Abstract
Background:
Preclinical in vitro experiments demonstrated that epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) might have synergistic effect in combination with radiotherapy on Non-small cell lung cancer (NSCLC), but the clinical trials showed inconsistence results in NSCLC patients with EGFR status unknow or mutations. This study aimed to determine if added TKIs to Thoracic radiotherapy (TRT) improve primary disease response rate (RR) and survival outcomes in advanced or metastatic NSCLC.
Methods:
We searched MEDLINE, EMBASE, and Cochrane Library from January 2000 to December 2017 for eligible studies where patients received concurrent EGFR TKIs and TRT or CRT. Concerned outcomes were primary tumor RR, overall survival (OS), and adverse events (AEs). The meta-analysis was performed using Stata software (version 12.0). Random effects models were used to pool outcomes across studies. Sensitivity analysis was performed to determine if the results would be different.
Results:
We found 16 prospective clinical trials with mature results for meta-analyses. Twelve studies including 446 patients reported the RR and survival outcomes of TRT combined TKIs. The CR, PR, SD, and PD, respectively, were 0.06 (95% CI 0.03–0.09, I2 = 0%), 0.44 (95% CI 0.38–0.49, I2 = 64.9%), 0.29 (95% CI 0.24–0.34, I2 = 78.4%), and 0.15 (95% CI 0.11–0.19, I2 = 84.2%). One- and 2-year OS, respectively, were 0.52 (95% CI 0.44–0.60, I2 = 38.8%) and 0.26 (95% CI 0.18–0.33, I2 = 0%). Four studies including 182 patients reported the RR and survival outcomes of CRT combined TKIs. The pooled CR, PR, SD, and PD, respectively, were 0.12 (95% CI 0.02–0.22, I2 = 69.1%), 0.41 (95% CI 0.27–0.55, I2 = 71.6%), 0.31 (95% CI 0.16–0.46, I2 = 79%), and 0.14 (95% CI −0.01–0.30, I2 = 87.8%). Only 1 study reported the survival event rate, 1- and 2-year OS, respectively, were 0.83 (95% CI 0.71–0.94) and 0.67 (95% CI 0.54–0.81). There were not severe adverse events (SAEs) reported either TRT combined TKIs or CRT combined TKIs.
Conclusion:
There is evidence, albeit of low quality, that added the TKIs to TRT or CRT may improve RR and survival outcomes in patients with EGFR mutant status unknown advanced or metastatic NSCLC relative to other studies of TKIs alone, TRT alone or CRT.
Keywords: EGFR TKIs, meta-analysis, pulmonary malignant tumor, radiotherapy, target therapy
1. Introduction
Worldwide, lung cancer is considered to be the most common type of malignancy in humans, and among lung cancer patients, some 80% are affected by non-small cell lung cancer (NSCLC).[1] In more than a half of all cases, NSCLC is detected after the disease has already progressed to an incurable stage. Pharmacotherapy has played a dominant role in the treatment of these patients, with platinum-based chemotherapy typically producing response rates of approximately 30% and median survival times of 8 to 10 months. Moreover, different chemotherapy regimens have been found to have similar efficacy.[2] Thoracic radiotherapy (TRT), as a main approach to local treatment, aims to control the primary lung lesions to reduce pulmonary symptoms, intrathoracic disease burden, and bronchial/vascular compression for metastatic NSCLC, and previous studies have shown that the combination of TRT and chemotherapy results in the better overall survival of patients with incurable NSCLC.[3–6]
Epidermal growth factor receptor (EGFR) is a transmembrane protein that functions as a receptor for members of the epidermal growth factor family, the overexpression of which plays a critical role in cellular proliferation, inhibition of apoptosis, angiogenesis, metastasis, and chemoradiotherapy resistance.[7] Abnormalities in EGFR signal or activity can lead to the unlimited proliferation of tumor cells, an increase in the aggressivity of tumor cells, inhibition of tumor cell apoptosis, and promotion of tumor angiogenesis, which are key factors in the process of cancer development. The mutation of EGFR is considered an effective predictor of advanced NSCLC when using tyrosine kinase inhibitor (TKI) therapy. In metastatic NSCLC patients harboring EGFR mutations, EGFR-TKIs such as gefitinib, erlotinib, or icotinib, are recommended as first-line systemic therapies.[8] In this regard, the Iressa Pan-Asia Study (IPASS) revealed that gefitinib showed better progression-free survival than chemotherapy in NSCLC patients with EGFR mutations. In contrast, in those patients with no EGFR mutation, chemotherapy has been found to be superior to treatment with gefitinib.[9] This study established a milestone in guiding the clinical selection of EGFR-TKI treatment. In the era of precise treatment of NSCLC, targeted therapy and radiotherapy have respectively played prominent roles in systematic or local treatment. To date, however, there have been no conclusive indications regarding the efficacy of using EGFR-TKI treatment combined with radiotherapy in patients with advanced NSCLC.
Previously, preclinical in vitro experiments have demonstrated that EGFR-TKIs might have synergistic effects on tumor control when employed in combination with radiotherapy, the underlying mechanisms of which are related to the regulation of cell cycle redistribution, promotion of tumor cell apoptosis, and interference with repair following radiotherapy.[10,11] Chang et al showed that combined first-line TKI therapy and early multi-target radiotherapy are very effective in selected patients who respond to TKIs, when the status of EGFR mutations is unknown prior to treatment.[12] However, many of the most recent clinical trials with long-term follow-up that have examined the efficacy of erlotinib or gefitinib combined with radiotherapy have failed to achieve desired results and have not significantly improve patient survival.[13] Given that EGFR-TKIs are most active against EGFR mutant-related NSCLC, it is possible that the synergistic effects between TKIs and radiotherapy can best be observed in this particular group of patients. Regrettably, however, most trials do not assess patient for the type of EGFR mutant NSCLC, nor do they determine which types of radiotherapy technique can used profitably in this combined treatment strategy. Furthermore, the published clinical trials are typically single arm with small sample sizes, and to date there has been a lack of large-sample prospective randomized controlled clinical trials, which is thus bound to affect the level of evidence.
On the basis of the aforementioned findings and research status, it still remains uncertain as to the optimal combined treatment strategy for the management of advanced or metastatic NSCLC in patients with different EGFR mutant status. It is against this background that we sought to conduct a systematic review and meta-analysis, with the aim of evaluating the efficacy and safety of combined EGFR-TKIs and thoracic primary lesion radiotherapy in patients with advanced or metastatic NSCLC. To this end, we undertook a comprehensive worldwide search of all available clinical trials that have examined the efficacy of EGFR-TKIs combined with TRT.
2. Materials and methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.[14] An ethical approval was not necessary since meta-analysis was based on secondary data and not involved individual patients.
2.1. Eligibility criteria
We assessed those studies that met the following inclusion criteria:
-
(1)
Locally advanced or metastatic NSCLC confirmed histologically;
-
(2)
Clinical stage III or IV;
-
(3)
No prior local treatment (surgery or radiation therapy) to the thoracic primary lesion.
-
(4)
Treatment with concurrent EGFR-TKIs and TRT, either conventional fraction, hypofraction, or stereotactic body radiotherapy (SBRT). EGFR-TKIs, including gefitinib, erlotinib, or icotinib.
-
(5)
Reported outcome of interest, that is, primary tumor response rate, survival, and toxicity.
-
(6)
Prospective clinical trials, such as randomized clinical controlled trial, nonrandomized clinical controlled trial, and single-arm clinical trial.
We excluded retrospective clinical trials, case reports based on fewer than 5 patients, reviews, letters, commentaries, and errata.
2.2. Search strategy
MEDLINE, EMBASE, and the Cochrane Library databases were searched for articles published between January 2000 and December 2017. The search strategy included the medical subject headings “lung neoplasms,” “radiotherapy,” “receptor, epidermal growth factor,” and “tyrosine kinase inhibitor,” We also tracked the references of eligible studies and manually searched the annual conferences of ASCO, ASTRO, and the World Conference on Lung Cancer from 2000.
2.3. Study selection and data collection
All searched records were imported into EndNote reference management software. Two reviewers (Liu and Wei) independently assessed the titles and abstracts. The full texts of all potential studies were retrieved for further selection. A third senior investigator (Wang) resolved any discrepancies between the 2 reviewers.
The same reviewers independently extracted the data of interest using standardized data collection forms, including the details of publications, methodological components, and the characteristics of studies such as sample size, interventions, duration of follow up and outcome measures. We defined outcomes of interest as response rate (RR) of primary disease (CR, PR, SD, or PD), progression-free survival (PFS), overall survival (OS) at 1 or 2 years, and toxicity.
2.4. Methodological quality assessment
In our systematic review, we adopted the case series quality assessment scale devised by the Agency for Healthcare Research and Quality (AHRQ) for assessment of quality,[15] which included the following items:
-
(1)
representativeness of the patients;
-
(2)
the outcome of interest was demonstrated to be absent at the start of the study;
-
(3)
there was adequate assessment of outcome;
-
(4)
there was a sufficient length of follow-up to allow outcomes to arise;
-
(5)
there was adequacy of follow-up (i.e., all patients in the study were accounted for).
2.5. Statistical analysis
We calculated the event rate of outcomes and 95% confidence intervals using the Jeffrey method; for example, the proportion of patients who developed outcomes of interest from the included cohorts.[16] We combined the individual log-transformed event rates and their variances using the generic inverse variance method. The meta-analysis was performed using Stata software (Stata version 12.0). Statistical heterogeneity among studies was assessed using the I2 statistic and forest plots. [17] An I2 value lower than 25% indicated a low level of heterogeneity. If there was a high level of heterogeneity, we would discard the studies with heterogeneous results and remerge the remaining studies.
2.6. Sensitivity analysis
Sensitivity analysis was performed to determine if the results showed differences, which we performed by excluding the trials for which the quality was very poor or those in which there was significant clinical heterogeneity. Publication bias is another common concern in meta-analysis that is related to the tendency of journals to favor the publication of large-scale studies and those showing positive results. In order to evaluate publication bias, we used funnel plots. We re-evaluated those articles showing imbalanced funnel plots, which is indicative of a large publication bias.
3. Results
3.1. Literature selection
On the basis of our database search, we obtained 2682 potentially relevant articles, of which 2602 were excluded after reviewing the title and abstract. The full texts of the remaining 80 articles were retrieved and subjected to further screening. Finally, for our meta-analysis, we selected 16 prospective clinical trials, [13,18–32] involving 628 patients with mature results (Fig. 1). Table 2 shows a list of the excluded trials and the reasons for exclusion. [35–49]. We identified a 3-arm and a 2-arm study, but for these we only focused on the TRT plus TKI arms.
Figure 1.
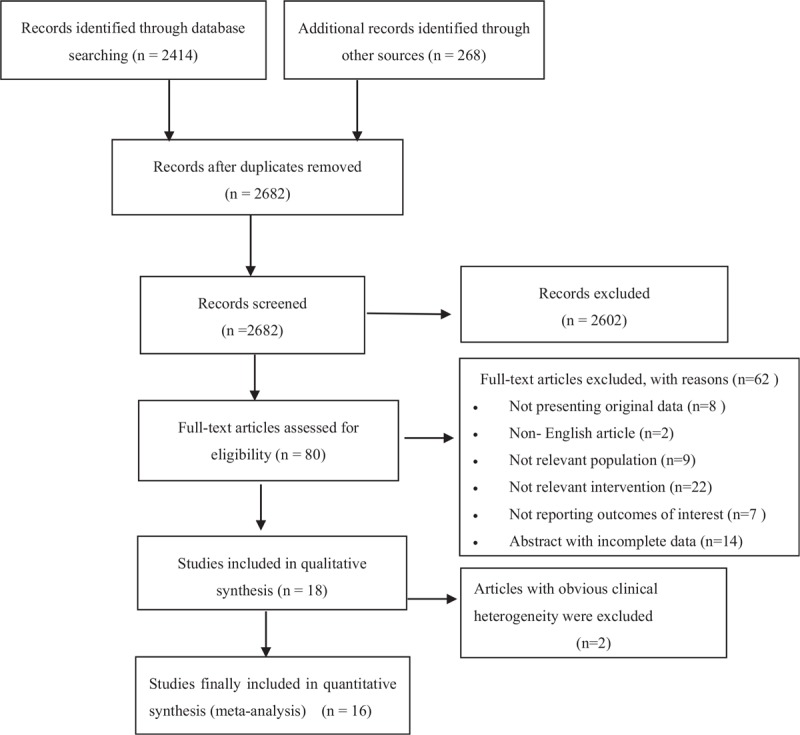
Flow chart for study selection.
Table 2.
The characteristics of excluded trials and excluded reasons.
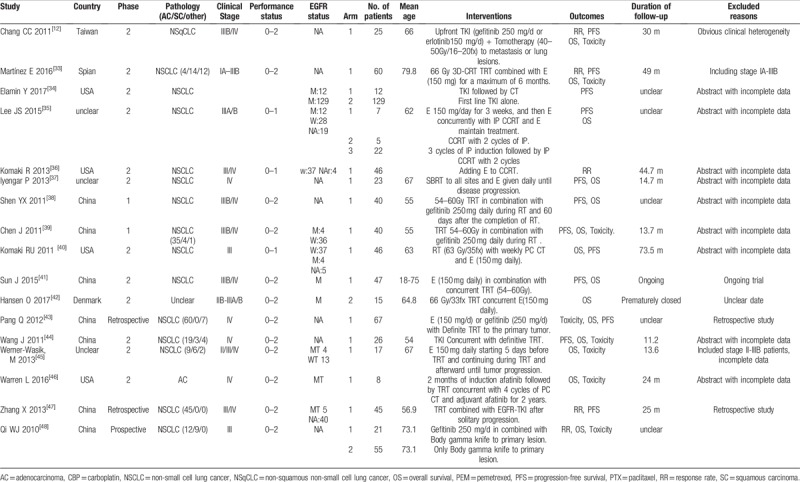
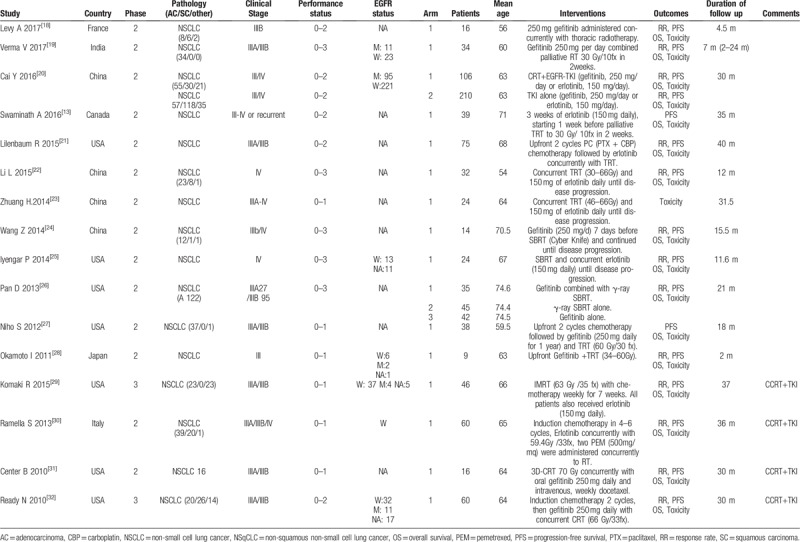
3.2. Characteristics of included studies
Table 1 shows the characteristics of the included studies. These studies were published in English between 2010 and 2017, and most of which were conducted in the USA, East Asia, or Europe. Among the 16 reports, 14 were phase II studies and the remaining 2 were phase III studies. Five included ECOG 0–1 cases, 6 included ECOG 0–2 cases, and the other 5 included ECOG 0–3 cases. Seven studies reported assessments of EGFR mutation status in tissues from primary lung tumors[19,20,25,28,29,30,32]; however, none of the studies analyzed results according to EGFR status. With regards to histological type, 6 studies reported the inclusion of NSCLC patients but did no differentiated among subtypes,[13,21,23,25,28,31] and the remaining 10 studies reported the inclusion of 339 adenocarcinoma patients, 91 squamous patients, and 64 patients with other pathological tissue types. All included trials received concurrent TKIs with TRT and then sequential TKI; however, the modalities of TRT were not accordant, with equivalent dose in 2 Grays per fraction ranging from 30 Gy to 70 Gy. One study arm reported using γ-ray SBRT as TRT,[26] whereas nine study arms used gefitinib (250 mg/day), 7 study arms used erlotinib (150 mg/day), and 1 study arm used gefitinib or erlotinib as EGFR-TKIs. All studies reported the duration of follow-up, with the median reported duration being from 2 to 40 months. Adverse events were reported using the Common Terminology Criteria for Adverse Events version 3.0.
Table 1.
The characteristics of patients in the included trials.
The methodological quality of the case series of the included studies was evaluated using the AHRQ assessment scale.[15] All studies enrolled a representative sample of patients and provided adequate follow-up, that is, all patients in the study arms were accounted for. Most studies provided adequate outcome assessment and had sufficient length of follow-up. We acknowledge that the evidence of single arms without comparative data is associated with a high risk of bias.
Publication bias was evaluated using funnel plots with pseudo 95% confidence limits in 1- or 2-year OS. The funnel plots were very asymmetrical because of superior survival outcomes in two studies. Chang et al[12] included those patients with stage IIIb or IV NSqCLC who responded to upfront TKI treatment and thereafter underwent multi-target radiotherapy as a subsequent therapy during TKI treatment. Martínez et al[33] included those patients with stage IA-IIIB NSCLC. Both these studies reported a superior response rate and survival results compared with the other studies we evaluated, and therefore, we excluded these studies from the subsequent meta-analysis (Fig. 2)
Figure 2.
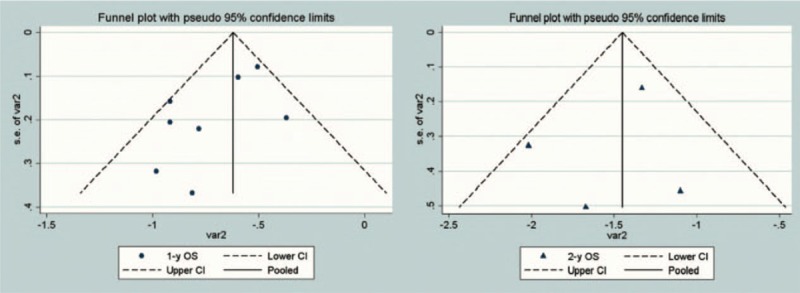
Funnel plots depicting the publication bias in included studies.
3.3. Efficacy and safety analysis
3.3.1. Thoracic radiotherapy combined with TKIs
Twelve studies, which included 446 patients, reported the outcomes of TRT combined with TKIs, 7 of which reported RR event rate. The pooled CR, PR, SD, and PD were 0.06 (95% CI 0.03–0.09, I2 = 0%), 0.44(95% CI 0.38–0.49, I2 = 64.9%), 0.29 (95% CI 0.24–0.34, I2 = 78.4%), and 0.15 (95% CI 0.11–0.19, I2 = 84.2%), respectively (Fig. 3). The pooled survival event rate was determined in seven studies, with 1- and 2-year OS of 0.52 (95% CI 0.44–0.60, I2 = 38.8%) and 0.26 (95% CI 0.18–0.33, I2 = 0%), respectively (Fig. 4). The reported median PFS (mPFS) ranged from 3.4 to 14.7 months, with a mean value of 8.1 ± 3.9 months, whereas median OS (mOS) ranged from 5.2 to 28.5 months, with a mean value of 15.2 ± 6.2 months.
Figure 3.
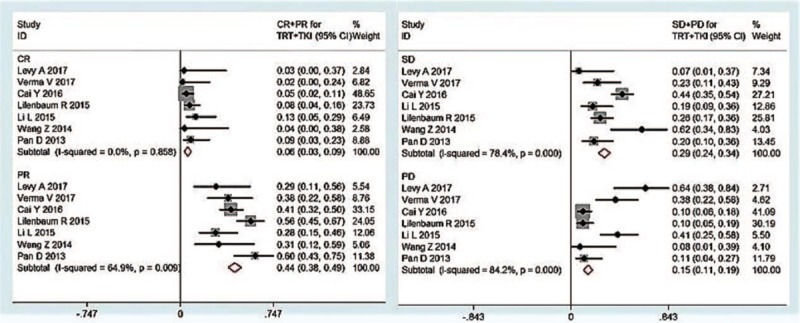
Forest plots depicting response rate in TRT+ TKI arm. TKI = tyrosine kinase inhibitor, TRT = thoracic radiotherapy.
Figure 4.
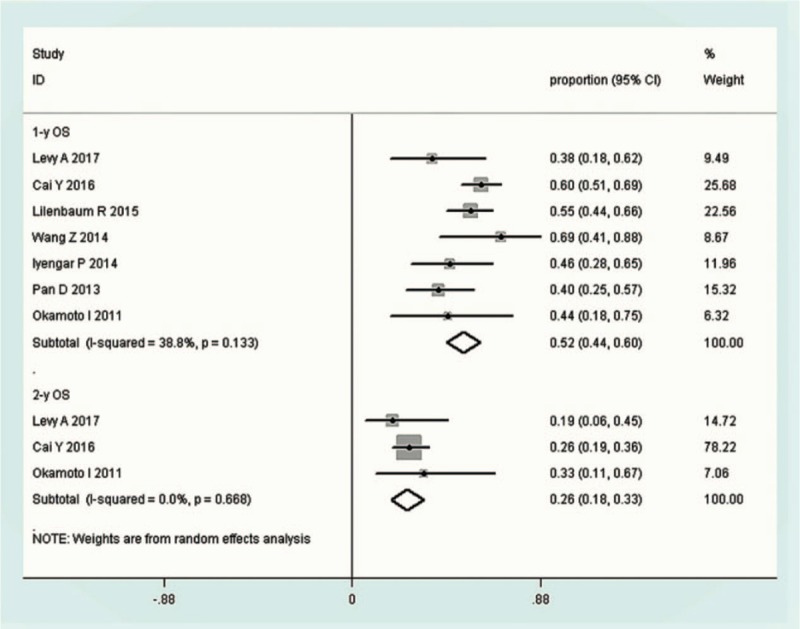
Forest plots depicting OS in TRT+ TKI arm. TKI = tyrosine kinase inhibitor, TRT = thoracic radiotherapy, OS = overall survival.
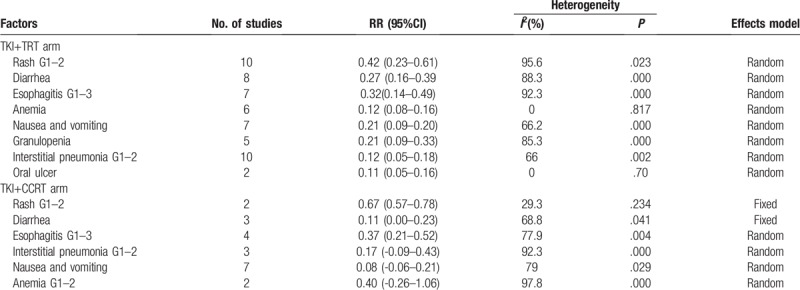
The pooled incidences of rash, diarrhea, esophagitis, animia, interstitial pneumonia, nausea and vomiting, granulopenia, and oral ulcer were 0.42 (95% CI 0.23–0.61, I2 = 95.6%), 0.27 (95% CI 0.16–0.39, I2 = 88.3%), 0.32 (95% CI 0.14–0.49, I2 = 92.3%), 0.12 (95% CI 0.08–0.16, I2 = 0%), 0.12 (95% CI 0.05–0.18, I2 = 66%), 0.21 (95% CI 0.09–0.20, I2 = 66.2%), 0.21 (95% CI 0.09–0.33, I2 = 85.3%) and 0.11 (95% CI 0.05–0.16, I2 = 0%), respectively (Table 3).
Table 3.
Meta-analysis results of depicting toxicity.
3.3.2. Concurrent chemoradiotherapy combined with TKIs
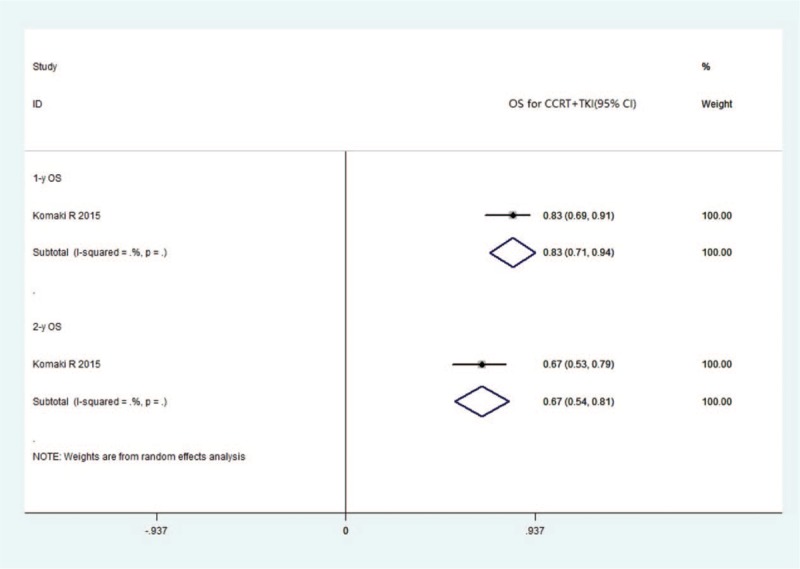
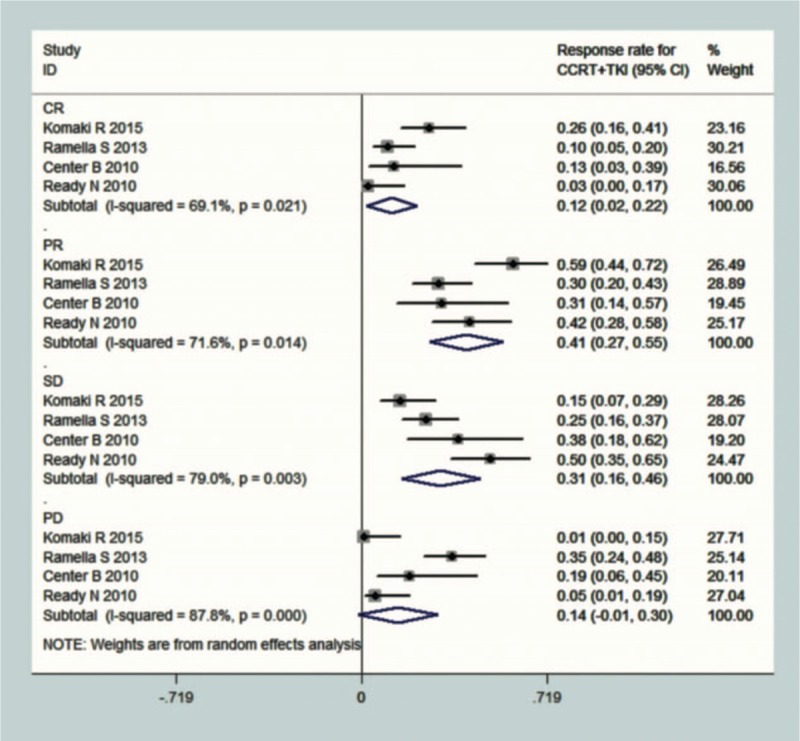
Four studies, which included 182 patients, reported the outcomes of concurrent chemoradiotherapy (CCRT) combined with TKIs. In all 4 studies, the pooled RR event rate was calculated, with CR, PR, SD, and PD values of 0.12 (95% CI 0.02–0.22, I2 = 69.1%), 0.41 (95% CI 0.27–0.55, I2 = 71.6%), 0.31 (95% CI 0.16–0.46, I2 = 79%), and 0.14 (95% CI −0.01–0.30, I2 = 87.8%), respectively (Fig. 5). Only a single study reported the survival event rate, with 1- and 2-year OS of 0.83 (95% CI 0.71–0.94) and 0.67 (95% CI 0.54–0.81), respectively (Fig. 6). The mPFS ranged from 4.7 to 14.0 months, with a mean value of 8.8 ± 3.4 months, whereas the mOS ranged from 13 to 36.5 months, with a mean value of 23.4 ± 8.4 months.
Figure 5.
Forest plots depicting response rate in CCRT+ TKI arm. CRT = chemo-radiotherapy, TKI = tyrosine kinase inhibitor.
Figure 6.
Forest plots depicting OS in CCRT+ TKI arm. CRT = chemo-radiotherapy, TKI = tyrosine kinase inhibitor, OS = overall survival.
The pooled incidences of the side effects of rash, diarrhea, esophagitis, interstitial pneumonia, nausea and vomiting, and anemia were 0.67 (95% CI 0.57–0.78, I2 = 29.3%), 0.11 (−0.00–0.23, I2 = 68.8%), 0.37 (95% CI 0.21–0.52, I2 = 77.9%), 0.17 (95% CI −0.09–0.43, I2 = 92.3%), 0.08 (95% CI −0.06–0.21, I2 = 79%), and 0.40 (95% CI −0.26–1.06, I2 = 97.8%), respectively (Table 3).
4. Discussion
The findings of this meta-analysis indicate that combined TRT [either Intensity Modulated Radiation Therapy (IMRT)/ conformal radiation therapy (CRT) or SBRT] or CCRT and TKIs can be an effective and safe approach to the treatment of advanced or metastatic NSCLC. This combination therapy model achieved favorable lung primary lesion response rates and superior survival rates with only mild side-effects. However, it should be noted that the quality of evidence supporting these findings is low because of our inclusion of non-controlled clinical studies.
Traditionally, chemotherapy has generally been the first-choice treatment option for advanced, locally advanced, and metastatic NSCLC. Some of the platinum regimens have been regarded as the standard first-line regimens for NSCLC with good performance status. The findings of large-scale network meta-analysis, performed to discern the best platinum regimens for chemo-naïve incurable NSCLC from 45 studies with 16,141 cases,[2] indicated that for the treatment of non-squamous NSCLC, carboplatin (CBP) + paclitaxel and CBP + pemetrexed resulted in the best OS with acceptable toxic side-effects. In a review that focused on pemetrexed clinical studies in in PS 2 patients with NSCLC,[49] the published completed studies that evaluated single-agent pemetrexed reported ORR values ranging from 4.5% to 15.8%, mPFS values ranging from 2.9 to 3.3 months, and mOS values ranging from 4.7 to 8.3 months. These outcomes are considerably inferior to those reported from clinical trials that have evaluated CCRT for the treatment of locally advanced or metastatic NSCLC. These findings are also notably inferior to the pooled results of EGFR-TKIs used in combination with TRT or CCRT obtained in the present meta-analysis. In the TRT combined with TKI arm, the pooled CR and PR were 6% and 44%, respectively, the mPFS ranged from 3.4 to 14. 7 months, and the mOS ranged from 5.2 to 28.5 months, whereas in the CCRT combined with TKI arm, the pooled CR and PR were 12% and 41%, respectively, the mPFS range from 4.7 to 14.0 months, and the mOS ranged from 13 to 36.5 months. Although it remains unclear why CCRT combined with TKI had better RR and survival benefits than TRT combined with TKI, one plausible explanation is that the most of included patients were either of unknown EGFR mutation status or lacked an EGFR mutation, who benefited to a greater extent from chemotherapy than from TKIs.
Three previously conducted meta-analyses have examined the efficacy of chemotherapy in combination with radiotherapy for locally advanced NSCLC,[50–52] and they found that the combined use of chemotherapy and radiotherapy improved survival compared with radiotherapy alone. However, the absolute benefit was relatively small, corresponding to a mean gain in median survival time of approximately 2 months and an increase in OS at 2 years of 3% to 4%. In a phase III randomized trial that compared combined chemoradiotherapy with radiotherapy alone in unresectable locally advanced NSCLC patients, Kim et al concluded that induction chemotherapy plus radiotherapy was superior to radiotherapy alone.[53] Furthermore, in a review conducted to assess the results of sequential versus concurrent chemoradiotherapy in inoperable stage III NSCLC patients, El-Sharouni concluded that the 5-year survival had increased from approximately 7% for radiotherapy alone to 10% for sequential therapy, and to approximately 15% for CCRT.[54] However, compared with sequential therapy using the same drug doses, CCRT schedules are associated with higher toxicity. Nevertheless, regardless of whether combined with EGFR-TKIs or chemotherapy, when compared with radiotherapy alone, the combination therapy modality had better survival benefits for advanced NSCLC patients. For patients with metastatic NSCLC, numerous randomized clinical trials have demonstrated the role of primary lesion radiotherapy in basic systematic therapy. The Cochrane Collaboration performed a systematic review involving 14 randomized clinical trials,[55] in which different dose schedules were used for the palliation of symptomatic primary lung cancer. In general, the results of these trials indicated that for patients with incurable NSCLC, radiotherapy can improve thoracic symptoms and prolong OS.
To date, there have been three generations of EGFR-TKIs, among which are gefitinib, erlotinib, icotinib, afatinib, and osimertinib. Recent data have indicated that screening patients for EGFR mutation is critical for appropriate selection of a first-line therapy. The first trial to confirm the utility of EGFR mutation as a predictor of anticancer efficacy was the Iressa Pan-Asia Study (IPASS), which investigated the outcomes of the overall study population (n = 1217) and subgroups [including those evaluable for EGFR mutation status (n = 437)] treated with gefitinib or carboplatin/paclitaxel.[56,57] The IPASS revealed superior PFS, ORR, symptom control, and quality of life with first-line gefitinib compared with carboplatin/paclitaxel in patients with EGFR mutation-positive tumors. Five additional phase III studies have subsequently verified the significantly increased PFS in response to treatment with EGFR-TKIs (gefitinib, erlotinib, and afatinib) compared with platinum-based chemotherapy in patients with EGFR mutation-positive tumors.[58–62] In all these trials, it was found that in NSCLC patients with EGFR mutations, TKIs produced a higher response rates and longer PFS than standard chemotherapy. However, for those NSCLC patients without EGFR mutations or for whom the EGFR mutation status is unknown, single-agent TKIs have not afforded superior ORR or OS compared with standard chemotherapy. Accordingly, further development of EGFR-TKI treatment in combination with TRT for molecularly selected patients is warranted.
The principle of administering EGFR-TKIs in combination with radiotherapy is based on the involvement of EGFR overexpression or mutation in radio-resistant cases of NSCLC. Baumann et al[63] summarized the findings of several pre-clinical studies and examined various combination mechanisms in addition to those mentioned above, including inactivating cancer stem cells directly to improve local tumor control, regulating several signaling pathways, suppressing DNA repair, decreasing cell repopulation, and improving re-oxygenation, all of which provided valid evidence for clinical practice. Although fundamental studies have shown that the radiosensitivity mechanism of TKIs is similar to that of EGFR monoclonal antibodies, the results of corresponding clinical trials have not been identical. This latter discrepancy could, in large part, be attributable to the fact that most of the studies evaluated have included NSCLC patients with unknown or negative EGFR mutation status, and have not conducted subgroup statistical analysis according to EGFR gene status. In the present meta-analysis, we identified 7 studies that have reported screening for EGFR mutation status in tissues from primary lung tumor. However, although EGFR mutation was detected in 123 of the 565 patients included in these studies, in none of the studies were the results analyzed according to EGFR status. Nevertheless, the pooled OS and RR of these trials were superior to those reported in previous chemoradiotherapy studies for advanced or metastatic NSCLC.
Theoretically, patients harboring an EGFR mutation should have better outcomes compared with EGFR wild-type patients among those NSCLC patients treated with TRT combined with TKIs. However, this expected difference has not been observed in the results obtained in clinical trials. In a systematic review to explore the impact of EGFR-sensitizing mutations on the outcomes of patients with NSCLC treated with definitive TRT,[64] Soon et al identified 7 studies that included 537 patients with stage III NSCLC. Up to 45% of the patients in these studies had mutations in exon 19 and 21. Compared with those patients characterized by EGFR wild-type status, those harboring EGFR-sensitizing mutations had a trend toward improvement in ORR. There were, however, no significant differences in LRR, DRR, RFS, OS, or adverse event outcomes between the EGFR mutant and EGFR wild-type groups. These authors thus concluded that EGFR-sensitizing mutations are not significant prognostic markers for patients with non-metastatic NSCLC undergoing definitive TRT, with or without chemotherapy. A more recent clinical trial has demonstrated the role of conventional radiotherapy and SBRT in patients with EGFR-mutant or anaplastic lymphoma kinase rearrangement-positive metastatic NSCLC.[65]. Among these patients, there were 50 with an mOS of 19.3 months and 1- and 2-year OS of 71.5% and 36.5%, respectively. The group treated with SBRT gained a significant benefit in terms of OS. These outcomes were significantly superior to those reported in the studies evaluated in the present meta-analysis. We believe that this inconsistency can be explained largely in terms of the use of the new generation of TKIs, such as osimertinib[66] or crizotinib,[67] which can overcome TKI resistance and reach higher concentrations in the cerebrospinal fluid.
To the best of our knowledge, there has been only one randomized phase II study that has incorporated tailored chemotherapy regimens into the TRT combined with TKI setting according to EGFR mutation status for unresectable stage III NSCLC patients.[68] The results showed that those patients with sensitive EGFR mutations had a significantly longer OS than EGFR wild-type patients (74.8 vs 25.3 months, P = .034), and that there were no unexpected toxicities. In addition, concurrent administration of erlotinib with standard double medicine containing platinum chemotherapy together with TRT was found to be safe and well tolerated without unexpected adverse events. In this regard, we are currently closely monitoring the results of on-going trials, such as RTOG 1306, to enable us to determine whether adding upfront EGFR-TKIs to standard CCRT would benefit patients with unresectable locally advanced EGFR mutant-related NSCLC.[69]
Another issue that we are paying close attention to is the adverse events associated with this combination treatment modality, particularly radiation-induced pneumonitis, because severe drug-induced interstitial lung disease, which has an incidence of approximately 1%, is the most concerning toxicity associated with the use of EGFR-TKIs.[70] In a Japanese clinical trial, 38 patients with stage III adenocarcinoma received 250 mg gefitinib daily in combination with TRT after induction therapy and one of 38 patients stopped CCRT due to grade 3 pneumonitis.[27] In another prospective study, 49 stage III NSCLC patients received erlotinib 150 mg daily for 6 days a week during TRT, but only three of 49 patients showed severe pneumonitis (2 with grade 3 and one with grade 4).[29] Although the number of subjects enrolled in these 2 studies were small, our meta-analysis indicated that studies with larger samples reported only mild rather than severe adverse events. The most common adverse events related to TKI use were rash and diarrhea, whereas the pooled incidence of Grade 1 to 3 esophagitis and Grade 1 to 2 interstitial pneumonia were, respectively, 32% and 12% in the TRT combined TKI arm and 37% and 17% in CCRT combined TKIs arm. Furthermore, no esophagitis or interstitial pneumonia higher than Grade 3 was reported in any of the included studies, even in the CCRT combined with TKI arm. Accordingly, we can conclude that TKIs can be safely used in combination with CCRT or TRT.
The current review does, nonetheless, have several limitations. First, without the inclusion of a controlled arm in prospective single-arm clinical trials, the quality of the summarized evidence is very low; moreover, we gathered information from published data rather than from individual patient data. We analyzed outcomes based on comparisons among single studies but not within the included studies, thereby raising concerns regarding the comparability of baseline characteristics between the studies. Second, the quality of the data was heterogeneous, as information on several important factors, including EGFR mutation status, organ distribution of metastatic lesions, number of metastases, status of extrapulmonary disease control, type of TKI medicine and application duration, difference of chemotherapy regimens in the CCRT arm, and the use of local salvage or systemic therapies, were not consistently reported. Third, the potential publication bias was reasonably high because we suspect that studies involving the use of TKI combination treatment that report negative results are unlikely to be published.
In summary, on the basis of the present meta-analysis, we can conclude that there is evidence, although the quality is not high, that the combination of TKIs with TRT or CCRT may improve the ORR and survival outcomes in advanced or metastatic NSCLC patients with unknown EGFR mutant status, relative to other studies that have evaluated the efficacy of TKIs alone, TRT alone, or CCRT. Although it was our intention to evaluate the difference between those patients with sensitive EGFR mutations and wild-type EGFR, we were unable to achieve this objective due to the lack of information on the EGFR genetic characteristics of individual patients. Several of the latest trials have, however, examined the efficacy of combination therapy based on EGFR-TKIs and radiotherapy according to EGFR genetic characteristics, and some have reported that the combination would be feasible, tolerated, and effective in certain populations of patients, indicating that the use of such combination therapy should be determined individually for specific cases based on the patient's genetic background.
Acknowledgments
The authors acknowledge methodology guiding by the center of evidence-based medicine of Lanzhou University in the process of research and language editing services by Wolters Kluwer Author Services in manuscript submission.
Author contributions
Conceptualization: Jinhui Tian.
Data curation: Ruifeng Liu, Shihong Wei, Hongtao Luo, Jinhui Tian.
Formal analysis: Ruifeng Liu, Jinhui Tian.
Investigation: Ruifeng Liu, Xueliang Zhang, Hongtao Luo.
Methodology: Ruifeng Liu, Qiuning Zhang.
Project administration: Xiaohu Wang.
Resources: Ruifeng Liu, Shihong Wei, Xueliang Zhang, Long Ge.
Software: Jinhui Tian, Yi Li, Long Ge.
Supervision: Shihong Wei, Qiuning Zhang, Xiaohu Wang.
Validation: Ruifeng Liu, Shihong Wei, Xiaohu Wang.
Visualization: Ruifeng Liu, Xueliang Zhang.
Writing – original draft: Ruifeng Liu.
Footnotes
Abbreviations: AE = adverse event, CR = complete response, CRT = chemo-radiotherapy, EGFR = epidermal growth factor receptor, NSCLC = non-small cell lung cancer, OS = overall survival, PD = progress disease, PR = partial response, RR = response rate, SD = stable disease, SAE = severe adverse event, TKI = tyrosine kinase inhibitor, TRT = thoracic radiotherapy.
This study was supported by National key research and development project of China (No. 2016YFC0105706), Talent innovation and venture project of Lanzhou city (No. 2017-RC-23), Gansu provincial healthcare industry science research project (No. GSWSKY-2017–17).
The authors have no conflicts of interest to disclose.
References
- [1].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [2].Horita N, Nagashima A, Nakashima K, et al. The best platinum regimens for chemo-naive incurable non-small cell lung cancer: network meta-analysis. Sci Rep 2017;7:13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].De Ruysscher D, Wanders R, van Baardwijk A, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: Long-term results of a prospective phase II trial (NCT01282450). J Thorac Oncol 2012;7:1547–55. [DOI] [PubMed] [Google Scholar]
- [4].Mehta N, Mauer AM, Hellman S, et al. Analysis of further disease progression in metastatic non-small cell lung cancer: Implications for locoregional treatment. Int J Oncol 2004;25:1677–83. [PubMed] [Google Scholar]
- [5].Higginson DS, Chen RC, Tracton G, et al. The impact of local and regional disease extent on overall survival in patients with advanced stage IIIB/IV non-small cell lung carcinoma. Int J Radiat Oncol Biol Phys 2012;84:e385–92. [DOI] [PubMed] [Google Scholar]
- [6].Su S, Li T, Lu B, et al. Three-dimensional radiation therapy to the primary tumor with concurrent chemotherapy in patients with stage IV non-small cell lung cancer: results of a multicenter phase 2 study from PPRA-RTOG, China. Int J Radiat Oncol Biol Phys 2015;93:769–77. [DOI] [PubMed] [Google Scholar]
- [7].Hirsch FR, Varella-Garcia M, Bunn PA, Jr, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol 2003;21:3798–807. [DOI] [PubMed] [Google Scholar]
- [8].Reck M, Popat S, Reinmuth N, et al. Metastatic non-small cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow up. Ann Oncol 2014. [DOI] [PubMed] [Google Scholar]
- [9].Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57. [DOI] [PubMed] [Google Scholar]
- [10].Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res 2005;65:3328–35. [DOI] [PubMed] [Google Scholar]
- [11].Das AK, Sato M, Story MD, et al. Non-small cell lung cancers with kinase domain mutations in the epidermal growth factor receptor are sensitive to ionizing radiation. Cancer Res 2006;66:9601–8. [DOI] [PubMed] [Google Scholar]
- [12].Chang CC, Chi KH, Kao SJ, et al. Upfront gefitinib/erlotinib treatment followed by concomitant radiotherapy for advanced lung cancer: A mono-institutional experience. Lung Cancer 2011;73:189–94. [DOI] [PubMed] [Google Scholar]
- [13].Swaminath A, Wright JR, Tsakiridis TK, et al. A Phase II trial of erlotinib and concurrent palliative thoracic radiation for patients with non-small-cell lung cancer. Clin Lung Cancer 2016;17:142–9. [DOI] [PubMed] [Google Scholar]
- [14].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [15].Rostom A, Dube C, Cranney A, et al. Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality(US); 2004. Sep. (Evidence Reports/Technology Assessment, No. 104) Appendix D. Quality Assessment Forms. http://ncbi.nlm.nih.gov/books/NBK35156. [Google Scholar]
- [16].Brown LD, Cat TT, DasGupta A. Interval estimation for a proportion. Stat Sci 2001;16:101–33. [Google Scholar]
- [17].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Levy A, Bardet E, Lacas B, et al. A phase II open-label multicenter study of gefitinib in combination with irradiation followed by chemotherapy in patients with inoperable stage III non-small cell lung cancer. Onco Target 2017;8:15924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Verma V, Kaur P, Chauhan A, et al. Palliative radiotherapy with concomitant and maintenance gefitinib for the management of locally advanced adenocarcinoma lung patients unfit for radical treatment. J Med Sci Clin Res 2017;5:24098–108. [Google Scholar]
- [20].Cai Y, Wang JY, Bai C. Clinical studies on conformal radiotherapy combined with epidermal growth factor receptor-tyrosine kinase inhibitor in second-line treatment of non-small cell lung cancer. Trop J Pharmaceut Res 2016;15:1053–60. [Google Scholar]
- [21].Lilenbaum R, Samuels M, Wang X, et al. A phase II study of induction chemotherapy followed by thoracic radiotherapy and erlotinib in poor-risk stage III non-small-cell lung cancer: Results of CALGB 30605 (Alliance)/RTOG 0972 (NRG). J Thorac Oncol 2015;10:143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li L, Liu LY, Chen M, et al. A pilot study of conformal radiotherapy combined with erlotinib-based multimodality therapy in newly diagnosed metastatic non-small-cell Lung cancer. Eur Rev Med Pharmacol Sci 2015;19:1812–20. [PubMed] [Google Scholar]
- [23].Zhuang H, Hou H, Yuan Z, et al. Preliminary analysis of the risk factors for radiation pneumonitis in patients with non-small-cell lung cancer treated with concurrent erlotinib and thoracic radiotherapy. Onco Targets Ther 2014;7:807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang Z, Zhu XX, Wu XH, et al. Gefitinib combined with stereotactic radiosurgery in previously treated patients with advanced non-small cell lung cancer. Am J Clin Oncol 2014;37:148–53. [DOI] [PubMed] [Google Scholar]
- [25].Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol 2014;32:3824–30. [DOI] [PubMed] [Google Scholar]
- [26].Pan D, Wang B, Zhou X, et al. Clinical study on gefitinib combined with γ-ray stereotactic body radiation therapy as the first-line treatment regimen for senile patients with adenocarcinoma of the lung (final results of JLY20080085). Mol Clin Oncol 2013;1:711–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Niho S, Ohe Y, Ishikura S, et al. Induction chemotherapy followed by gefitinib and concurrent thoracic radiotherapy for unresectable locally advanced adenocarcinoma of the lung: a multicenter feasibility study (JCOG 0402). Ann Oncol 2012;23:2253–8. [DOI] [PubMed] [Google Scholar]
- [28].Okamoto I, Takahashi T, Okamoto H, et al. Single-agent gefitinib with concurrent radiotherapy for locally advanced non-small cell lung cancer harboring mutations of the epidermal growth factor receptor. Lung Cancer 2011;72:199–204. [DOI] [PubMed] [Google Scholar]
- [29].Komaki R, Allen PK, Wei X, et al. Adding erlotinib to chemoradiation improves overall survival but not progression-free survival in stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2015;92:317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ramella S, Alberti AM, Cammilluzzi E, et al. Erlotinib and concurrent chemoradiation in pretreated NSCLC patients: radiobiological basis and clinical results. Biomed Res Int 2013;2013:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Center B, Petty WJ, Ayala D, et al. A phase i study of gefitinib with concurrent dose-escalated weekly docetaxel and conformal three-dimensional thoracic radiation followed by consolidative docetaxel and maintenance gefitinib for patients with stage III non-small cell lung cancer. J Thorac Oncol 2010;5:69–74. [DOI] [PubMed] [Google Scholar]
- [32].Ready N, Janne PA, Bogart J, et al. Chemoradiotherapy and gefitinib in stage III non-small cell lung cancer with epidermal growth factor receptor and KRAS mutation analysis: cancer and leukemia group B (CALEB) 30106, a CALGB-stratified phase II trial. J Thorac Oncol 2010;5:1382–90. [DOI] [PubMed] [Google Scholar]
- [33].Martínez E, Martínez M, Rico M, et al. Feasibility, tolerability, and efficacy of the concurrent addition of erlotinib to thoracic radiotherapy in locally advanced unresectable non-small-cell lung cancer: a phase II trial. Onco Targets Ther 2016;9:1057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Elamin Y, Gomez DR, Papadimitrakopoulou V, et al. Local consolidation therapy (LCT) after first line tyrosine kinase inhibitor (TKI) for patients with EGFR mutant metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 35D 2017: [DOI] [PubMed] [Google Scholar]
- [35].Lee JS, Moon SH, Lim KY, et al. Incorporating erlotinib into chemoradiation therapy for unresectable stage IIIa/b NSCLC: interim results of ongoing phase II randomized trial. J Thorac Oncol 2015;10:S288. [Google Scholar]
- [36].Komaki R, Allen P, Wei X, et al. Value of adding erlotinib to thoracic radiation therapy with chemotherapy for stage iii non-small cell lung cancer: a prospective phase ii study. J Thorac Oncol 2013;8:S136–7. [Google Scholar]
- [37].Iyengar P, Kavanagh BD, Smith I, et al. A phase II trial of second-line erlotinib in combination with stereotactic body radiation therapy (SBRT) for patients with metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 2013;31: [DOI] [PubMed] [Google Scholar]
- [38].Shen YX, Fan M, Chen JY, et al. Final report of a phase i study of concurrent thoracic radiotherapy and gefitinib in pre-treated patients with IIIB/IV non-small cell lung cancer (NSCLC). J Thorac Oncol 2011;6:S1584. [Google Scholar]
- [39].Chen J, Fan M, Jiang GL, et al. Concurrent thoracic radiotherapy (TRT) with single-agent gefitinib in patients with pre-treated IIIB/IV non-small cell lung cancer (NSCLC): Final report of a phase I study with EGFR mutation analyses. Int J Radiat Oncol Biol Phys 2011;81:S577–8. [Google Scholar]
- [40].Komaki RU, Blumenschein GR, Wistuba LL, et al. The Phase II trial of erlotinib and radiotherapy following chemoradiotherapy for patients with Stage III non-small cell lung cancer has shown a favorable response profile. Int J Radiat Oncol Biol Phys 2011;81:S591–2. [Google Scholar]
- [41].Sun J, Wang YM, Duan YZ. Concurrent erlotinib and thoracic radiotherapy as 1st-line treatment of stage IV NSCLC patients with EGFR active mutations (CERTAIN study). Ann Oncol 2015;26:ix145. [Google Scholar]
- [42].Hansen O, Knap M, Khalil A, et al. Phase II trial of concurrent erlotinib in locally advanced non-small cell lung cancer (LA-NSCLC). Radiother Oncol 2017;123:S663. [DOI] [PubMed] [Google Scholar]
- [43].Pang Q, Wang P, Wang J, et al. EGFR TKIs combined with definite radiotherapy for stage iv non-small cell lung cancer with bone-only metastases. Radiother Oncol 2012;103:S288. [Google Scholar]
- [44].Wang J, Xia T, Wang Y, et al. Phase II study of tyrosine kinase inhibitors concurrent with definitive thoracic radiotherapy for stage IV non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2011;81:S575–6. [DOI] [PubMed] [Google Scholar]
- [45].Werner-Wasik M, Pequignot E, Campling B, et al. A phase 2 study of erlotinib (ERL) and hypofractionated thoracic radiation therapy (HRT) in patients (PTS) with non-small cell lung cancer (NSCLC) and comorbidities. Int J Radiat Oncol Biol Phys 2013;87:S508. [Google Scholar]
- [46].Warren L, Turcotte JC, Wehrenberg-Klee E, et al. Phase 2 trial of afatinib sequenced with concurrent chemo/radiation in EGFR-mutant non-small cell lung cancer: Initial feasibility and response analysis. Int J Radiat Oncol 2016;96:E455. [Google Scholar]
- [47].Zhang X, Wang B, Lin L, et al. Local treatment combined with EGFR-TKIs for advanced non-small cell lung cancer with solitary progression during EGFR-TKI treatment. Zhongguo Fei Ai Za Zhi 2013;16:514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Qi WJ, Kang JB, Nie Q, et al. Body-gamma knife combined with gefitinib as the therapy in treating the elderly patients with advanced non-small cell lung cancer. Chin J Cancer Prevent Treat 2010;17:1301–3. [Google Scholar]
- [49].Zinner R, Visseren-Grul C, Spigel DR, et al. Pemetrexed clinical studies in performance status 2 patients with non-small cell lung cancer (Review). Int J Oncol 2016;48:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Marino P, Preatoni A, Cantoni A. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell lung cancer. A meta-analysis. Cancer 1995;76:593–601. [DOI] [PubMed] [Google Scholar]
- [51].Pritchard RS, Anthony SP. Chemotherapy plus radiotherapy compared with radiotherapy alone in the treatment of locally advanced, unresectable, non-small cell lung cancer: a meta-analysis. Ann Intern Med 1996;125:723–9. [DOI] [PubMed] [Google Scholar]
- [52].Non-small Cell Lung Cancer Collaborative Group: Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical, trials. BMJ 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- [53].Kim TY, Yang SH, Lee SH, et al. A phase III randomized trial of combined chemoradiotherapy versus radiotherapy alone in locally advanced non-small-cell lung cancer. Am J Clin Oncol 2002;25:238–43. [DOI] [PubMed] [Google Scholar]
- [54].El-Sharouni SY, Kal HB, Battermann JJ, et al. Sequential versus concurrent chemo-radiotherapy in inoperable stage III non-small cell lung cancer. Anti-cancer Res 2006;26(1B):495–505. [PubMed] [Google Scholar]
- [55].Stevens R, Macbeth F, Toy E, et al. Palliative radiotherapy regimens for patients with thoracic symptoms from non-small cell lung cancer. Cochrane Database Syst Rev 2015;1:CD002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866–74. [DOI] [PubMed] [Google Scholar]
- [57].Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57. [DOI] [PubMed] [Google Scholar]
- [58].Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–8. [DOI] [PubMed] [Google Scholar]
- [59].Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–8. [DOI] [PubMed] [Google Scholar]
- [60].Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46. [DOI] [PubMed] [Google Scholar]
- [61].Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–34. [DOI] [PubMed] [Google Scholar]
- [62].Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42. [DOI] [PubMed] [Google Scholar]
- [63].Baumann M, Krause M, Dikomey E, et al. EGFR-targeted anti-cancer drugs in radiotherapy: preclinical evaluation of mechanisms. Radiother Oncol 2007;83:238–48. [DOI] [PubMed] [Google Scholar]
- [64].Soon YY, Vellayappan B, Tey JCS, et al. Impact of epidermal growth factor receptor sensitizing mutations on outcomes of patients with non-small cell lung cancer treated with definitive thoracic radiation therapy: a systematic review and meta-analysis. Oncotarget 2017;8:109712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Borghetti P, Bonù ML, Roca E, et al. Radiotherapy and tyrosine kinase inhibitors in stage IV non-small cell lung cancer: real-life experience. In Vivo 2018;32:159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bollinger MK, Agnew AS, Mascara GP. Osimertinib: A third-generation tyrosine kinase inhibitor for treatment of epidermal growth factor receptor-mutated non-small cell lung cancer with the acquired Thr790Met mutation. J Oncol Pharm Pract 2018;24:379–88. [DOI] [PubMed] [Google Scholar]
- [67].Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167–77. [DOI] [PubMed] [Google Scholar]
- [68].Lee Y, Han JY, Moon SH, et al. Incorporating erlotinib or irinotecan plus cisplatin into chemoradiotherapy for stage III non-small cell lung cancer according to EGFR mutation status. Cancer Res Treat 2017;49:981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].RTOG Clinical Trials Study Number 1306. Rtog.Org. 2017. Available at: https://www.rtog.org/Clinical Trials/Protocol Table/Study Details. aspx? study=1306 [access date May 1, 2017]. [Google Scholar]
- [70].Qi WX, Sun YJ, Shen Z, et al. Risk of interstitial lung disease associated with EGFR-TKIs in advanced non-small-cell lung cancer: a meta-analysis of 24 phase III clinical trials. J Chemother 2015;27:40–51. [DOI] [PubMed] [Google Scholar]


