Abstract
Because rates of skin cancer are greater among adult survivors of childhood cancer who received radiation therapy than the general population, the National Cancer Institute recommends skin selfexaminations and annual physician examination. There has been no comprehensive assessment of survivor’s adherence with skin cancer screening guidelines associated with skin self-examination (SSE) and physician whole-body skin examination (PSE). We conducted a cross-sectional survey of radiation-treated, adult five-year survivors of childhood cancer, diagnosed between 1970-1986, in the Childhood Cancer Survivor Study (CCSS) cohort. Multivariate multinomial logit regression investigated the association between demographic, cancer diagnosis, patient activation, cancer treatment characteristics, and skin cancer screening practice. Among 728 survivors, 13.1% reported having had performed SSE in the prior 2 months plus received PSE in the prior 12 months, while 16.4% and 11.0% reported having had only a SSE or a PSE, respectively; 59.5% of survivors reported having had neither. Participants at the highest patient activation score were most likely to report SSE+PSE compared with neither (aRRR 4.16, 95% CI 1.34-12.85). Most adult survivors of childhood cancer who underwent radiation therapy do not practice strategies that promote early detection of skin cancer. Interventions designed to activate survivors to increase screening are needed.
Keywords: skin cancer, childhood cancer, survivor, radiation, early detection, patient activation
Introduction
With improvements in therapy designed to increase survival and reduce late mortality there are now greater than 420,000 adult survivors of childhood cancer in the United States (Robison and Hudson, 2014, Armstrong et al, 2016). Advances in the diagnosis and treatment of children with cancer have contributed to an overall five-year survival rate that currently exceeds 80%. 3 However, survivors are at risk for late effects of childhood cancer treatment, including an increased risk for subsequent neoplasms; most frequently occurring within a radiation field. (Turcotte et al, 2017)
Skin cancers, primarily basal cell carcinomas (BCCs), are the most common subsequent neoplasm, representing an estimated 58% of subsequent cancers. (Watt et al 2012, Friedman et al 2010, Braam et al, 2010, Perkins et al, 2005), Adult survivors of childhood cancer under age 35 years who were treated with radiation have nearly a 40-fold increased risk for non-melanoma skin cancer and nearly a 2.5-fold higher risk for melanoma compared with the general population (Watt et al, 2012, Pappo et al, 2013)
Because of the high rates of skin cancer among childhood cancer survivors, in April 2012 and again in April 2016, the National Cancer Institute released a PDQ® (evidence-based data summary) recommending the use of an annual dermatological exam to screen for early-onset skin cancer (National Cancer Institute, 2012 and National Cancer Institute 2016). Additionally, the Children’s Oncology Group, a National Cancer Institute supported clinical trials consortium, in its Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers , endorsed screening for early-onset skin cancer, recommending an annual dermatological exam focusing on skin lesions and pigmented nevi in the radiation field, monthly skin self-examination, and adherence to behaviors protecting the skin from excessive ultraviolet radiation exposure (Landier et al, 2004). Despite these clear risks and recommendations, in a previous study of survivor’s skin cancer early detection practices conducted in 2009, fewer than 30 percent of adult survivors of childhood cancer had received a physician skin cancer exam; there was no information on skin self-examination (Buchanan et al, 2009). Thus, it appears that there is a large gap between recently stated guidelines and early detection practices of this at-risk population.
We describe the prevalence of and factors associated with performance of a skin self-examination (SSE) in the last two months and receipt of a physician whole-body skin exam (PSE) in the last 12 months among a population of radiation-treated adult survivors of childhood cancer. These results are reported from the baseline survey of a large randomized intervention trial designed to improve skin cancer early detection rates. We also explored whether patient activation variables, awareness of basic warning signs of skin cancer, risk perception, and self-efficacy to perform a skin self-examination, were cross-sectionally associated with performance of SSE and/or PSE.
Results
Among 728 ASK participants, the median age at enrollment was 44 years (range 30-65 years), median age at diagnosis of childhood cancer was 7 years (range 0-20 years) and time from diagnosis to participation in the study was a median of 36 years (range 29-46 years). Forty-seven percent were male, 70% had at least a college degree and 73% had fair or very fair skin. Ninety-two percent were white. There were no differences between the participants (n=728) and non- participants (n=474) with the exception that participants had higher risks of post-graduate education (aRRR 1.48, 95% CI 1.09-2.01) (Table 1). Of the 709 survivors with non-missing outcome information, 13.1% reported having SSE+PSE, while 16.4% and 11.0% only reported having had a SSE only and PSE only, respectively; 59.5% of survivors reported not having had either.
Table 1.
Baseline demographic and treatment characteristics of participants and non-participants
| Overall | Non-participants | Participants | Odds Ratio for Participation |
||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | OR | 95% CI | ||
| Total | 1202 | 474 | 39.4 | 728 | 60.6 | ||
| Sex | |||||||
| Female | 635 | 246 | 51.9 | 389 | 53.4 | 1.00 | REF |
| Male | 567 | 228 | 48.1 | 339 | 46.6 | 0.93 | (0.73, 1.18) |
| Age at diagnosis, years | |||||||
| 0-4 | 462 | 193 | 40.7 | 269 | 37.0 | 1.00 | REF |
| 5-9 | 268 | 103 | 21.7 | 165 | 22.7 | 1.13 | (0.79, 1.63) |
| 10-14 | 256 | 97 | 20.5 | 159 | 21.8 | 0.98 | (0.62, 1.56) |
| 15+ | 216 | 81 | 17.1 | 135 | 18.5 | 0.91 | (0.52, 1.58) |
| Age at invitation, years | |||||||
| 29-34 | 124 | 49 | 10.3 | 75 | 10.3 | 1.00 | REF |
| 35-39 | 253 | 100 | 21.1 | 153 | 21.0 | 0.88 | (0.55, 1.42) |
| 40-44 | 317 | 130 | 27.4 | 187 | 25.7 | 0.73 | (0.45, 1.20) |
| 45-49 | 276 | 111 | 23.4 | 165 | 22.7 | 0.70 | (0.40, 1.24) |
| 50-54 | 157 | 59 | 12.4 | 98 | 13.5 | 0.74 | (0.38, 1.44) |
| 55+ | 75 | 25 | 5.3 | 50 | 6.9 | 0.89 | (0.40, 1.96) |
| Education | |||||||
| High School or less | 107 | 45 | 9.5 | 62 | 8.5 | 0.93 | (0.6, 1.45) |
| Some College | 278 | 126 | 26.6 | 152 | 20.9 | 1.00 | REF |
| College Graduate | 510 | 205 | 43.2 | 305 | 41.9 | 0.82 | (0.61, 1.11) |
| Post-Graduate | 307 | 98 | 20.7 | 209 | 28.7 | 1.48 | (1.09, 2.01) |
| Race/ethnicity | |||||||
| White, Non-Hispanic | 1091 | 419 | 88.4 | 672 | 92.3 | 1.00 | REF |
| Black, Non-Hispanic | 38 | 16 | 3.4 | 22 | 3.0 | 0.92 | (0.47, 1.80) |
| Hispanic | 44 | 23 | 4.9 | 21 | 2.9 | 0.60 | (0.32, 1.11) |
| Asian, Non-Hispanic | 20 | 11 | 2.3 | 9 | 1.2 | 0.47 | (0.19, 1.19) |
| Other | 4 | 2 | 0.4 | 2 | 0.3 | 0.70 | (0.09, 5.25) |
| Missing | 5 | 3 | 0.6 | 2 | 0.3 | 0.35 | (0.05, 2.32) |
| Diagnosis | |||||||
| Bone cancer | 64 | 21 | 4.4 | 43 | 5.9 | 1.00 | REF |
| Central Nervous System | 92 | 44 | 9.3 | 48 | 6.6 | 0.51 | (0.24, 1.07) |
| Hodgkin lymphoma | 218 | 68 | 14.3 | 150 | 20.6 | 0.98 | (0.52, 1.85) |
| Wilms tumor | 182 | 78 | 16.5 | 104 | 14.3 | 0.52 | (0.25, 1.06) |
| Leukemia | 350 | 147 | 31.0 | 203 | 27.9 | 0.56 | (0.29, 1.10) |
| Neuroblastoma | 77 | 34 | 7.2 | 43 | 5.9 | 0.46 | (0.20, 1.02) |
| Non-Hodgkin lymphoma | 108 | 40 | 8.4 | 68 | 9.3 | 0.72 | (0.35, 1.46) |
| Soft tissue Sarcoma | 111 | 42 | 8.9 | 69 | 9.5 | 0.85 | (0.43, 1.67) |
| Max RT dose in cGy | |||||||
| < 2000 | 301 | 122 | 25.7 | 179 | 24.6 | 1.00 | REF |
| 2000-3999 | 535 | 203 | 42.8 | 332 | 45.6 | 0.99 | (0.72, 1.38) |
| 4000+ | 323 | 128 | 27.0 | 195 | 26.8 | 0.75 | (0.45, 1.25) |
| Unknown | 43 | 21 | 4.4 | 22 | 3.0 | 0.70 | (0.35, 1.40) |
| Chemotherapy | |||||||
| No | 387 | 153 | 32.3 | 234 | 32.1 | 1.00 | REF |
| Yes | 790 | 310 | 65.4 | 480 | 65.9 | 0.90 | (0.67, 1.20) |
| Unknown | 25 | 11 | 2.3 | 14 | 1.9 | 0.73 | (0.31, 1.72) |
| Number of chronic conditions | |||||||
| 0 | 227 | 112 | 23.6 | 115 | 15.8 | 1.00 | REF |
| 1-2 | 532 | 198 | 41.8 | 334 | 45.9 | 1.56 | (1.13, 2.16) |
| 3-4 | 443 | 164 | 34.6 | 279 | 38.3 | 1.56 | (1.10, 2.21) |
Factors associated with skin cancer practice
Female survivors were more likely than males to complete SSE (aRRR 1.72, 95% CI 1.04-2.82) and survivors with older age (ages 55+)(aRRR 28.33 (95% CI 3.7-217.15) and a postgraduate education were more likely to have a PSE than those with a high school education (aRRR 3.63, 95% CI 1.24-10.66) (Table 2) . Notably, survivors who received higher doses of radiation (20-39 Gy, and ≥40Gy) did not have statistically significant increases in SSE, PSE or both, compared to those with <20 Gy. However, patients with the highest level of activation had an increased likelihood of SSE (aRRR 3.83, 95% CI 1.47-10) and SSE+PSE (aRRR 4.16, 95% CI 1.34-12.85).
Table 2.
Baseline demographic and treatment characteristics and associations with performance of Skin Self-Exam (SSE) and Physician Skin Exam (PSE)
| N | SSE vs Neither |
PSE vs Neither |
Both vs Neither |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| aRRR* | 95% CI | aRRR | 95% CI | aRRR | 95% CI | |||
| Sex | ||||||||
| Male | 339 | 1.00 | REF | 1.00 | REF | 1.00 | REF | 0.19 |
| Female | 389 | 1.72 | (1.04, 2.82) | 1.05 | (0.6, 1.85) | 1.24 | (0.74, 2.08) | |
| Education | ||||||||
| High School or less | 78 | 1.00 | REF | 1.00 | REF | 1.00 | REF | 0.001 |
| Some College | 160 | 1.17 | (0.51, 2.70) | 0.64 | (0.19, 2.16) | 1.29 | (0.45, 3.74) | |
| College Graduate | 303 | 1.14 | (0.53, 2.46) | 1.53 | (0.53, 4.41) | 2.15 | (0.81, 5.67) | |
| Post-Graduate | 187 | 0.75 | (0.31, 1.81) | 3.63 | (1.24, 10.66) | 2.43 | (0.87, 6.82) | |
| Age at survey, years | ||||||||
| < 35 | 70 | 1.00 | REF | 1.00 | REF | 1.00 | REF | 0.006 |
| 35-39 | 155 | 0.65 | (0.27, 1.60) | 3.48 | (0.69, 17.63) | 0.92 | (0.37, 2.31) | |
| 40-44 | 180 | 0.51 | (0.19, 1.37) | 5.44 | (1.07, 27.61) | 0.88 | (0.32, 2.38) | |
| 45-49 | 164 | 0.63 | (0.21, 1.90) | 12.97 | (2.34, 71.84) | 1.39 | (0.45, 4.27) | |
| 50-54 | 103 | 0.80 | (0.23, 2.80) | 18.41 | (2.87, 118.23) | 1.56 | (0.39, 6.22) | |
| 55+ | 53 | 0.44 | (0.10, 1.94) | 28.33 | (3.7, 217.15) | 3.33 | (0.72, 15.43) | |
| Race | ||||||||
| White | 672 | 1.00 | REF | 1.00 | REF | 1.00 | REF | 0.02 |
| Non-White** | 52 | 0.92 | (0.37, 2.32) | 1.04 | (0.3, 3.58) | 0.72 | (0.23, 2.27) | |
| Skin type | ||||||||
| Very fair/Fair | 530 | 1.00 | REF | 1.00 | REF | 1.00 | REF | < 0.001 |
| Olive/Dark/Very Dark | 184 | 1.34 | (0.79, 2.28) | 0.55 | (0.27, 1.14) | 0.79 | (0.42, 1.48) | |
| Age at diagnosis, years | ||||||||
| 0-4 | 269 | 1.00 | REF | 1.00 | REF | 1.00 | REF | 0.19 |
| 5-9 | 165 | 1.82 | (0.87, 3.81) | 1.73 | (0.73, 4.1) | 1.04 | (0.49, 2.21) | |
| 10-14 | 159 | 1.57 | (0.62, 3.93) | 1.24 | (0.44, 3.5) | 0.69 | (0.27, 1.78) | |
| 15+ | 135 | 1.45 | (0.49, 4.29) | 0.50 | (0.14, 1.81) | 0.32 | (0.1, 1.1) | |
| Diagnosis | ||||||||
| Bone cancer | 43 | 1.00 | REF | 1.00 | REF | 1.00 | REF | 0.07 |
| Central Nervous System | 48 | 1.92 | (0.4, 9.28) | 7.25 | (1.25, 42.23) | 1.69 | (0.33, 8.73) | |
| Hodgkin lymphoma | 150 | 2.85 | (0.8, 10.2) | 3.54 | (0.83, 15.16) | 1.91 | (0.51, 7.16) | |
| Wilms tumor | 104 | 1.65 | (0.36, 7.53) | 6.67 | (1.24, 36.02) | 2.07 | (0.44, 9.69) | |
| Leukemia | 203 | 4.15 | (1.05, 16.43) | 3.14 | (0.65, 15.26) | 5.14 | (1.23, 21.44) | |
| Neuroblastoma | 43 | 2.04 | (0.36, 11.51) | 8.30 | (1.33, 51.96) | 1.98 | (0.34, 11.4) | |
| Non-Hodgkin lymphoma | 68 | 4.14 | (1.02, 16.81) | 3.57 | (0.7, 18.31) | 3.23 | (0.72, 14.42) | |
| Soft tissue sarcoma | 69 | 1.60 | (0.42, 6.13) | 1.11 | (0.19, 6.42) | 1.14 | (0.28, 4.6) | |
| Chemotherapy | ||||||||
| No | 234 | 1.00 | REF | 1.00 | REF | 1.00 | REF | < 0.001 |
| Yes | 480 | 1.23 | (0.68, 2.23) | 1.57 | (0.82, 3.03) | 1.54 | (0.82, 2.89) | |
|
Highest CTCAE Grade Chronic Condition | ||||||||
| 0 | 115 | 1.00 | REF | 1.00 | REF | 1.00 | REF | 0.27 |
| 1-2 | 334 | 0.94 | (0.46, 1.94) | 0.48 | (0.22, 1.05) | 0.71 | (0.36, 1.43) | |
| 3-4 | 279 | 1.18 | (0.56, 2.51) | 0.90 | (0.41, 1.98) | 0.65 | (0.3, 1.38) | |
| Max RT dose in cGy | ||||||||
| < 2000 | 179 | 1.00 | REF | 1.00 | REF | 1.00 | REF | < 0.001 |
| 2000-3999 | 332 | 0.86 | (0.45, 1.65) | 0.70 | (0.34, 1.44) | 1.12 | (0.57, 2.18) | |
| 4,000+ | 195 | 1.64 | (0.6, 4.5) | 0.85 | (0.28, 2.57) | 3.08 | (1, 9.5) | |
| Patient activation measure | ||||||||
| 1 | 71 | 1.00 | REF | 1.00 | REF | 1.00 | REF | < 0.001 |
| 2 | 99 | 1.17 | (0.36, 3.82) | 1.77 | (0.56, 5.65) | 2.95 | (0.85, 10.22) | |
| 3 | 308 | 2.79 | (1.08, 7.22) | 1.30 | (0.47, 3.6) | 2.91 | (0.95, 8.96) | |
| 4 | 234 | 3.83 | (1.47, 10) | 1.69 | (0.61, 4.71) | 4.16 | (1.34, 12.85) | |
Adjusted relative risk ratio- Sex, education, age (years), race/ethnicity, skin type, age at diagnosis, diagnosis, chemotherapy (yes/no), Highest CTCAE Grade Chronic Condition, maximum radiotherapy dose, patient activation
White, Non- Hispanic, Black Non-Hispanic, Hispanic, Asian, Non-Hispanic
Prior and recent history of skin cancer screening
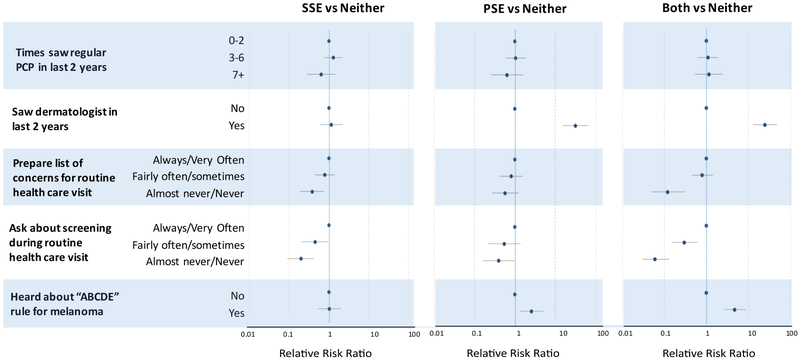
Having seen a dermatologist in the past two years was associated with increased PSE (aRRR 31.79, 95% CI 15.43-65.5) and SSE+PSE (aRRR 24.91, 95% CI 12.99-47.8). (Figure 1, e-table 1). Only 26% of patients routinely prepared a list of questions for their physicians at a routine visit and only 13% asked about cancer screenings that they may need (52% almost never or never do so) (data not shown). Participants who almost never or never prepared a list of concerns for routine health care visits were less likely to report SSE only (aRRR 0.38, 95% CI 0.19-0.73) or SSE+PSE (aRRR 0.12, 95% CI .05-0.31.) Likewise, those who almost never/never asked about screening during routine health care visits were least likely to report SSE only (aRRR 0.20, 95% CI 0.09-0.42) or SSE+PSE (aRRR 0.06, 95% CI 0.03-0.13). Physician exams were provided to only 36 of the 580 (6.2%) people who did not ask for an exam, while of the 141 participants who reported requesting such an exam, 137 received one (97.2%)(data not shown).
Figure 1 – Prior and recent history of routine health visit and performance of SSE and PSE.
Adjusted for sex, education, age (years), race/ethnicity, skin type, age at diagnosis, diagnosis, chemotherapy (yes/no), Highest CTCAE Grade Chronic Condition, maximum radiotherapy dose, patient activation
e-Table 1.
Baseline demographic and treatment characteristics of ParticiPants and non-participants
| N |
SSE vs Neither |
PSE vs Neither |
Both vs Neither |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| aRRR* | 95% CI | aRRR | 95% CI | aRRR | 95% CI | |||
| Number of times saw regular PCP in last 2 years | ||||||||
| 0-2 | 297 | 1.00 | REF | 1.00 | REF | 1.00 | REF | 0.55 |
| 3-6 | 326 | 1.29 | (0.78,2.14) | 1.05 | (0.59,1.85) | 1.10 | (0.63,1.89) | |
| 7+ | 105 | 0.64 | (0.28,1.42) | 0.64 | (0.26,1.6) | 1.16 | (0.54,2.47) | |
| Saw dermatologist in last 2 years | ||||||||
| No | 512 | 1.00 | REF | 1.00 | REF | 1.00 | REF | < 0.001 |
| Yes | 215 | 1.14 | (0.6,2.17) | 31.79 | (15.43,65.5) | 24.91 | (12.99,47.8) | |
| Asked HCP/Derm to check whole body in last 12 months | ||||||||
| No | 583 | 1.00 | REF | 1.00 | REF | 1.00 | REF | < 0.001 |
| Yes | 141 | 2.28 | (0.19,27.07) | 1331.33 | (234.24,7566.66) | 2370.27 | (405.84,13843.3) | |
| Prepare list of concerns for routine health care visit | ||||||||
| Always/Very often | 192 | 1.00 | REF | 1.00 | REF | 1.00 | REF | < 0.001 |
| Fairly often/Sometimes | 317 | 0.79 | (0.45,1.39) | 0.81 | (0.42,1.55) | 0.80 | (0.45,1.42) | |
| Almost never/Never | 219 | 0.38 | (0.19,0.73) | 0.57 | (0.27,1.21) | 0.12 | (0.05,0.31) | |
| Ask about screening during routine health care visit | ||||||||
| Always/Very often | 92 | 1.00 | REF | 1.00 | REF | 1.00 | REF | < 0.001 |
| Fairly often/Sometimes | 259 | 0.45 | (0.21,0.96) | 0.55 | (0.22,1.37) | 0.30 | (0.15,0.62) | |
| Almost never/Never | 377 | 0.20 | (0.09,0.42) | 0.40 | (0.16,0.98) | 0.06 | (0.03,0.13) | |
| Heard about "ABCDE" rule for melanoma? | ||||||||
| No | 578 | 1.00 | REF | 1.00 | REF | 1.00 | REF | < 0.001 |
| Yes | 145 | 1.03 | (0.53,2.01) | 2.59 | (1.32,5.1) | 4.72 | (2.57,8.66) | |
Adjusted for sex, education, age (years), race/ethnicity, skin type, age at diagnosis, diagnosis, chemotherapy (yes/no), Highest CTCAE Grade Chronic Condition, maximum radiotherapy dose, patient activation.
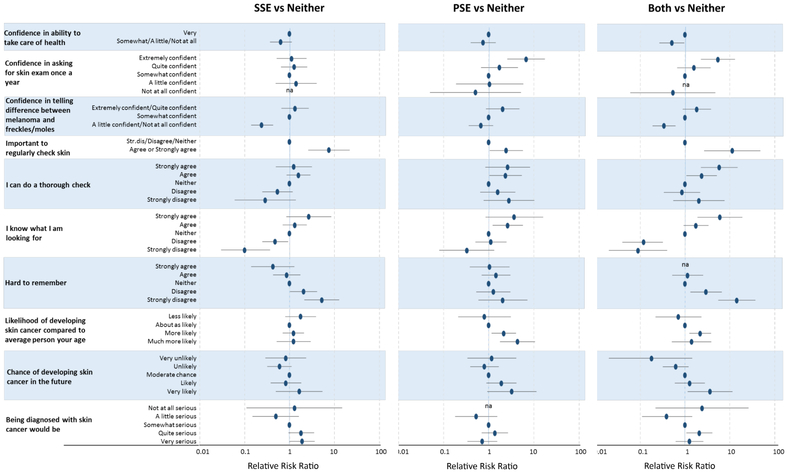
Risk perception, self-efficacy, and current SSE and PSE practices
Being very confident in asking for a skin exam once a year was highly associated with PSE only (aRRR 6.76, 95% CI 2.59-17.63) or SSE+PSE (aRRR 5.43, 95% CI 2.26-13.04); (Figure 2, e-Table 2). Understanding that it was important to regularly check the skin (aRRR 11.31, 95% CI 2.67-47.87), strongly agreeing that one can do a thorough check (aRRR 5.80, 95% CI 2.26-14.87) and that one knows what to look for when examining the skin (aRRR 6.00, 95% CI 1.9=18.93) were all strongly associated with completing SSE+PSE.
Figure 2 – Risk perception, self-efficacy, and performance of SSE and PSE.
Adjusted for sex, education, age (years), race/ethnicity, skin type, age at diagnosis, diagnosis, chemotherapy (yes/no), Highest CTCAE Grade Chronic Condition maximum radiotherapy dose patient activation
e-Table 2.
Baseline demographic and treatment characteristics and associations with performance of Skin Self-Exam (SSE) and Physician Skin Exam (PSE)
| N |
SSE vs Neither |
PSE vs Neither |
Both vs Neither |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| aRRR* | 95% CI | aRRR | 95% CI | aRRR | 95% CI | |||
| Confidence in ability to take care of health | ||||||||
| Very | 474 | 1.00 | REF | 1.00 | REF | 1.00 | REF | 0.09 |
| Somewhat/A little/Not at all | 252 | 0.64 | (0.37,1.12) | 0.75 | (0.4,1.43) | 0.51 | (0.27,0.96) | |
| Confidence in asking for skin exam once a year | ||||||||
| Extremely confident | 221 | 1.12 | (0.52,2.4) | 6.76 | (2.59,17.63) | 5.43 | (2.26,13.04) | |
| Quite confident | 305 | 1.27 | (0.65,2.49) | 1.74 | (0.68,4.45) | 1.58 | (0.67,3.73) | |
| Somewhat confident | 127 | 1.00 | REF | 1.00 | REF | 1.00 | REF | < 0.001 |
| A little confident | 43 | 1.40 | (0.49,4) | 1.04 | (0.19,5.85) | NA | NA | |
| Not at all confident | 24 | NA | NA | 0.51 | (0.05,5.18) | 0.54 | (0.06,4.74) | |
|
Confidence in telling difference between melanoma and freckles/moles | ||||||||
| Extremely confident/Quite confident | 106 | 1.33 | (0.66,2.67) | 2.05 | (0.87,4.8) | 1.84 | (0.89,3.81) | |
| Somewhat confident | 209 | 1.00 | REF | 1.00 | REF | 1.00 | REF | < 0.001 |
| A little confident/Not at all confident | 407 | 0.24 | (0.14,0.43) | 0.67 | (0.36,1.25) | 0.34 | (0.19,0.61) | |
| Important to regularly check skin | ||||||||
| Strongly disagree/Disagree/Neither | 115 | 1.00 | REF | 1.00 | REF | 1.00 | REF | < 0.001 |
| Agree or Strongly agree | 605 | 7.54 | (2.61,21.79) | 2.45 | (1.05,5.74) | 11.31 | (2.67,47.87) | |
| I can do a thorough check | ||||||||
| Strongly agree | 76 | 1.25 | (0.5,3.15) | 2.62 | (0.84,8.18) | 5.80 | (2.26,14.87) | |
| Agree | 283 | 1.59 | (0.86,2.91) | 2.37 | (1.04,5.43) | 2.35 | (1.08,5.14) | |
| Neither | 149 | 1.00 | REF | 1.00 | REF | 1.00 | REF | < 0.001 |
| Disagree | 175 | 0.54 | (0.25,1.17) | 1.59 | (0.65,3.89) | 0.86 | (0.34,2.16) | |
| Strongly disagree | 37 | 0.29 | (0.06,1.38) | 2.80 | (0.77,10.2) | 2.04 | (0.55,7.63) | |
| I know what I am looking for | ||||||||
| Strongly agree | 38 | 2.68 | (0.85,8.46) | 3.68 | (0.85,16.02) | 6.00 | (1.9,18.93) | |
| Agree | 215 | 1.31 | (0.71,2.43) | 2.64 | (1.22,5.7) | 1.76 | (0.93,3.33) | |
| Neither | 166 | 1.00 | REF | 1.00 | REF | 1.00 | REF | < 0.001 |
| Disagree | 214 | 0.48 | (0.25,0.93) | 1.12 | (0.51,2.48) | 0.12 | (0.04,0.32) | |
| Strongly disagree | 88 | 0.10 | (0.03,0.37) | 0.33 | (0.08,1.33) | 0.09 | (0.02,0.4) | |
| Hard to remember | ||||||||
| Strongly agree | 65 | 0.43 | (0.14,1.28) | 1.04 | (0.38,2.88) | 0.00 | (0,Inf) | |
| Agree | 240 | 0.87 | (0.43,1.74) | 1.46 | (0.7,3.01) | 1.14 | (0.52,2.52) | |
| Neither | 167 | 1.00 | REF | 1.00 | REF | 1.00 | REF | < 0.001 |
| Disagree | 165 | 2.06 | (1.03,4.13) | 1.27 | (0.53,3.01) | 2.95 | (1.32,6.6) | |
| Strongly disagree | 79 | 5.24 | (2.14,12.8) | 2.05 | (0.59,7.16) | 14.33 | (5.53,37.14) | |
| Likelihood of developing skin cancer compared to average person your age | ||||||||
| Less likely | 74 | 1.77 | (0.81,3.9) | 0.80 | (0.21,3.1) | 0.71 | (0.22,2.31) | |
| About as likely | 289 | 1.00 | REF | 1.00 | REF | 1.00 | REF | 0.005 |
| More likely | 294 | 1.23 | (0.71,2.11) | 2.16 | (1.16,4.04) | 2.18 | (1.25,3.8) | |
| Much more likely | 69 | 1.23 | (0.52,2.96) | 4.35 | (1.79,10.58) | 1.40 | (0.51,3.86) | |
| Chance of developing skin cancer in the future | ||||||||
| Very unlikely | 42 | 0.83 | (0.29,2.34) | 1.17 | (0.34,4.07) | 0.18 | (0.02,1.44) | |
| Unlikely | 166 | 0.60 | (0.32,1.11) | 0.80 | (0.39,1.66) | 0.62 | (0.32,1.2) | |
| Moderate chance | 391 | 1.00 | REF | 1.00 | REF | 1.00 | REF | 0.03 |
| Likely | 95 | 0.83 | (0.38,1.83) | 1.91 | (0.89,4.12) | 1.28 | (0.59,2.76) | |
| Very likely | 29 | 1.65 | (0.5,5.39) | 3.25 | (0.93,11.38) | 3.62 | (1.15,11.42) | |
| Being diagnosed with skin cancer would be | ||||||||
| Not at all serious | 6 | 1.30 | (0.11,14.83) | NA | NA | 2.40 | (0.22,26.03) | |
| A little serious | 60 | 0.50 | (0.15,1.61) | 0.53 | (0.18,1.54) | 0.39 | (0.11,1.45) | |
| Somewhat serious | 189 | 1.00 | REF | 1.00 | REF | 1.00 | REF | 0.003 |
| Quite serious | 246 | 1.82 | (0.95,3.51) | 1.37 | (0.7,2.68) | 2.10 | (1.08,4.07) | |
| Very serious | 223 | 1.91 | (1,3.65) | 0.72 | (0.34,1.54) | 1.26 | (0.62,2.56) | |
Adjusted for variables in Table 2- Sex, education, age (years), race/ethnicity, skin type, age at diagnosis, diagnosis, chemotherapy (yes/no), Highest CTCAE Grade Chronic Condition, maximum radiotherapy dose, patient activation.
Ninety-eight percent of participants had insurance. More than 90% of participants had ever had a blood pressure measurement and a Pap smear. Seventy-seven percent had a flu shot while 71% of female participants had a mammogram. With the four-level skin exam outcome, we included whether they had a flu shot in the multivariable multinomial regression and found it to not be statistically significant (p=0.993). We also evaluated the contribution of having a mammogram and found it to not be statistically significant (p=0.314). Only 10% of the participants indicated having seen a cancer survivorship care provider in the past two years.
Discussion
We provide the first report from the CCSS cohort to evaluate rates of SSE and PSE practices of adult survivors of childhood cancer since the publication and dissemination of recommendations for periodic screening. Despite clear guidance provided in these recommendations, 59.5% of survivors, not previously diagnosed with skin cancer, reported not having had either SSE or PSE. This report extends our knowledge of skin cancer screening practices of childhood cancer survivors by providing rates of SSE and factors associated with SSE+PSE not previously known and identifies major gaps in screening that remain despite the release of NCI and COG guidance to screen survivors for skin cancer.
While PSE rates were low among ASK participants (24.1% when PSE only and SSE+PSE are considered together), they were higher than those of the general US population, estimated to be 16% for PSE (Coups et al, 2010), providing some indication of greater early detection awareness among this subset of survivors. Likewise, SSE was considered performed if it were the only early detection practice or also done with PSE, rates for SSE were 29%, higher than general population rates of 18% in the only other large-scale study that asked about skin self-exams during the prior two months (Martin et al, 2007) However, given that radiation-treated survivors are a high-risk population for subsequent skin cancers, these data provide evidence that efforts are needed to improve screening rates.
Skin cancers, primarily basal cell carcinomas (BCCs), are the most common subsequent neoplasm among adult survivors of childhood cancer. However, there are no data on the clinical presentation and aggressiveness of BCCs among adult survivors of childhood cancer compared to that in the general population. Because of the disproportionate burden of skin cancer among this population, the early detection of BCCs may be particularly important for adult survivors of childhood cancer. Among individuals in the general population, cutaneous BCCs have poorer outcomes if treated when the diameter is large (Kricker et al, 2014, Chinem and Miot 2011, Weinstock and Still 2011, Hassanpour et al, 2006, Brown and Rzucidlo 2011, Allison, 1984). For example, Kricker et al. found that larger BCCs were identified among patients without a physician skin exam (Kricker et al, 2014) and concluded that earlier diagnosis of BCCs, perhaps through physician skin exams, may result in smaller size, earlier detection, and improved outcomes.
Skin cancer presents a unique opportunity as it and its precursors can be seen by the patient, their providers, and significant others (Koh et al, 1992, Berwick et al 1996, American Academy of Dermatology, 2012, Abbasi et al 2004, Geller et al, 2004). Therefore, professional and public educational programs that work to teach SSE and encourage patients to alert their physicians to skin changes provides a key opportunity for education, activation, and early detection. Thorough skin self-examination, although only practiced by 15% of participants, reduced mortality due to melanoma by an estimated 60% in one major case-control study (Berwick et al, 1996). The American Academy of Dermatology has recommended the practice of SSE to detect new and or changing lesions (American Academy of Dermatology, 2012) and individuals are encouraged to perform SSEs regularly (for example, monthly) using the ABCDE (Asymmetry, Border, Color, Diameter, Evolution) algorithm (Abbasi et al, 2004). A national study of US physicians indicated that they were more inclined to screen when requested to do so by their patients (Geller et al, 2004), as suggested herein.
Among the many factors associated with less than optimal adherence to SSE and PSE, we identified gaps in patient activation. Related to health care visits in general, few patients routinely prepared a list of questions for their physicians at a routine visit and fewer still asked about cancer screenings that they may need (52% almost never or never do so). Conversely, the fact that patients who frequently prepared a list of concerns for their routine health care visit were far more likely to do SSE and receive a PSE speaks to the importance of patient education and activation. Furthermore, the fact that nearly all participants who asked for an exam received one indicated the positive effects of a model that supports and encourages patient activation. Activated patients who are prepared to take a key role, such as asking their physician for a whole-body skin exam, typically receive better care, have better health outcomes and reduced health care costs (Hibbard et al, 2007).
Of note, for the planning of future interventions, female survivors were more likely than males to complete SSE; older survivors and those with a postgraduate education were more likely to have a PSE than those with a high school education. While conducting a physician skin examination of men, physicians can use the opportunity to counsel about the importance of the complementary practice of examining one’s skin for changes in any component of the ABCDE rule noted above. Interestingly, participants with higher doses of radiation were no more likely to practice early detection indicating future pathways for targeted education.
Early detection of skin cancer requires a three-pronged approach: patient vigilance in observing for changes in moles and lesions, patient request of a skin exam, and physician examination and referral. However, surveys of high-risk patients rarely examine factors associated with SSE+PSE. Notably, we found that many factors associated with SSE were also relevant for PSE providing an indication that educational efforts to raise awareness could improve both practices. This study also revealed higher rates of SSE and PSE for patients who saw the importance of a regular skin check, reported confidence in doing a skin check, and were more likely to report that they knew what to look for when examining their skin.
Limitations
A number of limitations should be considered. First, participants may not be representative of the larger survivor population with respect to early detection of skin cancer. However, eligible survivors in the CCSS cohort are largely similar in their distribution of key demographic characteristics to survivors in the population-based SEER database (Phillips et al, 2015). Second, our results must be interpreted within the context of a single cross-sectional analysis. Thus, there is always the possibility that for some participants their practice of the skin cancer examination preceded their reported confidence for doing the exam rather than confidence being an antecedent to screening. However, the associations provided in our analysis may be helpful in understanding and exploring avenues for intervention development. Finally, the outcomes SSE and PSE are self-reported without verification of the physician visit.
Conclusion
Despite the fact that survivors of childhood cancer who received radiation therapy are at high risk for skin cancer, we identified low rates of SSE and PSE. However, higher rates of SSE+PSE were observed among the most activated participants. Many findings from the baseline study, including the role of patient activation were fundamental to the ongoing randomized, controlled trial designed to enhance skin self-examination and increase requests for a physician examination of the skin.
Methods
Participants in the CCSS exposed to radiation therapy were invited to participate in a randomized intervention trial entitled Advancing Survivors’ Knowledge about Skin Cancer (ASK), designed to improve performance of SSE and PSE for skin cancer. Eligibility criteria included: (1) current age of 18 years or older; (2) treated with radiation for childhood cancer between 1970 and 1986; (3) having seen their primary care physician or oncologist in the previous two years or planning to do so in the next year; (4) having no previous history of a skin cancer diagnosis, and (5) access to a smartphone. Overall, 1,202 CCSS participants were eligible for the trial of whom 728 (61%) provided informed consent and completed the baseline survey between March 2015 and July 2016. For those who completed the survey by paper, written, informed patient consent was provided; for those online, participants would click a box indicating their consent, and phone respondents used a consent script and then documented the consent in the CCSS database.
The study () protocol was reviewed and approved by the Institutional Review Board of Harvard TH Chan School of Public Health and St. Jude Children’s Research Hospital. Datasets related to this article can be found at https://ccss.stjude.org/ and accessed through collaboration with the Childhood Cancer Survivor Study. The data cannot be posted for confidentiality reasons but researchers would be free to contact the data source (i.e. CCSS) and the code can be provided if asked for.
Outcome Variables
The primary outcome was a four-level “skin cancer practice” indicator of whether the patient reported: neither a SSE in the prior 2 months nor a PSE in the prior 12 months; a SSE in the prior 2 months only; a PSE in the prior 12 months only; or, both. SSE was assessed via the question: “How many times in the past two months have you carefully checked your whole body (including the skin on your back and back of your legs) for any sign of skin cancer?” Responses included never, once, and 2 or more times, with the latter two levels combined to give responses of never vs at least once. PSE was assessed by the question: “In the past 12 months, has your regular health care provider or your dermatologist carefully examined your whole body for any sign of skin cancer?” Responses were yes or no.
Covariates
For this analysis, a range of factors derived from conceptual models for early detection of skin cancer (Swetter et al, 2012, Pollitt et al, 2009) were considered, including: demographics and physical features (sex, age at diagnosis, age at survey, education, race/ethnicity and color of participant’s untanned skin ), skin cancer-related encounters with physicians and self-detection (saw dermatologist in last 2 years, asked physician to check whole body in past 12 months, patient activation score (assessed by the 13 point Patient Activation Measure (Hibbard et al 2005) that gauged the knowledge, skills, and confidence in managing one’s health scored from 1 to 4 with 4 as the highest) and whether the patient prepared a list of questions for the physician or asked about cancer screenings that they may need; awareness: (heard about ABCDE rule for melanoma); risk perception (likelihood of developing skin cancer compared to average person your age, the likelihood of developing skin cancer in the future, seriousness of a skin cancer diagnosis); self-efficacy (confidence in doing a thorough check, knowing what type of moles to look for), confidence in ability to take care of one’s health, in asking for physician skin exam once a year, in telling the difference between melanoma and freckles/moles, and in knowing the importance of regularly checking one’s skin.
Primary childhood cancer diagnosis and chemotherapy and radiotherapy exposures were identified through detailed medical record abstraction. For participants who underwent radiotherapy, the maximum tumor dose (maxTD) for seven body regions (brain, other head, neck, chest, abdomen, pelvis, and extremities) was determined. The maxTD was defined as the maximum prescribed dose within each region, which is taken as the total prescribed dose from all overlapping fields within the treated region. (Stovall et al, 2006) Participants were classified according to maximum radiation dose received to any body region (classified as <20, 20-39, and >=40 GY).. Chronic conditions were graded and categorized as 0, 1-2 (mild or moderate) and 3-4 (severe, disabling, or life-threatening) according to the Common Terminology Criteria for Adverse Events (version 4.02) (National Cancer Institute 2010, Oeffinger 2006).
Statistical Analysis
To assess the generalizability of results based on patients enrolled in ASK, multiple logistic regression analyses were conducted to investigate differences between participants and non-participants. Total counts were calculated for each independent measure across possible responses of the four-level skin cancer practice outcome. A multiple multinomial baseline category logit regression was then used to investigate the associations between demographic, cancer diagnosis, and cancer treatment variables, and skin cancer practice (Faraway 2016). For this model, three sets of adjusted relative risk ratios (aRRR) are reported, one for each of the outcome levels “SSE only”, “PSE only” and “Both”, with the “Neither SSE nor PSE” used as the baseline outcome category. To interpret the aRRR consider the relationship between sex and the four-level outcome. Specifically consider the risk of “SSE only” relative to “Neither SSE nor PSE”: RR(female) = P(SSE only ∣ female)/ P(Neither SSE nor PSE only ∣ female). Furthermore consider the corresponding relative risk for males: RR(male) = P(SSE only ∣ male)/ P(Neither SSE nor PSE only ∣ male). The aRRR indicates the extent to which these risk ratios differ relative to each other. If, for example, the aRRR > 1.0, then the risk ratio for “SSE only” vs “Neither” is higher for females than for males; females exhibit an increased skin cancer practice preference for “SSE only” versus “Neither” compared to males. Correspondingly, if the female aRRR for the “PSE only” outcome category is < 1.0, then females express decreased preference for “PSE only” versus “Neither” compared to males. Finally, if the female aRRR for the “Both” outcome category equals 1.0, then females express neither increased nor decreased preference for “Both” versus “Neither” compared to males. A series of multinomial logit models were fit to investigate a broad range of skin cancer related behavioral factors by additionally including them one at a time into the main multivariable model. There was some missingness in the outcome (n=19) and select covariates (range n=0 to n=22). Given the small degree of missingness, complete-case analyses were conducted and reported.
All statistical analyses were performed in the R version 3.4 (R Core Team). Throughout, associations between risk factors and the four-level outcome measure were evaluated for statistical significance via a likelihood ratio test.
Acknowledgments
Funding: This work was supported by the National Cancer Institute (CA55727, G.T. Armstrong, Principal Investigator; 1 R01 CA175231, A. Geller, Principal Investigator). Support to St. Jude Children’s Research Hospital also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese-Syrian Associated Charities (ALSAC).
Glossary
- BCC
Basal Cell Cancer
- PSE
Physician Skin Examination
- SSE
Skin Self-Examination
- ASK
Advancing Survivors’ Knowledge about Skin Cancer
Footnotes
Work performed at the Harvard TH Chan School of Public Health, Boston Massachusetts and St Jude Children's Research Hospital, Memphis Tennessee,
Conflicts of Interest
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alan C. Geller, Department of Social and Behavioral Sciences, Harvard TH Chan School of Public Health, Boston MA.
Robyn R. Keske, Department of Social and Behavioral Sciences, Harvard TH Chan School of Public Health, Boston MA.
Sebastien Haneuse, Department of Biostatistics, Harvard TH Chan School of Public Health, Boston MA.
Jessica A. Davine, Department of Social and Behavioral Sciences, Harvard TH Chan School of Public Health, Boston MA.
Karen M. Emmons, Department of Social and Behavioral Sciences, Harvard TH Chan School of Public Health, Boston MA.
Casey L. Daniel, Department of Social and Behavioral Sciences, Harvard TH Chan School of Public Health, Boston MA.
Todd M. Gibson, Department of Epidemiology and Cancer Control, St Jude Children's, Research Hospital, Memphis, TN.
Ashfaq Marghoob, Department of Dermatology, Memorial Sloan Kettering Cancer Center, NYC, NY.
Ann C. Mertens, Department of Pediatrics at Emory University School of Medicine in Atlanta, GA.
Aaron J. McDonald, Department of Epidemiology and Cancer Control, St Jude Children's, Research Hospital, Memphis, TN.
Leslie L. Robison, Department of Epidemiology and Cancer Control, St Jude Children's, Research Hospital, Memphis, TN.
Rebecca M. Howell, Department of Radiation Physics, The University of Texas at MD Anderson Cancer Center, Houston, Texas.
John A. Whitton, Fred Hutchinson Cancer Research Center, Seattle WA.
Adina Coroiu, Department of Social and Behavioral Sciences, Harvard TH Chan School of Public Health, Boston MA.
Wendy M. Leisenring, Fred Hutchinson Cancer Research Center, Seattle WA.
Gregory T. Armstrong, Department of Epidemiology and Cancer Control, St Jude Children's, Research Hospital, Memphis, TN.
References
- Abbasi NR, Shaw HM, Rigel DS, et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA 2004; 292:2771–6. Review [DOI] [PubMed] [Google Scholar]
- Allison JR Jr., Radiation-induced basal-cell carcinoma. J Dermatol Surg Oncol 1984;10:200–3. [DOI] [PubMed] [Google Scholar]
- American Academy of Dermatology; 2012. SPOT Skin Cancer [Website]. Washington (DC): [cited 2012 May 19]. Available from: http://www.aad.org/spot-skin-cancer. [Google Scholar]
- Armstrong GT Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, et al. Reduction in late mortality among 5-Year survivors of childhood cancer. N Engl Med 2016;374:833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick M, Begg CB, Fine JA, Roush GC, Barnhill RL. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst 1996;88:17–23. [DOI] [PubMed] [Google Scholar]
- Braam KI, Overbeek A, Kaspers GJ, Ronckers CM, Schouten-van Meeteren AY, Van Dulmen-Den Broeder E, et al. Malignant melanoma as second malignant neoplasm in long-term childhood cancer survivors: a systematic review. Pediatr Blood Cancer 2012;5:665–74. [DOI] [PubMed] [Google Scholar]
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg 2011. ;53(1 Suppl): 15S–21S. [DOI] [PubMed] [Google Scholar]
- Buchanan N, Leisenring W, Mitby PA, Meadows AT, Robison LL, Hudson MM, et al. Behaviors associated with ultraviolet radiation exposure in a cohort of adult survivors of childhood and adolescent cancer: a report from the Childhood Cancer Survivor Study. Cancer 2009;115(18 Suppl):4374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinem VP, Miot HA. Epidemiology of basal cell carcinoma. An Bras Dermatol 2011;86:292–305. [DOI] [PubMed] [Google Scholar]
- Coups EJ, Geller AC, Weinstock MA, Heckman CJ, Manne SL. Prevalence and correlates of skin cancer screening among middle-aged and older white adults in the United States. Am J Med 2010; 123:439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraway JJ. (2016). Extending the linear model with R: generalized linear, mixed effects and nonparametric regression models (Vol. 124). CRC press. [Google Scholar]
- Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 2010;102:1083–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller AC, O'Riordan DL, Oliveria SA, Valvo S, Teich M, Halpern AC. Overcoming obstacles to skin cancer examinations and prevention counseling for high-risk patients: results of a national survey of primary care physicians. J Am Board Fam Pract. 2004. Nov-Dec;17:416–23. [DOI] [PubMed] [Google Scholar]
- Hassanpour SE, Kalantar-Hormozi A, Motamed S, Moosavizadeh SM, Shahverdiani R. Basal cell carcinoma of scalp in patients with history of childhood therapeutic radiation: a retrospective study and comparison to nonirradiated patients. Ann Plast Surg 2006;57:509–12. [DOI] [PubMed] [Google Scholar]
- Hibbard JH, Mahoney ER, Stockard J. Development and testing of a short form of the patient activation measure. Health Serv Res 2005; 40:1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbard JH, Mahoney ER, Stock R, Tusler M. Do increases in patient activation result in improved self-management behaviors? Health Serv Res 2007;42:1443–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh HK, Miller DR, Geller AC, Clapp RW, Mercer MB, Lew RA. Who discovers melanoma? Patterns from a population-based survey. J Am Acad Dermatol 1992;26:914–9. [DOI] [PubMed] [Google Scholar]
- Kricker A, Armstrong B, Hansen V, Watson A, Singh-Khaira G, Lecathelinais C, et al. Basal cell carcinoma and squamous cell carcinoma growth rates and determinants of size in community patients. J Am Acad Dermatol 2014; 70:456–64. [DOI] [PubMed] [Google Scholar]
- Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol 2004;22: 4979–4990. [DOI] [PubMed] [Google Scholar]
- Martin RA, Weinstock MA, Risica PM, Smith K, Rakowski W. Factors associated with thorough skin self-examination for the early detection of melanoma. J Eur Acad Dermatol Venerol 2007; 21:1074–81. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute; 2012. Late Effects of Treatment for Childhood Cancer (PDQ®) [Website]. Bethesda, MD:. Available from: http://www.cancer. gov/cancertopics/pdq/treatment/lateeffects/HealthProfessional/page2. Accessed January 15, 2019 [Google Scholar]
- National Cancer Institute re-release. http://www. cancer. gov/types/childhood-cancers/late-effects-hp-pdq#section/_116. Accessed January 15, 2019
- National Cancer Institute. Common Terminology Criteria for Adverse Events v4.03. In: NCI, National Institutes of Health, Department of Health and Human Services, ed. 2010. [Google Scholar]
- Oeffinger KC, Mertens AC, Sklar CA, Yasui Y, Fears T, Stovall M, et al. Chronic health conditions in adult survivors of childhood cancer. New Engl J Med 2006;355:1572–1582. [DOI] [PubMed] [Google Scholar]
- Pappo AS, Armstrong GT, Liu W, Srivastava DK, McDonald A, Leisenring WM, et al. Melanoma as a subsequent neoplasm in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Pediatric Blood Cancer 2013; 60:461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins JL, Liu Y, Mitby PA, Neglia JP, Hammond S, Stovall M, et al. Nonmelanoma skin cancer in survivors of childhood and adolescent cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2005;23:3733–41. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Padgett LS, Leisenring WM, Stratton KK, Bishop K, Krull KR, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev;.2015; 24:653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt RA, Geller AC, Brooks DR, Johnson TM, Park ER, Swetter SM. Efficacy of skin self-examination practices for early melanoma detection. Cancer Epidemiol Biomarkers Prev 2009; 18:3018–23. [DOI] [PubMed] [Google Scholar]
- R Core Team (2017). R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 2014;14:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stovall M, Weathers R, Kasper C, Smith SA, Travis L, Ron E, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res 2006;166(1 Pt 2):141–57. [DOI] [PubMed] [Google Scholar]
- Swetter SM, Pollitt RA, Johnson TM, Brooks DR, Geller AC. Behavioral determinants of successful early melanoma detection: role of self and physician skin examination. Cancer 2012; 118; 3725–34. [DOI] [PubMed] [Google Scholar]
- Turcotte LM, Liu Q, Yasui Y, Arnold MA, Hammond S, Smith SA, et al. Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970-2015. JAMA 2017; 317:814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt TC, Inskip PD, Stratton K, Smith SA, Kry SF, Sigurdson AJ, et al. Radiation-related risk of basal cell carcinoma: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 2012;104:1240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock MA, Still JM. Assessing current treatment options for patients with severe/advanced basal cell carcinoma. Seminars in Cutaneous Medicine and Surgery. 2011;30(4 Suppl):S10–3. [DOI] [PubMed] [Google Scholar]