Abstract
Rationale:
Pulmonary hypertension (PH) is a complicated disease which has complex causes and poor outcome. Many factors are involved in the increase of pulmonary artery pressure. It is often difficult to identify the specific cause of a particular patient. However, identifying the etiology is of great importance for specifying treatment strategies and improving the prognosis of patients.
Patient concerns:
A 58-year-old male was admitted because of fatigue, breath shortness for 6 months, which got worse in the last 3 months. The ultrasound cardiogram (UCG) indicated a remarkably elevated pulmonary artery systolic pressure (PASP = 82 mm Hg). He had hypertension for 15 years. Besides, his spleen was found to be enlarged since 15 years ago. Bone marrow biopsy of the patient revealed myeloproliferative neoplasm (MPN) with severe myelofibrosis (MF).
Diagnosis:
Myeloproliferative neoplasm (MPN) with severe myelofibrosis (MF) which in turn caused PH and portal vein hypertension (PVH).
Interventions:
We treated the patient with diuretics and fosinopril, and also steroids and thalidomide for his MPN/MF.
Outcomes:
Two weeks later, the pulmonary artery pressure (PAP) was remarkably decreased (PASP = 53.1 mm Hg by UCG, mean PAP = 21 mm Hg by right cardiac catheterization). Within 2 years’ follow-up, his circulatory state and hematological state remained stable.
Lessons:
It is often difficult to define the cause of PH, but it is important for making the appropriate treatment at the same time.
Keywords: megalosplenia, myelofibrosis, myeloproliferative neoplasm, portal vein hypertension, pulmonary hypertension
1. Introduction
Pulmonary hypertension (PH) has had a significant increase in attention over the recent years. This has been driven simultaneously by the advent of new treatments for this condition and new insights into the genetic and pathological drivers of the disease. However, despite current therapeutics, the 3-year survival rate is about 50% to 70%, illustrates the ongoing challenges of managing this disease.[1–5] The poor prognosis of PH is partially due to its diverse etiology and complex pathophysiological mechanisms. There are hundreds of causes for PH,[6,7] and the therapies for PH caused by different causes are significantly different. Therefore, determining the cause for PH of a certain patient is critical to make the most optimal treatment plan for him.[8,9] However, to identify the causes of PH is often a challenging task in clinical practice. Careful analysis of the patient's condition is crucial to make clear the real etiology of the patient.
2. Case presentation
A 58-year-old male was admitted to the hospital because of fatigue, breath shortness for 6 months, and got worse in the last 3 months. He had taken an ultrasound cardiogram (UCG) in another hospital 1 week before admission which suggested a mildly enlarged left heart (left ventricular end-diastolic diameter [LVEDD] = 55 mm) with an otherwise normal left ventricular ejection fraction (54%) and a significantly enlarged right heart (right ventricular end-diastolic diameter [RVEDD] = 50 mm). The UCG also indicated a remarkably increased pulmonary artery systolic pressure (PASP = 82 mm Hg), accompanied by a widened inferior vena cava (22 mm) with decreased compression during inspiratory (<50%). After taking furosemide 20 mg Qd, spironolactone 20 mg Qd, and captopril 6.25 mg Bid for 1 week, the patient did not feel better. Previously, he had hypertension for 15 years. His spleen was found to be enlarged 15 years ago, and was referred to as “massive splenomegaly” since 6 years ago. Four years ago, his portal vein was found to be widened during abdominal ultrasound examination. However, examinations such as abdominal ultrasound and CT had not identified the cause for these abnormalities. He was not a smoker, but he used to drink about 100 g alcohols per day for more than 30 years, which he just quitted 2 months ago. The changes of his portal vein, spleen vein, and spleen are shown in Figure 1.
Figure 1.
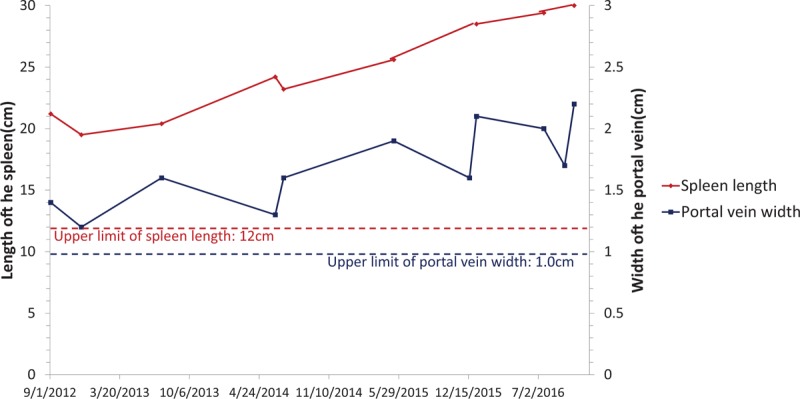
The changes of portal vein and spleen of the patient.
On admission, we found his enlarged spleen had reached the right lower abdomen. His lower extremities had moderate edema on both sides. The ECG (Fig. 2A) and cardiac markers test of the patient were otherwise normal. In order to get further information, we had another UCG which also suggested heart enlargement (LVEDD 58 mm, RVEDD 49 mm). But, we also found the thickness of his ventricular wall was slightly increased (left ventricular free wall and interventricular septum: 12 mm; right ventricular free wall: 0.6 cm). An increased main pulmonary artery diameter (36 mm by UCG) and significantly increased pulmonary artery pressure (PASP>90 mm Hg by UCG, Fig. 2B1, B2) had also been proved. Furthermore, the UCG detected slightly decreased systolic function of the RV (tricuspid annular plane systolic excursion, TAPSE=1.1 cm). A cardiac enhanced MR (CEMR, Fig. 2C1, C2) found abnormal delayed enhancement only in the root of the anterior papillary muscle, while other myocardial muscles seem to be normal. What is more, the cardiac output (CO) measured by the CEMR reached as 9.0 L/min.
Figure 2.
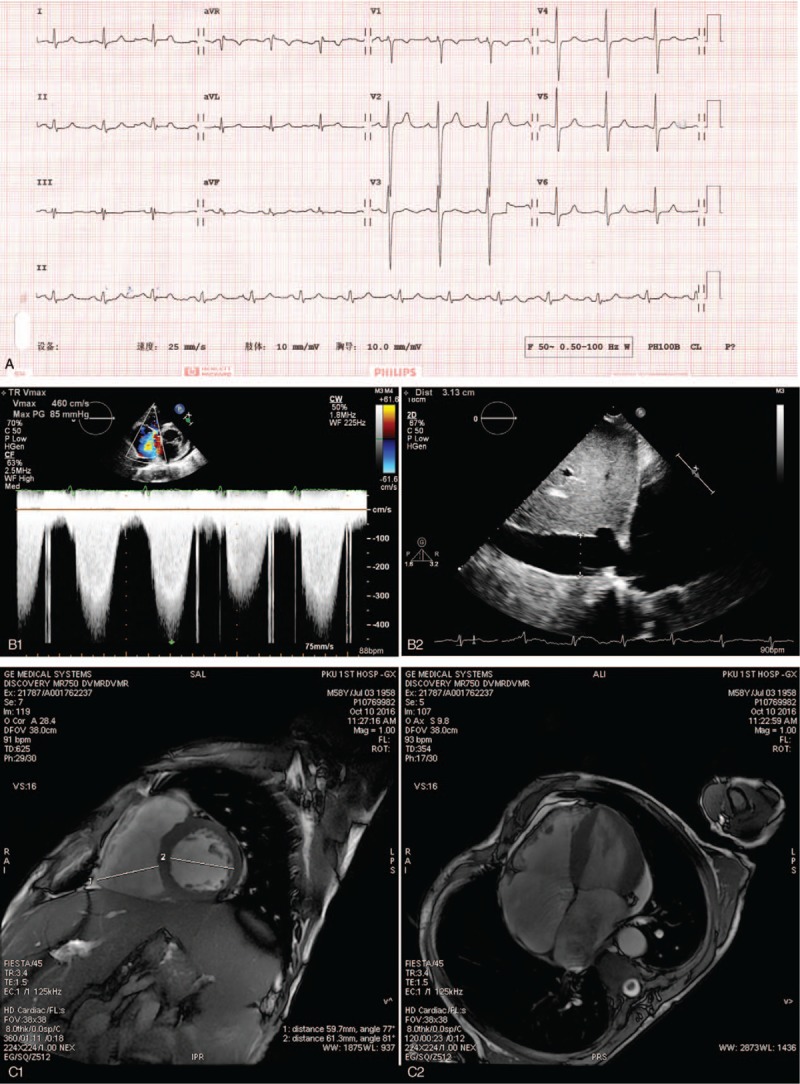
The results of ECG, UCG, and cardiac enhanced MR. A, It showed an otherwise normal ECG. B1, It showed the Doppler spectrum of the tricuspid valve regurgitation and the estimated tricuspid valve transvalvular pressure. B2, It showed the widened inferior vena cava, which suggested increased inferior vena cava and right atrial pressure. Combined B1 and B2, we can estimate the PASP. (C1) and (C2) were imagines of CEMR, and show the significantly enlarged heart.
As for other test results, the routine blood test showed a mild anemia (hemoglobin 105 g/L) and increased platelets (458×109/L) and basophile (0.8 × 109/L). His creatinine, transaminase, bilirubin and albumin levels were within normal limits, while LDH was increased (758IU/L). The abdominal ultrasound (Fig. 3) also found his spleen had entered the pelvic cavity (the intercostal thickness of spleen was 7.9 cm). An enlarged liver (oblique diameter of the right liver lobe 18.2 cm) and widened portal vein (1.8 cm) had also been found. Meanwhile, no signs of thrombosis could be found in the portal vein and inferior vena cava. Our imaging expert consulted the patient's previous abdominal enhanced CT and other images and came to similar conclusions.
Figure 3.
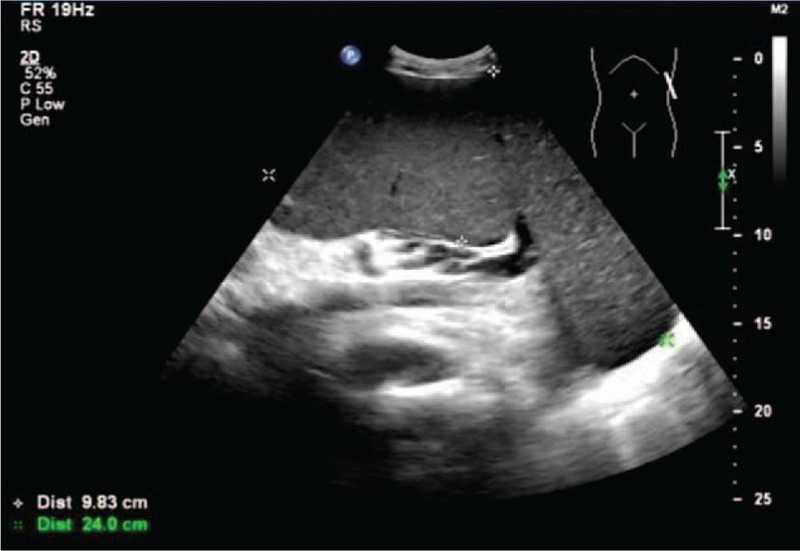
The B-mode ultrasonography of spleen.
As his clinical manifestations and examination results indicated severe pulmonary hypertension and right heart failure, we tried to identify the cause of these abnormalities. We excluded structural left heart diseases, serious lung diseases, hypoxia, severe chronic pulmonary embolism and other pulmonary artery obstructions by UCG, CEMR and coronary CTA, pulmonary function test, arterial blood gas test and pulmonary ventilation and perfusion scan. However, the PVH and massive splenomegaly of the patient attracted our attention. As for the reason for his PVH, the analysis of hepatic venous pressure gradient (inferior vena cava pressure = 9mmHg, hepatic vein pressure = 9mmHg, wedged hepatic vein pressure = 15mmHg, hepatic vein pressure gradient = 6mmHg) suggested that it was a hepatic portal hypertension. The most common cause of hepatic portal hypertension is cirrhosis. Although the patient had a long history of heavy drinking (100 g alcohol per day for 30 years), his biochemical tests and abdominal imaging showed no evidence of cirrhosis. Non-cirrhotic hepatic portal hypertension always a headache problem in clinical practice. However, the high platelet and basophil cells, high LDH and the massive splenomegaly led us to consider about hematological problems. A further analysis of his peripheral blood smears found the percentage of the myelocyte and the metamyelocyte were 8% and 2%. The results of bone marrow biopsy (shown in Fig. 4) showed myeloproliferative neoplasm (MPN) with severe myelofibrosis (MF). The result of his gene analysis showed the expression of a JAK2 (Janus kinase 2) mutation, V617F, which also supported the diagnosis of MPN.
Figure 4.
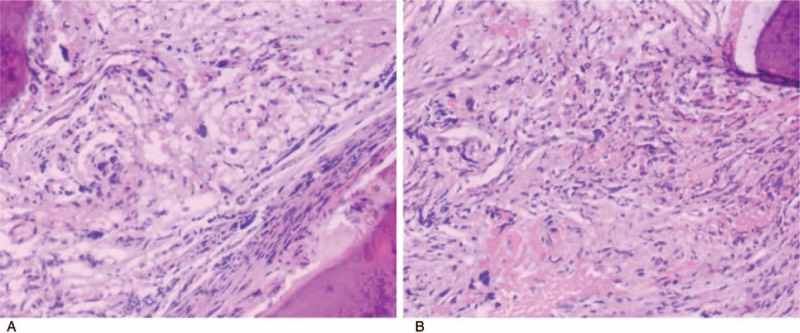
The result of his bone marrow biopsy. The bone marrow biopsy revealed significant hyperplasia of interstitial fibrous tissue and reticular fiber, and also hyperplasia of megakaryocyte, which distributed in a cluster. Naked megakaryocytes were visible. In conclusion, the patient had myeloproliferative neoplasms with severe myelofibrosis.
We treated the patient with furosemide, spironolactone and fosinopril in order to reduce the volume load of the patient and prevent cardiac remodeling. Steroids and thalidomide were given to the patient for his MPN and MF. Because of economic problem, the patient rejected to use targeting drugs for PH and MPN/MF.
2 weeks later, before his release from hospital, his symptom significantly released, and his pulmonary artery pressure was remarkably decreased (PASP=53.1 mm Hg by UCG; mean PAP=21 mm Hg, systolic PAP=34 mm Hg, diastolic PAP=15 mm Hg by right cardiac catheterization). During the 2-year follow-up, his symptom circulatory state and hematological state remained stable.
3. Discussion
This patient presented with severe edema and dyspnea which is considered to be associated with PH. PH is a complex clinical problem with various etiologies and unfavorable prognosis. Identifying the causes of PH is important for making the best therapy plan. However, it is difficult to determine the causes of PH sometimes. For this patient, we found that he had MPN and MF, which had caused his massive splenomegaly and PVH, and also probably was the cause of his PH, as no other reason of PH was found.
The relationship between MPN/MF and PH has long been discovered, but still not been fully understood. The prevalence of PH in the context of MPNs was still poorly defined. It ranged from 5% to about 50% among different studies, while in most studies it is about 40%.[10–17] Precapillary PH in patients affected by MPN/MF may be caused by several factors: PVH, pulmonary veno-occlusive disease, tumor microembolism, pulmonary myeloid infiltration, enhanced angiogenesis, and a hypermetabolic state with high cardiac output and left ventricular dysfunction.[16,18–20] PVH is a common complication and seen in up to 17% of patients of MPN with MF.[16,21] It is reported that patients with PVH caused by MPN/MF have some unique characteristics compared with PVH caused by other reasons: normal liver function (normal albumin level), splenomegaly but no hypersplenism (normal white blood cell and platelet count), and immature blood cells can be seen in peripheral blood.[22] All these features were present in this patient. Hyperdynamic circulation and high cardiac output were an important change of this patient. This change was also reported in other studies about MPN/MF.[16,23] The causes of hyperdynamic circulation include excessive blood volume caused by extramedullary hematopoiesis, and also anemia. It explained the generally enlarged and slightly thickened left and right ventricular of this patient. More importantly, hyperdynamic circulation could be a cause of both PVH and PH and is commonly seen in portopulmonary hypertension.[24] Overall, the progress and pathogenesis of the patient can be seen in Figure 5.
Figure 5.
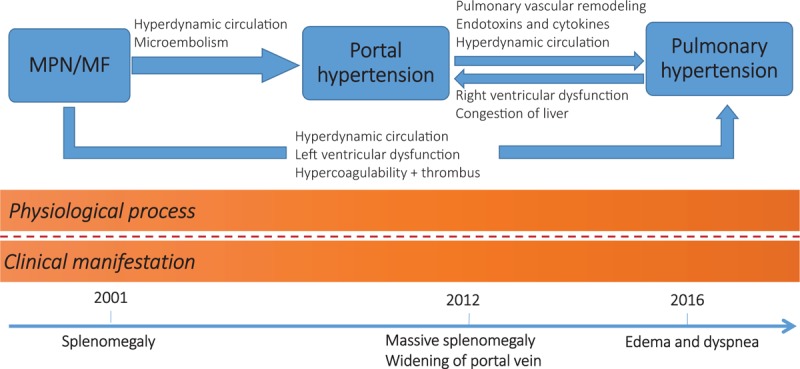
The flow chart of the progress and pathogenesis of the patient.
The treatment strategy for MPN/MF patients with PH remains controversial. For these patients, we should pay attention to their MPN/MF and PH at the same time. Conventional agents for MPN/MF, such as hydroxyurea and oral alkylating agents, may reduce splenomegaly in some patients, but its benefit is usually of short duration and tolerability is poor.[18,19,25] Ruxolitinib is currently the only approved treatment for patients with MF that has been shown in pivotal randomized clinical trials to be highly effective in alleviating symptom burden and splenomegaly.[26,27] However, it may have an adverse effect on pulmonary hypertension .[28,29] The combination of thalidomide and prednisone has been evaluated in patients with MF and has shown remarkable efficiency.[30] Splenectomy and transjugular intrahepatic shunt (TIPS) are treatments that can be considered for splenomegaly and portal hypertension caused by MF.[31–33] However, both splenectomy[34,35] and TIPS[36,37] seems to have adverse effects on pulmonary circulation on long-term follow up, which limited their application in patients with elevated pulmonary pressure. Splenic irradiation is a palliative treatment for symptomatic splenomegaly due to myelofibrosis [25,38,39], which may also have a positive effect on pulmonary hypertension.[40] Hematopoietic stem cell transplantation might also be a resolution of myelofibrosis-associated PAH.[41]
For this patient, we gave him diuretics to reduce his circulation volume, thalidomide for his MPN/MF. During the 2-year follow-up, his symptoms were stable, and the pulmonary artery systolic pressure measured by UCG remained at 40 to 50 mm Hg.
In general, it is a rare condition that PH coexisted with massive splenomegaly. In this case, after having MPN combined with severe MF without appropriate treatment for more than 10 years, the patient got massive splenomegaly. Under the influence of high volume, PVH and other possible factors, the patient developed PH. This case suggests that to find out the final cause of PH is often difficult, but important for providing the best therapy for patients.
Acknowledgments
The authors also thank all who helped during the writing of this thesis.
Author contributions
Funding acquisition: Tieci Yi.
Investigation: Tieci Yi, Wei Ma, Jianxing Qiu, Wenhui Ding.
Resources: Tieci Yi.
Software: Jianxing Qiu.
Supervision: Wei Ma, Wenhui Ding.
Writing – original draft: Tieci Yi.
Writing – review & editing: Tieci Yi.
Tieci Yi orcid: 0000-0001-9080-7745.
Footnotes
Abbreviations: MF = myelofibrosis, MPN = myeloproliferative neoplasm, PAP = pulmonary artery pressure, PASP = pulmonary artery systolic pressure, PH = pulmonary hypertension, PVH = portal vein hypertension, UCG = ultrasound cardiogram.
The patient has provided informed consent to publication of the case.
This work is supported by grants from the Scientific Research Seed Fund of Peking University First Hospital (2018SF065).
The authors have no conflicts of interest to disclose.
References
- [1].Lim Y, Low TT, Chan SP, et al. Pulmonary arterial hypertension in a multi-ethnic Asian population: characteristics, survival and mortality predictors from a 14-year follow-up study. Respirology 2019;24:162–70. [DOI] [PubMed] [Google Scholar]
- [2].Farber HW, Miller DP, Poms AD, et al. Five-year outcomes of patients enrolled in the REVEAL registry. Chest 2015;148:1043–54. [DOI] [PubMed] [Google Scholar]
- [3].Strange G, Lau EM, Giannoulatou E, et al. Survival of idiopathic pulmonary arterial hypertension patients in the modern era in Australia and New Zealand. Heart Lung Circ 2018;27:1368–75. [DOI] [PubMed] [Google Scholar]
- [4].Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156–63. [DOI] [PubMed] [Google Scholar]
- [5].Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012;186:790–6. [DOI] [PubMed] [Google Scholar]
- [6].Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- [8].Taichman DB, Mandel J. Epidemiology of pulmonary arterial hypertension. Clin Chest Med 2013;34:619–37. [DOI] [PubMed] [Google Scholar]
- [9].Yildiz M, Sahin A, Behnes M, et al. An expanding role of biomarkers in pulmonary arterial hypertension. Curr Pharm Biotechnol 2017;18:491–4. [DOI] [PubMed] [Google Scholar]
- [10].Reisner SA, Rinkevich D, Markiewicz W, et al. Cardiac involvement in patients with myeloproliferative disorders. Am J Med 1992;93:498–504. [DOI] [PubMed] [Google Scholar]
- [11].Garypidou V, Vakalopoulou S, Dimitriadis D, et al. Incidence of pulmonary hypertension in patients with chronic myeloproliferative disorders. Haematologica 2004;89:245–6. [PubMed] [Google Scholar]
- [12].Gupta R, Perumandla S, Patsiornik Y, et al. Incidence of pulmonary hypertension in patients with chronic myeloproliferative disorders. J Natl Med Assoc 2006;98:1779–82. [PMC free article] [PubMed] [Google Scholar]
- [13].Altintas A, Karahan Z, Pasa S, et al. Pulmonary hypertension in patients with essential thrombocythemia and reactive thrombocytosis. Leuk Lymphoma 2007;48:1981–7. [DOI] [PubMed] [Google Scholar]
- [14].Cortelezzi A, Gritti G, Del Papa N, et al. Pulmonary arterial hypertension in primary myelofibrosis is common and associated with an altered angiogenic status. Leukemia 2008;22:646–9. [DOI] [PubMed] [Google Scholar]
- [15].Chebrek S, Aissi K, Frances Y, et al. Pulmonary hypertension in patients with chronic myeloproliferative neoplasms. Leuk Lymphoma 2014;55:223–5. [DOI] [PubMed] [Google Scholar]
- [16].Adir Y, Elia D, Harari S. Pulmonary hypertension in patients with chronic myeloproliferative disorders. Eur Respir J 2015;24:400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Salit RB, Edwards R, Baker K, et al. Application of noninvasive methods to detect pulmonary hypertension in pre-transplant patients with myelofibrosis. Biol Blood Marrow Transpl 2018;24(3 suppl):S291. [Google Scholar]
- [18].Tefferi A. Primary myelofibrosis: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol 2016;91:1262–71. [DOI] [PubMed] [Google Scholar]
- [19].Mughal TI, Vaddi K, Sarlis NJ, et al. Myelofibrosis-associated complications: pathogenesis, clinical manifestations, and effects on outcomes. Int J Gen Med 2014;7:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mathew R, Huang J, Wu JM, et al. Hematological disorders and pulmonary hypertension. World J Cardiol 2016;8:703–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yan M, Geyer H, Mesa R, et al. Clinical features of patients with Philadelphia-negative myeloproliferative neoplasms complicated by portal hypertension. Clin Lymphoma Myeloma Leuk 2015;15:e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Song ZQ, Zhou L Y. A clinical analysis of six cases of portal hypertension secondary to primary myelofibrosis and review of literatures. Zhonghua Nei Ke Za Zhi 2010;49:845–7. [PubMed] [Google Scholar]
- [23].Ziakas PD, Voulgarelis M, Felekouras E, et al. Myelofibrosis-associated massive splenomegaly: a cause of increased intra-abdominal pressure, pulmonary hypertension, and positional dyspnea. Am J Hematol 2005;80:128–32. [DOI] [PubMed] [Google Scholar]
- [24].Katsuta Y, Zhang XJ, Aramaki T. Pulmonary hypertension complicating portal hypertension: portopulmonary hypertension. Nihon Rinsho 2001;59:1186–92. [PubMed] [Google Scholar]
- [25].Iurlo A, Cattaneo D. Treatment of myelofibrosis: old and new strategies. Clin Med Insights Blood Disord 2017;10: 1179545X17695233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Leung M, Highsmith K, Rexwinkle A. Pharmacologic management of myelofibrosis. J Oncol Pharmacy Pract 2017;23:591–601. [DOI] [PubMed] [Google Scholar]
- [27].Stahl M, Zeidan AM. Management of myelofibrosis: JAK inhibition and beyond. Expert Rev Hematol 2017;10:459–77. [DOI] [PubMed] [Google Scholar]
- [28].Low AT, Howard L, Harrison C, et al. Pulmonary arterial hypertension exacerbated by ruxolitinib. Haematologica 2015;100:e244–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McGee M, Whitehead N, Martin J, et al. Drug-associated pulmonary arterial hypertension. Clin Toxicol (Phila) 2018;56:801–9. [DOI] [PubMed] [Google Scholar]
- [30].Luo X, Xu Z, Li B, et al. Thalidomide plus prednisone with or without danazol therapy in myelofibrosis: a retrospective analysis of incidence and durability of anemia response. Blood Cancer J 2018;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Belohlavek J, Schwarz J, Jirásek A, et al. Idiopathic myelofibrosis complicated by portal hypertension treated with a transjugular intrahepatic portosystemic shunt (TIPS). Wiener klinische Wochenschrift 2001;113:208–11. [PubMed] [Google Scholar]
- [32].Alvarez-Larrán A, Abraldes JG, Cervantes F, et al. Portal hypertension secondary to myelofibrosis: a study of three cases. Am J Gastroenterol 2005;100:2355. [DOI] [PubMed] [Google Scholar]
- [33].Lukie BE, Card RT. Portal hypertension complicating myelofibrosis: reversal following splenectomy. Can Med Assoc J 1977;117:771–2. [PMC free article] [PubMed] [Google Scholar]
- [34].Palkar AV, Agrawal A, Verma S, et al. Post splenectomy related pulmonary hypertension. World J Respirol 2015;5:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rørholt M, Ghanima W, Farkas DK, et al. Risk of cardiovascular events and pulmonary hypertension following splenectomy: a Danish population-based cohort study from 1996-2012. Haematologica 2017;102: 1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wannhoff A, Hippchen T, Weiss CS, et al. Cardiac volume overload and pulmonary hypertension in long-term follow-up of patients with a transjugular intrahepatic portosystemic shunt. Aliment Pharmacol Ther 2016;43:955–65. [DOI] [PubMed] [Google Scholar]
- [37].Busk TM, Bendtsen F, Henriksen JH, et al. Effects of transjugular intrahepatic portosystemic shunt (TIPS) on blood volume distribution in patients with cirrhosis. Dig Liver Dis 2017;49:1353–9. [DOI] [PubMed] [Google Scholar]
- [38].Mesa RA. How I treat symptomatic splenomegaly in patients with myelofibrosis. Blood 2009;113:5394–400. [DOI] [PubMed] [Google Scholar]
- [39].de la Pinta C, Fernández Lizarbe E, Montero Luis Á, et al. Treatment of symptomatic splenomegaly with low doses of radiotherapy: retrospective analysis and review of the literature. Tech Innovations Patient Support Radiation Oncol 2017;3:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Steensma DP, Hook CC, Stafford SL, et al. Low-dose, single-fraction, whole-lung radiotherapy for pulmonary hypertension associated with myelofibrosis with myeloid metaplasia. Br J Haematol 2002;118:813–6. [DOI] [PubMed] [Google Scholar]
- [41].Faiz SA, Iliescu C, Lopez-Mattei J, et al. Resolution of myelofibrosis-associated pulmonary arterial hypertension following allogeneic hematopoietic stem cell transplantation. Pulmonary Circ 2016;6:611–3. [DOI] [PMC free article] [PubMed] [Google Scholar]


