclaughlin EJ, Karamyan VT. Angiotensin II type 1 receptor antagonists in the treatment of hypertension in elderly patients: focus on patient outcomes. Patient Relat Outcome Meas 2011;2:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wu MY, Ma XJ, Yang C, et al. Effects of allisartan, a new AT(1) receptor blocker, on blood pressure and end-organ damage in hypertensive animals. Acta Pharmacol Sin 2009;30:307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Amini H, Ahmadiani A, Moazenzadeh M. Pharmacokinetics of losartan and its active metabolite EXP3174 in healthy Iranian subjects. Clin Drug Investig 2004;24:619–23. [DOI] [PubMed] [Google Scholar]
- [16].Zhao Q, Wei J, Zhang H. Effects of quercetin on the pharmacokinetics of losartan and its metabolite EXP3174 in rats. Xenobiotica 2018;1–6. [DOI] [PubMed] [Google Scholar]
- [17].Li H, Liu L, Xie L, et al. Effects of berberine on the pharmacokinetics of losartan and its metabolite EXP3174 in rats and its mechanism. Pharm Biol 2016;54:2886–94. [DOI] [PubMed] [Google Scholar]
- [18].Sun J, Yang GH, Liu JX, et al. Discordance between VASP phosphorylation and platelet aggregation in defining high on-clopidogrel platelet reactivity after ST-segment elevation myocardial infarction. Clin Appl Thromb Hemost 2018;24:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang GH, Zhou X, Ji WJ, et al. Effects of a low salt diet on isolated systolic hypertension: a community-based population study. Medicine (Baltimore) 2018;97:e0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jafary FH. Devereux formula for left ventricular mass—be careful to use the right units of measurement. J Am Soc Echocardiogr 2007;20:783. [DOI] [PubMed] [Google Scholar]
- [21].Schulte-Altedorneburg G, Droste DW, Felszeghy S, et al. Accuracy of in vivo carotid B-mode ultrasound compared with pathological analysis: intima-media thickening, lumen diameter, and cross-sectional area. Stroke 2001;32:1520–4. [DOI] [PubMed] [Google Scholar]
- [22].Yamaki M, Sato T, Fujii H. Lower ankle-brachial index is associated with poor sleep quality in patients with essential hypertension. Am J Cardiovasc Dis 2015;5:77–82. [PMC free article] [PubMed] [Google Scholar]
- [23].Takase H, Dohi Y, Toriyama T, et al. Brachial-ankle pulse wave velocity predicts increase in blood pressure and onset of hypertension. Am J Hypertens 2011;24:667–73. [DOI] [PubMed] [Google Scholar]
- [24].Oluwatowoju IO, Ajuluchukwu JN, Afonja OA. Clinical usefulness of a timed overnight (8 hours) Urine Albumin (microalbumin) excretion in monitoring treatment in benign essential hypertension. Niger Postgrad Med J 2014;21:177–80. [PubMed] [Google Scholar]
- [25].Nessler J, Nessler B, Kitlinski M, et al. Concentration of BNP, endothelin 1, pro-inflammatory cytokines (TNF-alpha, IL-6) and exercise capacity in patients with heart failure treated with carvedilol. Kardiol Pol 2008;66:144–51. discussion 52-3. [PubMed] [Google Scholar]
- [26].Bellien J, Iacob M, Remy-Jouet I, et al. Epoxyeicosatrienoic acids contribute with altered nitric oxide and endothelin-1 pathways to conduit artery endothelial dysfunction in essential hypertension. Circulation 2012;125:1266–75. [DOI] [PubMed] [Google Scholar]
- [27].Zhong B. How to calculate sample size in randomized controlled trial? J Thorac Dis 2009;1:51–4. [PMC free article] [PubMed] [Google Scholar]
- [28].Zheng H, Li N, Ding Y, et al. Losartan alleviates hyperuricemia-induced atherosclerosis in a rabbit model. Int J Clin Exp Pathol 2015;8:10428–35. [PMC free article] [PubMed] [Google Scholar]
- [29].Jin H, Zhang HN, Hou XL, et al. Clinical study of double dose of valsartan combined with tacrolimus in treatment of diabetic nephropathy. Eur Rev Med Pharmacol Sci 2016;20:174–9. [PubMed] [Google Scholar]
- [30].Marinsek M, Sinkovic A. Ramipril and losartan exert a similar long-term effect upon markers of heart failure, endogenous fibrinolysis, and platelet aggregation in survivors of ST-elevation myocardial infarction: a single centre randomized trial. Biomed Res Int 2016;2016:9040457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li Y, Li XH, Huang ZJ, et al. A randomized, double blind, placebo-controlled, multicenter phase II trial of Allisartan Isoproxil in essential hypertensive population at low-medium risk. PLoS One 2015;10:e0117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering on outcome incidence in hypertension: 5. Head-to-head comparisons of various classes of antihypertensive drugs - overview and meta-analyses. J Hypertens 2015;33:1321–41. [DOI] [PubMed] [Google Scholar]
- [33].Yasunari K, Maeda K, Watanabe T, et al. Comparative effects of valsartan versus amlodipine on left ventricular mass and reactive oxygen species formation by monocytes in hypertensive patients with left ventricular hypertrophy. J Am Coll Cardiol 2004;43:2116–23. [DOI] [PubMed] [Google Scholar]
- [34].Kucukler N, Kurt IH, Topaloglu C, et al. The effect of valsartan on left ventricular myocardial functions in hypertensive patients with left ventricular hypertrophy. J Cardiovasc Med (Hagerstown) 2012;13:181–6. [DOI] [PubMed] [Google Scholar]
- [35].Wong M, Staszewsky L, Latini R, et al. Severity of left ventricular remodeling defines outcomes and response to therapy in heart failure: valsartan heart failure trial (Val-HeFT) echocardiographic data. J Am Coll Cardiol 2004;43:2022–7. [DOI] [PubMed] [Google Scholar]
- [36].Thomopoulos C, Parati G, Zanchetti A. Effects of blood-pressure-lowering treatment on outcome incidence. 12. Effects in individuals with high-normal and normal blood pressure: overview and meta-analyses of randomized trials. J Hypertens 2017;35:2150–60. [DOI] [PubMed] [Google Scholar]
- [37].Menne J, Izzo JL, Jr, Ito S, et al. Prevention of microalbuminuria in patients with type 2 diabetes and hypertension. J Hypertens 2012;30:811–8. discussion 18. [DOI] [PubMed] [Google Scholar]
- [38].Asselbergs FW, Diercks GF, Hillege HL, et al. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation 2004;110:2809–16. [DOI] [PubMed] [Google Scholar]
- [39].Ghiadoni L, Virdis A, Magagna A, et al. Effect of the angiotensin II type 1 receptor blocker candesartan on endothelial function in patients with essential hypertension. Hypertension 2000;35:501–6. [DOI] [PubMed] [Google Scholar]
- [40].Suzuki R, Fukuda N, Katakawa M, et al. Effects of an angiotensin II receptor blocker on the impaired function of endothelial progenitor cells in patients with essential hypertension. Am J Hypertens 2014;27:695–701. [DOI] [PubMed] [Google Scholar]
- [41].Benetos A, Laurent S, Asmar RG, et al. Large artery stiffness in hypertension. J Hypertens Suppl 1997;15:S89–97. [DOI] [PubMed] [Google Scholar]
- [42].Vriz O, Bertin N, Ius A, et al. Carotid artery stiffness and development of hypertension in people with paraplegia and no overt cardiovascular disease: a 7-year follow-up study. J Cardiovasc Echogr 2017;27:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Blacher J, Safar ME. Large-artery stiffness, hypertension and cardiovascular risk in older patients. Nat Clin Pract Cardiovasc Med 2005;2:450–5. [DOI] [PubMed] [Google Scholar]
- [44].Park JB, Intengan HD, Schiffrin EL. Reduction of resistance artery stiffness by treatment with the AT(1)-receptor antagonist losartan in essential hypertension. J Renin Angiotensin Aldosterone Syst 2000;1:40–5. [DOI] [PubMed] [Google Scholar]
- [45].Cadeddu C, Piras A, Mantovani G, et al. Protective effects of the angiotensin II receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress, and early ventricular impairment. Am Heart J 2010;160:487.e1–7. [DOI] [PubMed] [Google Scholar]
- [46].Dahiya R, Shultz SP, Dahiya A, et al. Relation of reduced preclinical left ventricular diastolic function and cardiac remodeling in overweight youth to insulin resistance and inflammation. Am J Cardiol 2015;115:1222–8. [DOI] [PubMed] [Google Scholar]
- [47].Zhang C, Tan Y, Guo W, et al. Attenuation of diabetes-induced renal dysfunction by multiple exposures to low-dose radiation is associated with the suppression of systemic and renal inflammation. Am J Physiol Endocrinol Metab 2009;297:E1366–77. [DOI] [PubMed] [Google Scholar]
- [48].Li JJ, Chen JL. Inflammation may be a bridge connecting hypertension and atherosclerosis. Med Hypotheses 2005;64:925–9. [DOI] [PubMed] [Google Scholar]


