Abstract
Auditory P300 oddball and novel components index working memory operations and salience processing, respectively, and are regarded as biomarkers of neurocognitive changes in both chronic and first-episode schizophrenia. Much less is known about whether P300 abnormalities exist in individuals at clinical high risk for psychosis (CHR) and if they are predictors of both transition to psychosis and remission from symptoms. One hundred and four CHR and 69 healthy control individuals (HC) completed P300 oddball paradigm, and 131 CHR and 69 HC subjects completed P300 novel paradigm. All CHR subjects were followed up for one year and stratified into CHR converters (CHR-C) and non-converters (CHR-NC), with CHR-NC further stratified into remitted and non-remitted subgroups. Between-group comparisons of P300 oddball and novel amplitude and latency were performed among CHR-C, CHR-NC and HC, as well as among CHR-C, non-remitted CHR, remitted CHR and HC. CHR converters had lower fronto-central P300 novel amplitude as well as marginally lower P300 oddball amplitude relative to HC. When CHR non-converters were stratified into remitted and non-remitted subgroups, P300 novel amplitude in remitted CHR subjects was comparable to HC, and it was higher than that in CHR subjects who converted to psychosis or who did not remit. Thus, reduced P300 novel amplitude indexing impaired salience processing marked both conversion to psychosis and remission from psychotic symptoms.
Keywords: Clinical high risk for psychosis, P300 novel, P300 oddball, transition to psychosis, remission from psychotic symptoms
Introduction
P300 oddball component, an index of working memory operations has been widely investigated in schizophrenia (Polich, 2007). Decreased P300 oddball amplitude and prolonged latency are consistently reported in chronic (Jeon and Polich, 2003; Javitt et al., 2008), first-episode (McCarley et al., 2002; Wang et al., 2010; del Re et al., 2015) schizophrenia patients and in their first-degree relatives (Turetsky et al., 2009; Kim et al., 2017). The P300 abnormality, associated with gray matter volume reduction of the left superior temporal gyrus, has been suggested to be an important pathophysiological feature of psychosis (McCarley et al., 2002; Wang et al., 2010; Fusar-Poli et al., 2011a).
The presence of reduced P300 amplitude in first-episode schizophrenia motivated research to examine P300 in individuals at clinical high risk (CHR) for developing psychosis in order to investigate when the P300 abnormality appears initially. CHR individuals are defined clinically by meeting Structured Interview for Prodromal Criteria (SIPS) criteria for the Brief Intermittent Psychotic Symptom Prodromal Syndrome, the Genetic Risk and Deterioration Prodromal Syndrome or the Attenuated Positive Symptom Prodromal Syndrome (Miller et al., 2002; Miller et al., 2003). Small to medium effect size declines in several cognitive functions including attention and working memory among others, are also common in CHR subjects, especially in those who convert into psychosis later (Seidman et al., 2016). However, evidence of P300 abnormalities in this population coming from large cohort studies is still limited (see Table 1) and somewhat mixed.
Table 1.
Overview of P300 oddball and novel studies in subject at clinical high risk for psychosis.
| Citation | Subjects | P300 Paradigm | ERP Measures | Results |
|---|---|---|---|---|
| van der Stelt et al. (2005) | 10 at-risk subjects, 14 recent-onset SZ, 14 CSZ patients and 14 HC | An active auditory oddball task; Pressing a button for target tones |
P300 oddball | Smaller P300 amplitude in high-risk subjects than HC, but comparable with recent-onset and chronic SZ patients. |
| Bramon et al. (2008) | 35 ARMS (7 ARMS-C and 28 ARMS-NC) and 57 HC; | An active auditory oddball task; Pressing a button for target tones |
P300 oddball and N100 | Smaller P300 amplitude in ARMS than HC; No significant differences of P300 amplitude between ARMS-C and ARMS-NC groups. |
| Ozgurdal et al. (2008) | 54 prodromal subjects, 31 EFP, 27 CSZ patients and 54 HC | An active auditory oddball task; Pressing a button for target tones |
P300 oddball | Smaller P300 amplitude in prodromal, FEP and CSZ patients than HC; Large P300 amplitude in prodromal FEP than CSZ patients. |
| Van Tricht et al (2010) | 61 UHR subjects and 28 HC; 18 UHR-Tand43UHR-NT | An active auditory oddball task; Counting target tones |
P300 oddball, N1, P2, N2b | Smaller P300 amplitude in UHR-T than UHR-NT and HC; Reduced P300 amplitude related to increased scores of social anhedonia and withdrawal; Smaller N2b in both UHR-T and UHR-NT than HC. |
| Fusar-Poli et al. (2011) | 39 ARMS subjects and 41 HC; 10 ARMS-C and 29 ARMS-NC | An active auditory oddball task; Pressing a button for target tones |
P300 oddball, gray matter volume | Smaller P300 amplitude in ARMS than HC; Positive correlation between P300 amplitude and gray matter loss of the right supramarginal gyrus/ superior temporal gyms. |
| Atkinson et al. (2012) | 30 UHR, 11 FEPand20HC | A passive auditory oddball task; | MMN, P3a | Smaller P3a amplitude in UHR than HC, but marginally smaller P3a amplitude in FEP than HC; Smaller MMN amplitude in both UHR and FEP than HC. |
| Jahshan et al. (2012) | 26 prodromal patients, 31 recent-onset SZ patients, 33 CSZ patients and 28 HC | A passive auditory oddball task | MMN, P3a, RON | Intermediate P3a and MMN amplitude of prodromal patients between those of HC and recent-onset group; Reduced RON in recent-onset and chronic groups than HC. |
| Mondragon-Maya et al. (2013) | 23CHR, 20FEPand24HC | A passive auditory oddball task | MMN, P3a | Smaller P3a amplitude in FEP and CHR than HC; No significant group difference on MMN amplitude. |
| Nieman et al. (2014) | 61 CHR subjects (18 CHR-C and 43 CHR-NC) | An active auditory oddball task; Counting target tones | P300 oddball | A decrease of P300 oddball amplitude of 1 uv related to an increase of the relative risk of developing a first psychotic disorder of 27% |
| Higuchi et al. (2014) | 19 ARMS, 19 FEP, 19 chronic SZ patients and 19 HC; 4 ARMS-C and 15ARMS-NC | A passive auditory oddball task | MMN, P3a, RON | No significant difference of P3a amplitude between ARMS and HC. Smaller MMN amplitude in ARMS-C than both ARMS-NC and HC while no differences between ARMS-NC and HC; Reduced RON in FEP and chronic SZ patients than HC, but not in ARMS. |
| del Re et al. (2015) | 21 CHR, 20 FEP and 25 HC; | An active auditory oddball task and an active auditory novel task | P300 oddball, P300 novel, N100, P200 | Smaller P300 oddball amplitude in CHR and FEP groups than HC; Smaller P300 novel amplitude in CHR and FEP groups than HC. |
| Kim et al. (2015) | 45 CHR non-converters; 19 CHR-R and 26 CHR-NR after 2-year follow-up | An active auditory oddball task; Pressing a button for target tones | P300 oddball | No significant differences of oddball P300 amplitude between CHR-R and CHR-NR; P300 oddball amplitude associated with improvement in negative and general symptoms. |
| Atkinson et al. (2017) | 80UHRand58HC; 7 UHR-T after 1-year follow-up | A passive auditory oddball task | MMN, P3a | No significant differences of either MMN or P3a between UHR and HC. |
| Kim et al. (2018) | 32 CHR, 32 GHR, 42 SZ and 42 HC | An active auditory oddball task; Pressing a button for target tones | P300 oddball, inter-trial variability | Smaller P300 oddball amplitude in CHR, GHR and SZ than HC; |
ARMS, subjects at-risk for mental state; ARMS-C, ARMS subjects who made a transition to psychosis; ARMS-NC, ARMS subjects who did not make a transition to psychosis; CHR, clinical high risk for psychosis; CHR-R, CHR subjects who remitted; CHR-NR, CHR subjects who did not remit; CSZ, chronic patients with schizophrenia; FEP, patients with first-episode psychosis; GHR, genetic high-risk for psychosis; MMN, Mismatch negativity; RON, reorienting negativity; UHR, subjects at ultra-high risk for psychosis; UHR-T, UHR subjects who made a transition to psychosis; UHR-NT, UHR subjects who did not make a transition to psychosis.
Some CHR studies suggested that subtle reduction of P300 oddball amplitude precede the onset of psychosis (van der Stelt et al., 2005; Bramon et al., 2008; Ozgurdal et al., 2008; van Tricht et al., 2010; Fusar-Poli et al., 2011b, a). Van Tricht et al. (van Tricht et al., 2011) first reported lower P300 amplitude in CHR converters than non-converters, whereas Kim et al. (Kim et al., 2015) found no P300 group differences between CHR remitters and non-remitters. Nieman et al. (Nieman et al., 2014) improved an individual risk estimation developed in this study after adding P300 oddball amplitude and premorbid adjustment to the model.
In addition to reports of abnormal P300 oddball, a frontally distributed P300 novel component elicited by novel, non-target sounds and indexing salience processing (Friedman et al., 2001), has also been found to be reduced in chronic schizophrenia (Mathalon et al., 2000; Jahshan et al., 2012) and anti-psychotic naïve first-episode psychosis (Mondragon-Maya et al., 2013; del Re et al., 2015). Likewise, smaller P300 novel amplitude was observed in CHR subjects relative to healthy controls (HC) (Atkinson et al., 2012; Jahshan et al., 2012). In contrast, Higuchi et al. (2014) found no differences in P300 novel amplitude between CHR subjects and HC, but four CHR subjects who converted to psychosis had smaller P300 novel amplitude than non-converters. In addition, Atkinson et al. (Atkinson et al., 2017) did not find P300 novel reduction in a group of ultra-high risk individuals.
To further establish whether both sets of neurocognitive processes indexed by these two P300 components or only one of them is abnormal in CHR, both the P300 oddball and novel need to be examined in the same CHR cohort, which is rarely done in the previous literature. A previous cross-sectional study suggested that P300 oddball and novel abnormalities differed across the course of psychosis and that the P300 novel may be more sensitive to prediction of a diagnostic group (del Re et al., 2015). Mathalon et al. (Mathalon et al., 2000) found that P300 oddball reduction tracked positive symptoms while P300 novel reduction tracked mood symptoms in schizophrenia patients.
Importantly, only approximately 20–35% of CHR individuals will develop psychosis at 2-year follow up (Yung and McGorry, 1996; Fusar-Poli et al., 2012; Fusar-Poli et al., 2015; Cannon et al., 2016). Therefore, it is of interest to examine whether P300 at the pre-psychosis stage can predict the conversion to psychosis. Further, among the majority of CHR subjects who will not convert to full-blown psychosis, not all are doing equally well (Addington et al., 2017). Thus, it is worth exploring whether P300 can be an index distinguishing those who will remit from psychotic symptoms from those who will not, so as to guide the development of effective interventions. Both cross-sectional and longitudinal studies with large samples examining these questions are lacking and, to the best of our knowledge, studies contrasting P300 as an index of both transition to psychosis and of remission using large sample of subjects are non-existent.
The aim of the present longitudinal study has been twofold: we set out to address a question of both P300 oddball and novel abnormalities as predictors of transition to psychosis as well as predictors of remission from psychotic symptoms as documented one year later. Accordingly, two sets of analyses were performed in a relatively large sample of Chinese CHR cohort (most drug-naive) from the Shanghai At Risk for Psychosis (SHARP) program: (1) between-group comparisons with CHR converters (CHR-C), CHR non-converters and HC, and (2) between group comparisons with CHR-C, non-remitted CHR, remitted CHR and HC, respectively. We hypothesized that: (1) CHR subjects, in particular CHR-C subjects, would have smaller P300 oddball and novel amplitudes and/or longer latencies than HC; (2) For CHR who did not convert to psychosis, either both P300 oddball and novel components or only one of them would differentiate between CHR subjects who remitted from psychotic symptoms from those who did not.
Materials and methods
Subjects
All CHR and HC subjects were from the SHARP program, and were assessed as described in detail in previous publications (Zhang et al., 2014; Zhang et al., 2018). The study protocol and consent form were reviewed and approved by the local Ethics Committee at Shanghai Mental Health Center and the Beth Israel Deaconess Medical Center (Boston, Massachusetts). Written informed consent was obtained from each participant. All procedures complied with the Helsinki Declaration. Shanghai Mental Health Center is one of the largest psychiatric hospitals in China with its psychiatry clinic receiving over 800 000 visits a year. Prospective participants are recruited from this pool of clients. The initial screening for CHR was conducted using self-report Prodromal Questionnaire-Brief version (PQ-B) (Loewy et al., 2011). For individuals who screened positive for CHR, the inclusion criteria for CHR syndrome were assessed with the Chinese version of the SIPS and the Scale of Prodromal Syndromes (SOPS) in a face-to-face interview (Zheng et al., 2012). Healthy controls were recruited through online advisements and interviewed using the SIPS. They were excluded if they met DSM-IV criteria for a psychotic disorder, or SIPS criteria for a CHR syndrome. The exclusion criteria for both CHR and HC subjects included: head injury with loss of consciousness of any duration, and any history of substance use, neurological disease, severe somatic diseases, mental retardation, and / or dementia.
One hundred and four CHR subjects and 69 HCs completed the P300 oddball paradigm: 19 CHR subjects (18%) used an anti-psychotic (18) or anti-depressant (1) medication (Table S2 in the Supplement). One hundred and thirty-one CHR subjects and 69 HCs completed the P300 novel paradigm: 21 CHR subjects (16%) used anti-psychotic (20) or anti-depressant (1) medication.
To examine whether the P300 oddball and P300 novel at baseline predict conversion to psychosis, all CHR subjects were followed-up for one year to assess whether they converted to psychosis or not, as defined by the criteria for the Presence of Psychotic Symptoms (POPS) in SIPS (McGlashan et al., 2010; Zhang et al., 2016). CHR subjects were divided into two subgroups based on their clinical outcome at one-year follow up: (1) CHR-C who had at least one of the SIPS positive symptoms scale rated “6”; (2) CHR-NC whose positive symptoms scale rated less than “6”. Demographic and clinical characteristics are summarized in Table 2.
Table 2.
Demographic characteristics and neurocognitive performances for CHR converters, CHR non-converters and HC.
| CHR converters |
CHR Non-converters |
Healthy controls |
Statistical significance |
|
|---|---|---|---|---|
| Samples completed the P300 oddball paradigm | ||||
| Cases | 19 | 85 | 69 | / |
| Age (years) | 19.7±5.3 | 18.3±4.7 | 18.4±3.5 | F(2,170)=0.84, p=0.43 |
| Gender (M/F) | 12/7 | 43/42 | 32/37 | X2=1.68, p=0.43 |
| Education (years) | 10.8±2.5 | 10.40±2.83 | 10.8±2.2 | F(2,170)=0.49, p=0.62 |
| IQ* | 95.5±13.2 | 101.8±11.9 | 103.0±11.5 | F(2,149)=2.44, p=0.09 |
| Medicated | 7/19 | 12/19 | / | / |
| SoP_T | 53.1±9.2 | 56.2±7.3 | 60.4±7.0 | F(2,159)=9.13, p<0.001 |
| AV_T | 47.2±11.6 | 51.5±8.6 | 52.6±8.3 | F(2,159)=2.47, p=0.09 |
| WM_T | 45.3±10.0 | 46.1±9.5 | 51.7±9.2 | F(2,159)=8.09, p<0.001 |
| Samples completed the P300 novel paradigm | ||||
| Cases | 23 | 108 | 69 | / |
| Age (years) | 19.7±5.5 | 18.6±5.0 | 18.5±3.7 | F(2,197)=0.62, p=0.54 |
| Gender (M/F) | 14/9 | 53/55 | 32/37 | X2=1.47, p=0.48 |
| Education (years) | 10.5±2.8 | 10.5±2.9 | 10.8±2.2 | F(2,197)=0.33, p=0.72 |
| IQ** | 91.9±18.9 | 99.6±12.6 | 104.0±10.1 | F(2,173)=6.74, p<0.01 |
| Medicated | 7/21 | 14/21 | / | / |
| SoP_T | 51.3±10.2 | 54.4±7.3 | 60.0±6.8 | F(2,186)=14.14, p<0.001 |
| AV_T | 45.5±11.4 | 51.0±9.1 | 52.3±8.1 | F(2,186)=4.31, p<0.05 |
| WM_T | 42.6±8.5 | 45.2±10.2 | 50.8±9.6 | F(2,186)=8.68, p<0.001 |
In samples who completed the P300 oddball paradigm, 15 CHR converters, 75 CHR non-converters and 62 HC performed IQ tests; 16 CHR converters, 80 CHR non-converters and 66 HC completed MCCB tests.
In samples who completed the P300 novel paradigm, 17 CHR converters, 96 CHR non-converters and 63 HC performed IQ tests; 19 CHR converters, 103 CHR non-converters and 67HC completed MCCB tests.
In addition, to examine whether the P300 oddball and P300 novel index remission, CHR non-converters were further divided into two subgroups: (1) non-remitted CHR subjects who still met the CHR criteria; (2) remitted CHR subjects whose positive symptoms improved and who did not meet CHR criteria anymore. Demographic and clinical characteristics are summarized in Table 3.
Table 3.
Demographic, clinical characteristics and neurocognitive performances among CHR converters, non-remitted CHR and remitted CHR subjects.
| CHR converters |
Non-remitted CHR |
Remitted CHR |
Statistical significance |
|
|---|---|---|---|---|
| Samples completed the P300 oddball paradigm | ||||
| Cases | 19 | 32 | 53 | / |
| Age (years) | 19.7±5.3 | 18.7±5.16 | 18.0±4.5 | F(2,101)=0.86, p=0.43 |
| Gender (M/F) | 12/7 | 17/15 | 26/27 | X2=1.12, p=0.57 |
| Education | 10.8±2.5 | 10.4±3.1 | 10.4±2.7 | F(2,101)=0.15, p=0.86 |
| IQ | 95.5±13.2 | 103.3±13.2 | 101.0±11.1 | F(2,87)=2..00, p=0.14 |
| DUP (months) | 4.4±3.9 | 5.2±4.0 | 6.2±4.5 | F(2,101)=1.44, p=0.24 |
| Medicated | 7/19 | 4/19 | 8/19 | / |
| SOPS_P | 9.7±3.0 | 10.2±3.4 | 9.4±3.4 | F(2,101)=0.56, p=0.57 |
| SOPS_N | 13.11±6.5 | 11.5±5.7 | 10.9±5.6 | F(2,101)=1.02, p=0.37 |
| SOPS_D | 6.9±3.3 | 6.2±3.0 | 6.7±2.8 | F(2,101)=0.46, p=0.63 |
| SOPS_G | 8.2±3.5 | 9.8±2.6 | 8.7±3.2 | F(2,101)=2.01, p=0.14 |
| Highest GAF | 77.5±2.2 | 77.8±3.3 | 76.9±6.1 | F(2,101)=1.25, p=0.29 |
| Current GAF | 53.1±8.1 | 53.5±2.6 | 55.7±8.0 | F(2,101)=0.42, p=0.66 |
| SoP_T | 53.1±9.1 | 55.5±7.3 | 56.7±7.4 | F(2,93)=1.31, p=0.27 |
| AV_T | 47.2±11.6 | 51.0±9.7 | 51.9±8.0 | F(2,93)=1.58, p=0.21 |
| WM_T | 45.3±10.0 | 48.2±8.9 | 44.8±8.1 | F(2,93)=1.48, p=0.23 |
| Samples completed the P300 novel paradigm | ||||
| Cases | 23 | 40 | 68 | / |
| Age (years) | 19.7±5.5 | 18.3±5.0 | 18.8±5.1 | F(2,128)=0.57, p=0.57 |
| Gender (M/F) | 14/9 | 21/19 | 32/36 | X2=1.35, p=0.51 |
| Education | 10.5±2.8 | 10.3±3.0 | 10.6±2.9 | F(2,128)=0.14, p=0.87 |
| IQ | 91.9±18.9 | 100.7±14.0 | 99.1±11.8 | F(2,110)=2.46, p=0.09 |
| DUP (months) | 5.5±5.2 | 5.0±4.2 | 5.5±4.0 | F(2,128)=0.15, p=0.86 |
| Medicated | 7/21 | 4/21 | 10/21 | / |
| SOPS_P | 9.8±3.1 | 10.5±3.2 | 9.3±3.5 | F(2,128)=1.59, p=0.21 |
| SOPS_N | 13.3±6.2 | 11.1±5.5 | 11.3±6.0 | F(2,128)=1.14, p=9.32 |
| SOPS_D | 6.8±3.2 | 6.4±3.1 | 6.7±3.3 | F(2,128)=0.12, p=0.89 |
| SOPS_G | 8.2±3.2 | 9.4±2.6 | 8.8±3.3 | F(2,128)=1.27, p=0.29 |
| Highest GAF | 77.4±2.5 | 78.1±3.3 | 77.0±5.9 | F(2,128)=0.45, p=0.64 |
| Current GAF | 51.9±7.6 | 53.9±6.4 | 53.6±9.6 | F(2,128)=0.66, p=0.52 |
| SoP_T | 51.3±10.2 | 54.0±7.6 | 54.7±7.2 | F(2,119)=1.37, p=0.26 |
| AV_T | 45.5±11.4 | 49.9±8.8 | 51.7±9.2 | F(2,119)=3.20, p<0.05 |
| WM_T | 42.6±8.5 | 45.8±9.9 | 44.8±10.4 | F(2,119)=0.66, p=0.52 |
In samples who completed the P300 oddball paradigm, 16 CHR converters, 31 non-remitted CHR and 49 remitted CHR completed MCCB tests; In samples who completed the P300 novel paradigm, 19 CHR converters, 38 non-remitted CHR and 65 remitted CHR completed MCCB tests.
Auditory P300 tasks
Two auditory P300 tasks were used. In the P300 oddball paradigm, stimuli consisted of 144 standard tones (1 kHz, 75 dB SPL, 82-ms duration) and 36 target tones (1.5 kHz, 75dB SPL, 82-ms duration). Standard and target tones were delivered randomly with an interval of 976 ms. In the P300 novel paradigm, in addition to 108 standard tones and 36 target tones, 6 environmental sounds such as dog barking or door slamming were included and were presented six times each as deviant stimuli (36 total). The environmental sounds lasted 300–320 ms (75 dB SPL). In both paradigms, subjects were asked to silently count the targets.
EEG Recording and Processing
Subjects were seated in a sound-attenuated and electrically shielded chamber with dim light. The EEG was recorded with 64-channel surface electrodes mounted in an elastic cap (EasyCap, Brain Products Inc., Bavaria, Germany). Two electro-oculogram electrodes were placed, one above the arch of right eye and the other below the left eye. The reference electrode was placed on the tip of the nose. The EEG was recorded with a sample rate of 1000 Hz and a 0.016 to 200 Hz bandpass filter (BrainAmp, Brain Products Inc., Bavaria, Germany). Impedance was kept below 5 kΩ.
The EEG was re-referenced to the average of bilateral electrodes TP9 and TP10 (mastoid electrodes were not available in the cap) and segmented into epochs of −100 to 800 ms relative to stimulus onset. All epochs were baseline corrected using −100 to 0 ms pre-stimulus baseline. Ocular artifacts were corrected (Miller et al., 1988) and epochs with amplitudes exceeding ±100μv were excluded. Artifact-free epochs were averaged separately for each stimulus type (standard, target and novel ones). Average ERPs were filtered between 0.05–30 Hz using a zero phase-shift IIR filter (24 dB/Oct).
In the P300 oddball paradigm, individual subject’s P300 oddball amplitude and latency were measured at centro-parietal electrodes (CP1, CPz, CP2, P1, Pz, and P2) within the latency of 280–450 ms. In the P300 novel paradigm, individual P300 novel amplitude and latency were measured at fronto-central electrodes (F1, Fz, F2, FC1, FCz, FC2, C1, Cz and C2) within 250–450 ms. P300 oddball/novel latency was defined as the most positive data point within each latency window. The individual P300 oddball/novel amplitude was the mean amplitude of ±20 ms around each peak.
Neurocognitive Assessments
The majority of CHR and HC subjects received the neurocognitive assessments, which included the Wechsler Abbreviated Scale of Intelligence for general intellectual ability (Wechsler and Manual, 1999) and the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognition Battery (MCCB) (Nuechterlein and Green, 2006) for the assessment of cognitive abilities. The Chinese version of the MCCB consists of 9 subtests assessing 7 cognitive domains: Speed of Processing (SoP), Verbal Learning, Working Memory (WM), Reasoning, Visual Learning, Social Cognition, Attention/Vigilance (AV) (Shi et al., 2015).
Statistical Analysis
All statistical analyses were performed using SPSS (version 19.0, SPSS Inc., Chicago, USA). Conventional between-group comparisons between the whole CHR group and HC were summarized in the supplementary materials.
Comparisons between CHR-C, CHR-NC and HC:
P300 oddball amplitude and latency were assessed using a two-way repeated measures analysis of variance (ANOVA) with group (CHR-C, CHR-NC, HC) as the between-group factor and electrodes (CPz, Pz) as the within-group factor for midline P300, and a three-way repeated measures ANOVA with group as the between-group factor and region (centro-parietal, parietal) and laterality (left, right) as within-group factors for lateral P300 at electrodes CP1, CP2, P1 and P2.
P300 novel amplitude and latency were assessed using a two-way repeated measures ANOVA with group (CHR-C, CHR-NC, HC) as the between-group factor and electrode (Fz, FCz, Cz) as the within-group factor for midline P300, and a three-way repeated measures ANOVA with group as the between-group factor and region (frontal, fronto-central, central) and laterality as within-group factors for lateral P300 at electrodes F1, F2, FC1, FC2, C1 and C2.
Comparisons between CHR-C, non-remitted CHR, remitted CHR and HC:
To further assess differences among CHR subgroups, the similar statistical design as described above was used except for the fact that the between-group factor consisted of four levels: CHR converters, non-remitted CHR, remitted CHR and HC.
Correlations:
Pearson’s correlations were performed to examine the relationships between these ERP measures where group differences were found (P300 oddball latency averaged over 6 electrodes and P300 novel amplitude averaged over 9 electrodes), and clinical characteristics (SOPS scores) and neurocognitive function including SoP, AV and WM scores since all the above measures were normally distributed. Multiple comparisons were corrected with Bonferroni correction.
Results
Demographic Differences between Groups
Age, gender and years of education of CHR-C, CHR-NC and HC were well matched as shown in Table 2. There were no significant between-group IQ differences in subjects who completed the P300 oddball task (F(2, 149)=2.44, p=0.09). For the subjects who completed the P300 novel task, there was a significant between-group difference in IQ scores (F(2,173)=6.74, p<0.01). IQ score of CHR-C group (91.9±18.9) was significantly lower than HC (104.0±10.1, p<0.01), whereas IQ score of CHR-NC group (99.6±12.6, p=0.06) was lower than HC at trend level.
When CHR subjects were divided into CHR-C, non-remitted CHR and remitted CHR, all characteristics including age, gender, years of education, IQ, duration of untreated psychosis, scores of SOPS and Global Assessment of Functioning (GAF) were comparable among the three CHR subgroups as shown in Table 3.
P300 as Predictor of Conversion
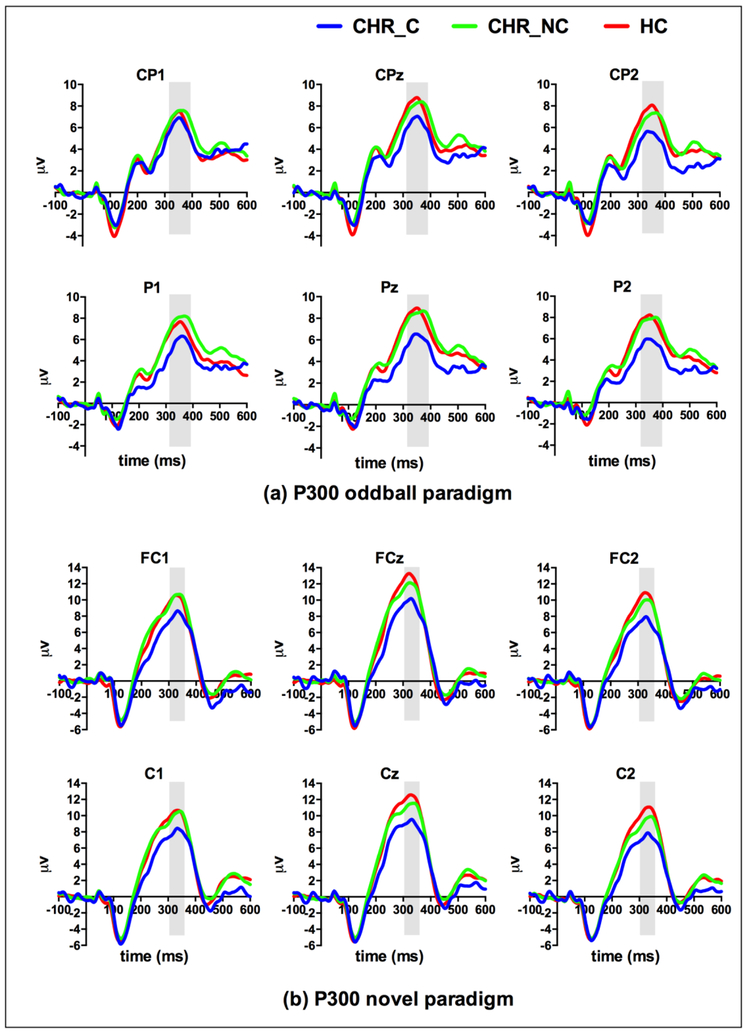
Grand average waveforms to the target stimuli in the P300 oddball task and to the novel stimuli in the P300 novel task are depicted in Figure 1.
Figure 1.
Grand average ERPs at baseline to target stimuli in the P300 oddball paradigm (a) and to novel stimuli in the P300 novel paradigm (b). CHR-C, subjects at clinical high risk for psychosis who converted into psychosis; CHR-NC, CHR subjects who did not convert into psychosis; HC, healthy controls.
The P300 oddball:
Amplitude: there were trend-level between-group differences at midline (F(2,170)=2.92, p=0.06, η2=0.03) and lateral electrodes (F(2,170)=2.81, p=0.06, η2=0.03). Latency: CHR-C, CHR-NC and HC did not show significant group differences at the midline or lateral electrodes.
The P300 novel:
Amplitude: there was a significant group difference among CHR-C, CHR-NC and HC (F(2, 197)=3.87, p<0.05, η2=0.04) at the midline electrodes (Fz, FCz and Cz). CHR-C subjects had lower novel P300 amplitudes (10.72±1.08μv) than HC (14.20±0.63μv, p<0.05), whereas CHR-NC subjects (12.98±0.51μv) had comparable novel P300 amplitudes with HC (p=0.41) and CHR-C (p=0.19) groups. At the lateral electrodes (F1, F2, FC1, FC2, C1 and C2), there was also a significant group difference (F(2,197)=4.22, p<0.05, η2=0.04). CHR-C (8.52±0.95μv) had significantly lower novel P300 amplitudes than HC (11.74±0.55μv, p<0.05) and lower than CHR-NC (11.07±0.44μv, p=0.051), whereas CHR-NC had comparable novel P300 amplitudes with HC (p=1.00).
There was also a significant interaction of group × laterality (F(2, 197)=4.82, p<0.01, η2=0.05), with the left hemisphere showing less robust group difference (p<0.05) between CHR-C and HC than the right (p<0.01) hemisphere.
Latency: there were no significant between-group differences or significant interaction of group × region or group ×laterality for the P300 novel latency at either midline or lateral electrodes.
P300 as a Predictor of Remission
The P300 oddball:
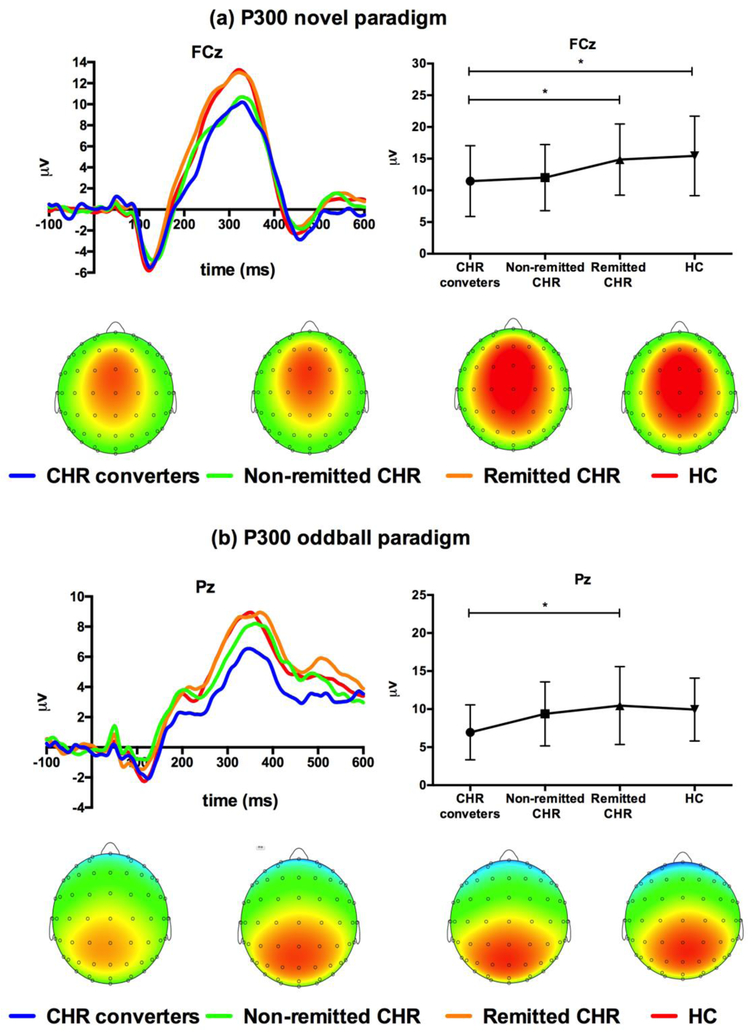
Amplitude: Only a trend level group difference (F(3,169)=2.14, p=0.10, η2=0.04) was found at midline. Latency: No significant group differences were found in the P300 oddball latency either at the midline or lateral electrodes.
The P300 novel:
Amplitude: At the midline electrodes, there was a significant group difference (F(3, 196)=4.94, p<0.01, η2=0.07) as shown in Figure 2. Post-hoc t-tests showed that the novel P300 amplitude in CHR-C (10.72±1.08μv) was significantly lower than in HC (14.20±0.62μv, p<0.05) and in remitted CHR (13.98±0.63μv, p<0.01). The P300 novel amplitude in non-remitted CHR (11.28±0.82μv) was lower than in HC (p<0.05), trend-level lower than in remitted CHR (p=0.06) and comparable with that in CHR-C (p>0.99), while that in remitted CHR (13.98±0.63μv) was comparable to HC (p>0.99).
Figure 2.
Group differences in the P300 novel and oddball amplitudes. (a) CHR converters and non-remitted CHR subjects had lower P300 novel amplitudes at fronto-central electrodes (e.g. FCz) than remitted CHR subjects and HC. (b) Few group differences were observed in the P300 oddball amplitude.
At the lateral electrodes, the group effect was also significant (F(3, 196)=5.34, p<0.01, η2=0.08). CHR-C (8.52±0.95μv) had significantly lower novel P300 amplitude than HC (11.74±0.55μv, p<0.05) and remitted CHR (11.98±0.55μv, p<0.05). Non-remitted CHR subjects (9.53±0.72μv) had lower novel P300 amplitudes than remitted CHR (p<0.05), and a tendency of lower amplitudes than HC (p=0.09). Remitted CHR had comparable P300 amplitude with HC (p>0.99), while non-remitted CHR had P300 novel amplitude comparable with CHR-C (p=1.00). There was also a significant interaction of group and laterality (F(3, 196)=3.41, p<0.05, η2=0.05). The simple effect tests showed more prominent group differences in the right hemisphere.
In the left hemisphere, only CHR-C had significantly lower amplitudes than remitted CHR (p<0.05). In the right hemisphere, P300 novel amplitude was significantly lower in CHR-C compared to remitted CHR (p<0.05) and to HC (p<0.01), and non-remitted CHR had lower P300 novel compared to remitted CHR (p<0.05) and to HC (p<0.05).
Latency: There was no significant group difference in the P300 novel latency at either midline or lateral electrodes.
Correlations with Neurocognitive Function and Clinical Symptoms
Neurocognitive function:
the P300 novel amplitude was not correlated with the SoP, AV or WM scores in either CHR-C, remitted CHR, non-remitted CHR or HC groups.
As shown in Figure 3, there was a significant correlation between P300 oddball latency and SoP scores (r=−0.30, p<0.05) in remitted CHR, while there were significant correlations between P300 oddball latency and AV (r=−0.49, p<0.01) and WM (r=−0.50, p<0.01) scores in non-remitted CHR, but not in CHR-C and HC groups.
Figure 3.
Significant correlations of the P300 oddball latency and scores of the speed of processing, attention/vigilance and working memory in CHR non-converters (CHR-NC) rather than CHR converters (CHR-C) and HC.
Clinical symptoms:
No correlations with clinical symptoms were observed for either the P300 oddball or novel in CHR subjects.
Global Assessment of Functioning:
The correlation between P300 oddball amplitude and current GAF scores was significant in HC (r=0.32, p<0.01), but not in any CHR subgroup (all p>0.05). There was a significant correlation between P300 oddball latency and current GAF scores in non-remitted CHR (r=−0.42, p<0.05), but not in HC, CHR-C or remitted CHR (all p>0.05). The P300 novel amplitude was not correlated with current GAF scores in either CHR-C, remitted CHR, non-remitted CHR or HC group.
Discussion
To the best of our knowledge, the present paper is the first to describe the extent of abnormalities in both P300 oddball and novel components in a relatively large sample of CHR individuals. Compared to HC, at baseline we found that CHR converters had lower fronto-central P300 novel amplitude as well as marginally lower P300 oddball amplitude. When the CHR non-converters were stratified into remitted and non-remitted subgroups, it became apparent that group differences in the CHR non-converters were influenced by those individuals who remitted: P300 novel amplitude in remitted CHR subjects were comparable to that in HC, and were significantly higher than in CHR subjects who converted to psychosis or who did not remit. In this study, P300 novel rather than P300 oddball was a better predictor of both conversion and remission.
Reduced fronto-central P300 novel amplitude was observed in the whole CHR group, and in particular, in the CHR subjects who converted to psychosis. This result is in line with the previous small-sample studies of at-risk individuals (Atkinson et al., 2012; Jahshan et al., 2012; Mondragon-Maya et al., 2013; Higuchi et al., 2014; del Re et al., 2015). Atkinson et al. (Atkinson et al., 2012) first reported a reduction of P300 novel amplitude in individuals at ultra-high risk for psychosis and smaller amplitude in six individuals who transitioned than those who did not. Jahshan et al. and our previous study confirmed the finding of abnormal P300 novel amplitude in at-risk subjects (Jahshan et al., 2012; del Re et al., 2015). Moreover, the P300 novel abnormality was progressive across the illness course, being most severe in chronic patients and followed by recent-onset patients (Jahshan et al., 2012; Mondragon-Maya et al., 2013; del Re et al., 2015). These consistent results of reduced P300 novel amplitude suggest that orienting attention and salience processing is impaired prior to the onset of psychosis (Friedman et al., 2001; Polich, 2007). Our much larger sample demonstrated a reduction of P300 novel amplitude in the CHR converters, suggesting that the extent of orienting attention deficit may be crucial to developing psychosis.
Moreover, we have examined CHR non-converters in greater detail given the fact that clinically this group consisted of the remitted CHR group who no longer fulfilled CHR definition and the non-remitted CHR group who still fulfilled the CHR criteria. Again, P300 novel index worked well in distinguishing these two CHR subgroups. Compared with HC, intact P300 novel amplitude was observed in remitted CHR, but reduced P300 novel amplitude characterized both CHR converters and non-remitted CHR. To the best of our knowledge, this is the first study to report this result for the P300 novel component. The present findings were based on one-year follow-up for our CHR sample. Long-term follow-up is ongoing and we need to further address three questions: 1. Would CHR remitters with a normal P300 novel maintain their remitted status over a longer period than a year? 2. Given comparable P300 novel abnormality in the CHR-non-remitters and CHR converters, what are the protective mechanisms that shielded the CHR non-remitters from conversion to frank psychosis? And 3. Would greater rates of conversion be observed in the non-remitted than in the remitted group over a longer period of time? Longitudinal data in this subject group will help address their long term clinical and neurocognitive trajectory.
The P300 novel reduction also showed a significant interaction of group and laterality, suggesting more prominent P300 novel reduction in the right hemisphere. The neural loci of the fronto-central P300 generation have been localized to the dorsolateral prefrontal, anterior cingulate (ACC) and inferior parietal cortices (Mathalon et al., 2000; Polich, 2007). Neuroimaging studies showed a steeper rate of gray matter loss in the right frontal regions in CHR converters than non-converters (Cannon et al., 2015). Aberrant frontal gyrification was found in the right hemisphere in patients with first-episode schizophrenia and was correlated with impaired executive function (Sasabayashi et al., 2017). Since the ACC is a part of the salience network and plays an important role in attention shifting and control, more prominent abnormality of P300 novel amplitude may reflect deficits in salience processing of as supported by the right frontal cortex and ACC and leading to the conversion to psychosis (McTeague et al., 2017).
Abnormal P300 oddball response was more widely explored across schizophrenia spectrum in clinical high-risk, first-episode to chronic stages (van der Stelt et al., 2005; Bramon et al., 2008; Ozgurdal et al., 2008; van Tricht et al., 2010; Wang et al., 2010; Fusar-Poli et al., 2011b, a; Nieman et al., 2014; Kim et al., 2015; Kim et al., 2017). Longitudinal follow-up of CHR subjects by Nieman et al. (Nieman et al., 2014) suggested that the risk for developing psychosis increased by 27% with a decrement of the oddball P300 amplitude of 1μv. However, in the present study, only converted CHR subjects had marginally smaller amplitudes relative to non-converted CHR subjects. Instead, in CHR non-converters, significantly delayed P300 oddball latency was associated with slower speed of processing and worse AV performance. As we included a larger sample than previous studies, the inconsistency may be due to the heterogeneity of CHR samples (Nieman et al., 2014; Kim et al., 2015; Cannon et al., 2016). Another reason could be the task procedure we used in which subjects were asked to count the number of target tone instead of pressing a button (Mathalon et al., 2000; Mathalon et al., 2010).
Few studies have explored both the P300 novel and oddball activities in a CHR population (del Re et al., 2015). Mathalon et al.’s longitudinal study found the P300 novel component tracked depression-anxiety symptoms while the effortful P300 oddball component tracked negative symptoms in patients with schizophrenia (Mathalon et al., 2000). Our previous cross-sectional study observed similar reductions in both P300 novel and oddball amplitudes in CHR subjects and first-episode patients, but only reduced P300 novel amplitude contributed to the regression model distinguishing CHR group from HC (del Re et al., 2015). In the present study, in line with our hypothesis, aberrant P300 novel amplitude reflecting automatic attention orienting was associated with both transition to psychosis and remission from symptoms, whereas P300 oddball abnormality suggesting deficit in effortful memory processing did not quite reach significance in comparisons between HC and CHR. These findings suggest that P300 novel amplitude may be a more sensitive index of predicting the outcomes in CHR subjects and may be a potential target for early intervention.
In addition to the ERP measures, the current study also collected neurocognitive assessments of attention/vigilance. Of note, when the entire CHR group was compared to HC, no differences in AV measures were noted. They emerged only when CHR converters were compared to HC and remitted CHR. The association of deficits in attention with transition to psychosis was also reported in other neurocognitive studies, in which CHR converters had large deficits in attention and performed worse than non-converters (Seidman et al., 2016). There was no significant correlation between P300 novel amplitude and any clinical or neurocognitive measures, suggesting that P300 novel amplitude could be a relatively independent biomarker for predicting the outcome of CHR subjects.
Supplementary Material
Acknowledgement
Role of funding source
This work was supported by Ministry of Science and Technology of China, National Key R&D Program of China (2016YFC1306803), the National Institute of Mental Health (NIMH RO1MH101052 and R01MH111448), National Nature Science Foundation of China (81361120403, 81671332, 81671329 and 81871050), Shanghai Science and Technology Committee (17411953100 and 16JC1420200), the Clinical Research Center at Shanghai Mental Health Center (CRC2018ZD01, CRC2018ZD04 and CRC2018YB01) and Shanghai Jiao Tong University (YG2016MS36). Y. Tang was funded by a Municipal Human Resources Development Program for Outstanding Young Talents in Medical and Health Sciences in Shanghai (2017YQ069). J. Wang was funded by Suzhou Municipal Science and Technology Bureau, China (sys2018097).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None.
References:
- Addington J, Piskulic D, Liu L, Lockwood J, Cadenhead KS, Cannon TD, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Bearden CE, Mathalon DH, Woods SW, 2017. Comorbid diagnoses for youth at clinical high risk of psychosis. Schizophr. Res 190, 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RJ, Fulham WR, Michie PT, Ward PB, Todd J, Stain H, Langdon R, Thienel R, Paulik G, Cooper G, Schall U, 2017. Electrophysiological, cognitive and clinical profiles of at-risk mental state: The longitudinal Minds in Transition (MinT) study. PloS One 12(2), e0171657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RJ, Michie PT, Schall U, 2012. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol. Psychiatry 71(2), 98–104. [DOI] [PubMed] [Google Scholar]
- Bramon E, Shaikh M, Broome M, Lappin J, Berge D, Day F, Woolley J, Tabraham P, Madre M, Johns L, Howes O, Valmaggia L, Perez V, Sham P, Murray RM, McGuire P, 2008. Abnormal P300 in people with high risk of developing psychosis. Neuroimage 41(2), 553–560. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Chung Y, He G, Sun DQ, Jacobson A, van Erp TGM, McEwen S, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, McGlashan T, Perkins D, Jeffries C, Seidman LJ, Tsuang M, Walker E, Woods SW, Heinssen R, S, N.A.P.L., 2015. Progressive reduction in cortical thickness as psychosis develops: A multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol. Psychiatry 77(2), 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, Heinssen R, Jeffries CD, Mathalon DH, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Kattan MW, 2016. An individualized risk calculator for research in prodromal psychosis. Am. J. Psychiatry 173(10), 980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Re EC, Spencer KM, Oribe N, Mesholam-Gately RI, Goldstein J, Shenton ME, Petryshen T, Seidman LJ, McCarley RW, Niznikiewicz MA, 2015. Clinical high risk and first episode schizophrenia: Auditory event-related potentials. Psychiatry Res. 231(2), 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H, 2001. The novelty P3: An event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci. Biobehav. Rev 25(4), 355–373. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Cappucciati M, Borgwardt S, Woods SW, Addington J, Nelson B, Nieman DH, Stahl DR, Rutigliano G, Riecher-Rossler A, Simon AE, Mizuno M, Lee TY, Kwon JS, Lam MM, Perez J, Keri S, Amminger P, Metzler S, Kawohl W, Rossler W, Lee J, Labad J, Ziermans T, An SK, Liu CC, Woodberry KA, Braham A, Corcoran C, McGorry P, Yung AR, McGuire PK, 2015. Heterogeneity of psychosis risk within individuals at clinical high risk: A meta-analytical stratification. JAMA Psychiatry, 1–8. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Crossley N, Woolley J, Carletti F, Perez-Iglesias R, Broome M, Johns L, Tabraham P, Bramon E, McGuire P, 2011a. Gray matter alterations related to P300 abnormalities in subjects at high risk for psychosis: Longitudinal MRI-EEG study. Neuroimage 55(1), 320–328. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Crossley N, Woolley J, Carletti F, Perez-Iglesias R, Broome M, Johns L, Tabraham P, Bramon E, McGuire P, 2011b. White matter alterations related to P300 abnormalities in individuals at high risk for psychosis: An MRI-EEG study. J. Psychiatry Neurosci 36(4), 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Deste G, Smieskova R, Barlati S, Yung AR, Howes O, Stieglitz RD, Vita A, McGuire P, Borgwardt S, 2012. Cognitive functioning in prodromal psychosis: A meta-analysis. Arch. Gen. Psychiatry 69(6), 562–571. [DOI] [PubMed] [Google Scholar]
- Higuchi Y, Seo T, Miyanishi T, Kawasaki Y, Suzuki M, Sumiyoshi T, 2014. Mismatch negativity and P3a/reorienting complex in subjects with schizophrenia or at-risk mental state. Front. Behav. N neurosci 8, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahshan C, Cadenhead KS, Rissling AJ, Kirihara K, Braff DL, Light GA, 2012. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychol. Med 42(1), 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M, 2008. Neurophysiological biomarkers for drug development in schizophrenia. Nat. Rev. Drug Discov 7(1), 68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YW, Polich J, 2003. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology 40(5), 684–701. [DOI] [PubMed] [Google Scholar]
- Kim M, Lee TH, Kim JH, Hong H, Lee TY, Lee Y, Salisbury DF, Kwon JS, 2017. Decomposing P300 into correlates of genetic risk and current symptoms in schizophrenia: An inter-trial variability analysis. Schizophr Res. [DOI] [PubMed] [Google Scholar]
- Kim M, Lee TY, Lee S, Kim SN, Kwon JS, 2015. Auditory P300 as a predictor of short-term prognosis in subjects at clinical high risk for psychosis. Schizophr. Res 165(2–3), 138–144. [DOI] [PubMed] [Google Scholar]
- Loewy RL, Pearson R, Vinogradov S, Bearden CE, Cannon TD, 2011. Psychosis risk screening with the Prodromal Questionnaire--brief version (PQ-B). Schizophr. Res 129(1), 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Ford JM, Pfefferbaum A, 2000. Trait and state aspects of P300 amplitude reduction in schizophrenia: A retrospective longitudinal study. Biol. Psychiatry 47(5), 434–449. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Hoffman RE, Watson TD, Miller RM, Roach BJ, Ford JM, 2010. Neurophysiological distinction between schizophrenia and schizoaffective disorder. Front. Hum. Neurosci 3, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW, Salisbury DF, Hirayasu Y, Yurgelun-Todd DA, Tohen M, Zarate C, Kikinis R, Jolesz FA, Shenton ME, 2002. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Arch. Gen. Psychiatry 59(4), 321–331. [DOI] [PubMed] [Google Scholar]
- McGlashan T, Walsh B, Woods S, 2010. The psychosis-risk syndrome: Handbook for diagnosis and follow-up. Oxford University Press. [Google Scholar]
- McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A, 2017. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am. J. Psychiatry 174(7), 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Gratton G, Yee CM, 1988. Generalized implementation of an eye movement correction procedure. Psychophysiology 25(2), 241–243. [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW, 2003. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: Predictive validity, interrater reliability, and training to reliability. Schizophr. Bull 29(4), 703–715. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW, 2002. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: Preliminary evidence of interrater reliability and predictive validity. Am. J. Psychiatry 159(5), 863–865. [DOI] [PubMed] [Google Scholar]
- Mondragon-Maya A, Solis-Vivanco R, Leon-Ortiz P, Rodriguez-Agudelo Y, Yanez-Tellez G, Bernal-Hernandez J, Cadenhead KS, de la Fuente-Sandoval C, 2013. Reduced P3a amplitudes in antipsychotic naive first-episode psychosis patients and individuals at clinical high-risk for psychosis. J. Psychiatr. Res 47(6), 755–761. [DOI] [PubMed] [Google Scholar]
- Nieman DH, Ruhrmann S, Dragt S, Soen F, van Tricht MJ, Koelman JH, Bour LJ, Velthorst E, Becker HE, Weiser M, Linszen DH, de Haan L, 2014. Psychosis prediction: Stratification of risk estimation with information-processing and premorbid functioning variables. Schizophr. Bull 40(6), 1482–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, 2006. MATRICS consensus cognitive battery manual. Los Angeles, CA: MATRICS Assessment Inc. [Google Scholar]
- Ozgurdal S, Gudlowski Y, Witthaus H, Kawohl W, Uhl I, Hauser M, Gorynia I, Gallinat J, Heinze M, Heinz A, Juckel G, 2008. Reduction of auditory event-related P300 amplitude in subjects with at-risk mental state for schizophrenia. Schizophr. Res 105(1–3), 272–278. [DOI] [PubMed] [Google Scholar]
- Polich J, 2007. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol 118(10), 2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabayashi D, Takayanagi Y, Nishiyama S, Takahashi T, Furuichi A, Kido M, Nishikawa Y, Nakamura M, Noguchi K, Suzuki M, 2017. Increased frontal gyrification negatively correlates with executive function in patients with first-episode schizophrenia. Cereb. Cortex 27(4), 2686–2694. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Mathalon DH, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, 2016. Association of neurocognition with transition to psychosis: Baseline functioning in the second phase of the North American Prodrome Longitudinal Study. JAMA Psychiatry 73(12), 1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Kang L, Yao S, Ma Y, Li T, Liang Y, Cheng Z, Xu Y, Shi J, Xu X, 2015. The MATRICS consensus cognitive battery (MCCB): Co-norming and standardization in China. Schizophr. Res 169(1–3), 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Bilker WB, Siegel SJ, Kohler CG, Gur RE, 2009. Profile of auditory information-processing deficits in schizophrenia. Psychiatry Res. 165(1–2), 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt O, Lieberman JA, Belger A, 2005. Auditory P300 in high-risk, recent-onset and chronic schizophrenia. Schizophr. Res 77(2–3), 309–320. [DOI] [PubMed] [Google Scholar]
- van Tricht MJ, Nieman DH, Koelman JH, Bour LJ, van der Meer JN, van Amelsvoort TA, Linszen DH, de Haan L, 2011. Auditory ERP components before and after transition to a first psychotic episode. Biol. Psychology 87(3), 350–357. [DOI] [PubMed] [Google Scholar]
- van Tricht MJ, Nieman DH, Koelman JH, van der Meer JN, Bour LJ, de Haan L, Linszen DH, 2010. Reduced parietal P300 amplitude is associated with an increased risk for a first psychotic episode. Biol. Psychiatry 68(7), 642–648. [DOI] [PubMed] [Google Scholar]
- Wang J, Tang Y, Li C, Mecklinger A, Xiao Z, Zhang M, Hirayasu Y, Hokama H, Li H, 2010. Decreased P300 current source density in drug-naive first episode schizophrenics revealed by high density recording. Int. J. Psychophysiol 75(3), 249–257. [DOI] [PubMed] [Google Scholar]
- Wechsler D, Manual W, 1999. San Antonio, CA: Psychological Corp. Harcourt Brace & Co.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.