Abstract
The aim of the study was to introduce our in-house software to measure the muscle and adipose area on axial computed tomography (CT) scans and to compare with various quantification methods.
Our institutional review board approved this retrospective study and informed consent was waived. We developed in-house software to identify body composition analysis on CT scan, which semiautomatically operates 3 image processing steps. Abdominal images were obtained using multidetector row CT (MDCT). Two radiologists analyzed the same cross-sectional areas of subcutaneous fat, muscle, and visceral fat using the following techniques: manual measurements, Aquarius, ImageJ, and our newly developed software. We calculated an intraclass correlation coefficient (ICC) for comparison of muscle and fat areas quantified by various measurement methods using a 2-way random model. Interobserver agreement between the radiologists was also evaluated.
Agreements in the measurement of subcutaneous fat and muscle areas were excellent among the methods (ICC = 0.962 and 0.897, respectively), and that of the visceral fat area was good (ICC = 0.822). In the subgroup analysis, ICC of the visceral fat area in the female group and in subjects with ascites was slightly lower than the other group (ICC = 0.742 and 0.787, respectively). The correlation coefficients between our software and other methods were relatively high (r = 0.854–0.996). Additionally, ICCs between both observers of our program for quantification of subcutaneous fat, muscle, and visceral fat areas were 0.999, 0.980, and 0.999, respectively.
In conclusion, our method showed be reliable in quantifying muscle and adipose tissue using cross-sectional areas of MDCT with high reproducibility.
Keywords: body composition, muscles, subcutaneous fat, tomography, visceral fat, X-ray computed
1. Introduction
Sarcopenia is defined as the progressive and generalized loss of skeletal muscle mass and strength. Recent studies have shown that change in body composition, such as sarcopenia, is a significant factor that correlates to poor outcomes in various clinical conditions.[1–6] There have been various studies about sarcopenia or increased visceral fat as a significant prognostic factor in patients with cirrhosis or gastrointestinal cancer.[5,7,8] In particular, sarcopenia became to known as an independent predictor related with mortality for patients with cirrhosis, and is also associated with postliver transplantation mortality and recurrent hepatocellular carcinoma (HCC) after treatment.[7–12] Sarcopenia patients with nonalcoholic steatohepatitis (NASH) have larger amounts of visceral fat, and visceral fat is also an independent risk factor for the recurrence of HCC after treatment in patients with NASH.[13] In addition, sarcopenia is associated with HCC development in patients with nonalcoholic fatty liver disease.[14] Therefore, verification of agreement in various muscle and adipose tissue measurement methods is warranted to robust standard of sarcopenia and obesity, and this will be instrumental in the development of imaging biomarker.
Computed tomography (CT) is a useful radiologic technique for differentiating and quantifying various soft tissues, including muscle and fat at any site within the body.[15–20] Software programs for analyzing CT images have been developed over the past decades, and several reports about quantifying body composition using various methods of segmentation have been published.[19,21,22] In general, attenuation-based segmentation is usually used to differentiate fat from other soft tissue on a CT, and various software programs have been used to quantify body composition using this method.[21–23] Moreover, deep learning methods recently have been used for segmentation of body composition on cross-sectional image.[24–28]
We developed a new customized program using attenuation-based segmentation for semiautomatically quantification of muscle, subcutaneous fat, and visceral fat. The purpose of the present study was to correlate muscle and adipose tissue quantified using our software with quantification results obtained by manual measurements and commercially available programs. We further sought to evaluate the influence of gender and the presence of ascites on the measurement.
2. Materials and methods
2.1. Development of semiautomatic software
In this study, we developed software in-house to identify subcutaneous fat, muscle, and visceral fat in CT images for body composition analysis complied by MATLAB version R2014a (Mathworks Inc, Natick, MA). This open-source software (BMI_CT) is available on following URL (https://sourceforge.net/projects/muscle-fat-area-measurement/). Our software is composed of 3 image processing steps: a preprocessing step, a boundary detection step, and an identification step. In the preprocessing step, the background image (including the CT table and noise) was removed from the original CT image (Fig. 1A) using Hounsfield unit (HU) thresholds and morphologic operations (Fig. 1B). In the boundary detection step, the boundary between the muscle and the inner organs (including the liver, spleen, and soft tissues) was semiautomatically detected using the active contour method and morphologic image processing techniques. To highlight the boundary between the muscle and other tissues, the intensity of preprocessed CT image was linearly transformed into +10 to +100 HU (Fig. 1C). Using the active contour method,[29] the initial curve (defined in a semiautomatic manner) was manipulated to detect the boundary between the muscle and the inner tissues by minimizing a cost function that summed external and internal forces (Fig. 1D). In our study, the external force was minimized when the curve was at the object boundary position, and the internal force was minimized when the curve shape was as smooth as possible. In the identification step, subcutaneous fat, muscle, and visceral fat were detected on the preprocessed CT image using fuzzy c-means clustering algorithms. The preprocessed CT image was then segmented into the inner and outer CT images based on the detected muscle boundary. Pixels in the outer CT image were segmented into 4 clusters based on the HU of pixels using the fuzzy c-mean clustering algorithm; these clusters included pixels in the background, subcutaneous fat, muscle, and bone (Fig. 1E). Pixels in the visceral fat were identified using HU thresholds of −300 to −50 in the inner CT image (Fig. 1F). The areas were measured in the L3 section by multiplying the number of pixels by the pixel surface area for each type (i.e., subcutaneous fat, muscle, and visceral fat).
Figure 1.
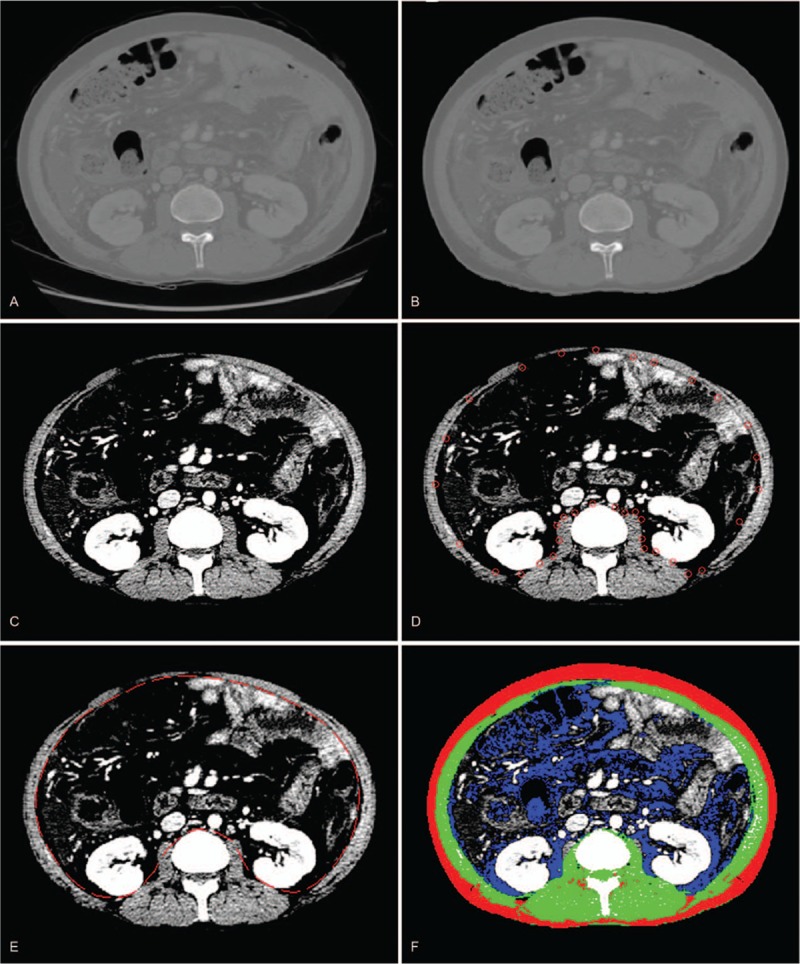
The process of semiautomatic quantification of body composition using the software developed in-house: preprocessing step (A and B), boundary detection step (C and D), and identification step (E and F).
Subsequently, we compared the measured areas of the patients who had liver cirrhosis (LC) with other established measurement methods, including manual measurements, measurements using a widely utilized open-source program (ImageJ, National Institutes of Health, Bethesda, MD), and a commercial program (Aquarius iNtuition, Terarecon, Forster City, CA). For convenience, we refer to these data as originating from the “Manual method,” “ImageJ method,” and “Aquarius method,” respectively.
2.2. Subjects
Thirty subjects (15 male and 15 female patients) whose preoperative CT data were available were randomly selected among the patients who underwent treatment for advanced LC at our institution. Our institutional review board approved this retrospective study, and informed consent was waived for all patients.
The CT examinations were performed using multidetector row CT (MDCT) scanners including a 128-section CT system Somatom Definition Flash (Siemens Medical Systems, Forchheim, Germany) (n = 1), an Emotion 6 (Siemens Medical Systems) (n = 1), a Philips Brilliance-40 detector (Philips Medical Systems, Cleveland, OH) (n = 11), a Toshiba Aquilion 64 detector (Toshiba Medical Systems, Tokyo, Japan) (n = 3), a Lightspeed 16 (GE Healthcare, Waukesha, WI) (n = 2), a LightSpeed ultra (GE Healthcare) (n = 1), a Lightspeed QX/I (GE Healthcare) (n = 1), or a GE LightSpeed VCT 64 (GE Healthcare) (n = 10). The scanning parameters were as follows: 120 kVp, 189–200 mAs, 5 mm slice thickness with an increment (overlap) of 2.5 mm, a table speed of 26.5 to 39.37 mm per rotation (pitch of 0.828–1.07), and a single-breath hold helical acquisition time of 4 to 6 seconds (depending on liver size). Images were obtained in the craniocaudal direction. The portal venous phase CT scans were obtained 70 seconds after injection of 120 mL of a nonionic iodinated contrast material (iopamidol, Iopamiro 300; Bracco, Milan, Italy), which was injected at a rate of 3 to 4 mL/s. The contrast material was injected through the antecubital vein using a power injector.
2.3. Measurement of muscle and adipose areas
Two radiologists (SSK and WKJ) independently measured muscle, and subcutaneous and visceral adipose tissue areas at the upper endplate of the L3 transverse section using the new software. The average time for each measurement was less than 1 minute per patient.
Then, one of the radiologists (SSK) used the other 3 methods (manual, ImageJ, and Aquarius) to measure abdominal muscle and adipose areas. Manual measurements of subcutaneous fat tissue and muscle were taken as references to correlate with other methods, and the boundaries of the muscle and subcutaneous fat were drawn using the Picture Archiving and Communications System (PACS; Centricity, GE Healthcare). The ImageJ method was used to measure subcutaneous fat, muscle, and visceral fat areas. The same cut-off levels (−300 to −50 HU for fat tissue; +10 to +100 HU for muscle) used in the new program were applied to extract the muscle and 2 adipose areas. To separate between abdominal muscle and the peritoneal cavity (visceral fat), a line was drawn manually. The Aquarius method was used to measure subcutaneous and visceral fat areas. All measured areas were divided by the square of the patient height (m2) for standardization.
2.4. Statistical analysis
Intraclass correlation coefficients (ICCs) were calculated to compare muscle and fat areas using a 2-way random model.[30] We compared them under various conditions (i.e., for all subjects, male vs female, and whether or not ascites were present). The agreement scale was classified as follows: an ICC of <0.50 indicated poor agreement, an ICC of 0.5 to 0.71 showed fair agreement, an ICC of 0.71 to 0.86 was classified as good agreement, and an ICC of ≥0.87 was excellent agreement.[31] We performed a Pearson correlation test to compare the correlations for each modality. The ICC between the 2 radiologists was also calculated using the new software to assess the interobserver agreement. A value of P < .05 was considered statistically significant. All analyses were performed using SPSS Statistics software (version 23; IBM, Armonk, NY).
3. Results
The mean age of the subjects was 55 ± 8.4 years (range, 41–66 years). Ascites were identified in 17 of the 30 subjects. Table 1 summarizes the ICCs between muscle and fat areas quantified by the various methods. Considering all the subjects, the measurement agreement in subcutaneous fat and muscle areas was excellent among the methods (ICC = 0.962 and 0.897, respectively), and the agreement in visceral fat area was good (ICC = 0.822). The agreement between muscle and visceral fat areas in the female subgroup was slightly lower than in the remaining group (ICC = 0.869 and 0.742, respectively), and that of the visceral fat area in patients with ascites was also lower than that in the nonascites group (ICC = 0.787). All the correlation coefficients were relatively high (r = 0.854–0.996). The results obtained using our software correlated relatively well with those of other methods, especially manual measurements. However, muscle area measured using the ImageJ method and visceral fat area measured using the Aquarius method were relatively less correlated to our software results (Table 2 and Fig. 2). Interobserver agreement with our program was also relatively very high. The ICC values for subcutaneous fat, muscle, and visceral fat areas were 0.999, 0.980, and 0.999, respectively (Table 3).
Table 1.
ICC comparison values among the computed tomography areas of subcutaneous fat, muscle, and visceral fat.

Table 2.
Correlation between our software and other measurement methods.

Figure 2.
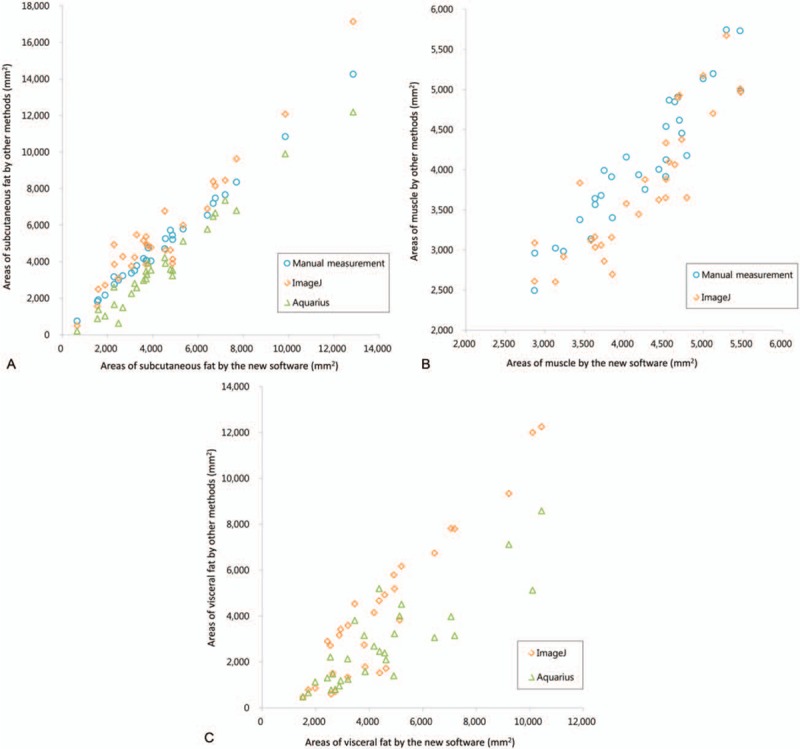
Scatter plot comparisons of areas measured using the new software and other measurement methods. Subcutaneous fat (A), muscle (B), and visceral fat (C).
Table 3.
Interobserver agreement in the area measurements using our software.

4. Discussion
In the present study, we used our in-house software for assessing abdominal fat and muscle mass using MDCT, and our software showed acceptable results. There were good or excellent correlations between subcutaneous fat, muscle, and visceral fat mass quantified by our new program and those quantified by Aquarius, ImageJ, or manual measurements. In addition, interobserver correlation was excellent.
There have been several studies on the radiologic assessment of muscle mass using cross-sectional images such as CT.[32,33] It has been shown that CT can provide an exact quantification of subcutaneous fat and muscle.[34] It can also provide reproducible, objective, and accurate measurements. Previous reports revealed that the total abdominal muscle areas on a CT section at the L3 or L4 level correlated well with whole body muscle mass; therefore, we quantified the muscle and fat areas at the upper endplate of the L3 level.[35,36] Our study showed excellent correlation coefficients for measurement of subcutaneous fat and muscle with the other techniques. The results were especially good for subcutaneous fat (ICC = 0.962), and no differences were observed based on gender or the presence of ascites. Actually, the ICC of muscle areas between the methods (ICC = 0.897) was slightly lower than that of subcutaneous fat areas, but this might be dependent on excluding intramuscular fatty change in the paravertebral muscle from the muscle areas and the proportion of intramuscular fat is not significantly large in the muscle area. Our new program also showed excellent interobserver correlations for subcutaneous fat, muscle, and visceral fat (ICC = 0.999, 0.980, and 0.999, respectively). These data suggest that trained persons produce highly reproducible measurements using our new program protocol. Additionally, our new software can be used to quantify subcutaneous fat, muscle, and visceral fat separately in less than 1 minute per patient. This method was already used in a published article and will be applied in daily practice.[37]
Several investigators have tried to quantify body composition using CT, and a variety of methods have been developed over the years. Yoshizumi and his colleagues developed a standardized technique for fat measurement using manual measurements of CT data,[38] and they showed that manual measurements were almost identical for quantifying abdominal fat area. But manual tracing of contours of visceral organs is complicated and may have a high probability of error. Thus, visceral fat was not assessed with manual measurements in the present study. NIH ImageJ is a widely used opened source program, and previous reports showed excellent correlation between measurements of adipose tissue and muscle area using cross-sectional areas in the abdomen.[19,22] In our study, the results of visceral fat area using our software were highly correlated with those of ImageJ and Aquarius (r = 0.937 and 0.854, respectively). With regard to subcutaneous fat and muscle, correlation coefficients between our software and other methods were higher than those in the result of visceral fat (Table 2).
Although there was very high interobserver agreement for measurement of visceral fat between both observers (ICC = 0.999), ICC values among various methods were relatively low, especially in females and in patients with ascites (ICC = 0.742 and 0.787, respectively). Women have relatively little visceral fat compared with men, and preferentially employ fat stores rather than skeletal muscle stores.[39,40] Malnutrition is a common complication of LC.[41] Therefore, women with LC may have a small amount of visceral fat, and this may be a reason for the relatively low ICC in the female group. Subjects with ascites also showed low ICCs for quantification of visceral fat among the various measurement methods. We suggest that edematous changes in intraabdominal organs and ascites may increase attenuation of visceral fat, and this may make it harder to discriminate fat from other soft tissue. It may be difficult to differentiate visceral fat in patients with cirrhosis because ascites are common complications of chronic liver disease. Nemoto and his colleagues described a newly developed automatic visceral fat volume calculation software,[23] which showed excellent correlation coefficients between true visceral fat volumes and that quantified using the software (r = 0.999). However, they grouped subjects based on BMI categories, and there was no mention of ascites. To our knowledge, there have been no previous published reports regarding effect of ascites on the quantification of visceral fat. Further research on quantifying visceral fat in patients with ascites may be needed.
From our perspective, the new software has the following advantages: it can be adapted to analyze a single-slice CT image; it can quantify muscle, subcutaneous fat, and visceral fat at the same time after simply drawing the peritoneal boundary. It takes less than a minute per patient; and the ranges of HU for segmentation are adjustable, so the researcher can easily change the range of segmentation for target tissue in different situations.
In conclusion, the results of this study reveal that all the methods including our software provide reliable measurement of muscle and adipose tissue using cross-sectional areas of MDCT with high reproducibility. We expect that our open-source software will be widely used for evaluation of metabolic status of the patients with malignancies, chronic illness, and critical diseases.
Author contributions
All authors had contributed to the patient care and had access to the data and a role in writing this manuscript.
Conceptualization: Seung Soo Kim, Woo Kyoung Jeong.
Data curation: Seung Soo Kim, Woo Kyoung Jeong.
Formal analysis: Woo Kyoung Jeong.
Investigation: Seung Soo Kim, Woo Kyoung Jeong.
Methodology: Jea-Hun Kim, Woo Kyoung Jeong.
Software: Jea-Hun Kim.
Supervision: Young Kon Kim, Dongil Choi, Won Jae Lee.
Writing – original draft: Seung Soo Kim.
Writing – review & editing: Seung Soo Kim, Woo Kyoung Jeong, Jisun Lee.
Seung Soo Kim orcid: 0000-0002-1763-8217.
Footnotes
Abbreviations: CT = computed tomography, HCC = hepatocellular carcinoma, HU = Hounsfield unit, ICC = intraclass correlation coefficient, LC = liver cirrhosis, MDCT = multidetector row computed tomography, NASH = nonalcoholic steatohepatitis.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Written informed consent was waived by the Institutional Review Board because of the retrospective nature of the study.
SSK and JHK contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Delmonico MJ, Harris TB, Lee JS, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 2007;55:769–74. [DOI] [PubMed] [Google Scholar]
- [2].Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61:1059–64. [DOI] [PubMed] [Google Scholar]
- [3].Honda H, Qureshi AR, Axelsson J, et al. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am J Clin Nutr 2007;86:633–8. [DOI] [PubMed] [Google Scholar]
- [4].Landi F, Liperoti R, Fusco D, et al. Prevalence and risk factors of sarcopenia among nursing home older residents. J Gerontol A Biomed Sci Med Sci 2011;67:48–55. [DOI] [PubMed] [Google Scholar]
- [5].Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629–35. [DOI] [PubMed] [Google Scholar]
- [6].Sabel MS, Lee J, Cai S, et al. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol 2011;18:3579–85. [DOI] [PubMed] [Google Scholar]
- [7].Guiu B, Petit JM, Bonnetain F, et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut 2010;59:341–7. [DOI] [PubMed] [Google Scholar]
- [8].Montano-Loza AJ, Meza-Junco J, Prado CM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012;10:166–73. [DOI] [PubMed] [Google Scholar]
- [9].DiMartini A, Cruz RJ, Dew MA, et al. Muscle mass predicts outcomes following liver transplantation. Liver Transpl 2013;19:1172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hanai T, Shiraki M, Nishimura K, et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 2015;31:193–9. [DOI] [PubMed] [Google Scholar]
- [11].Meza-Junco J, Montano-Loza AJ, Baracos VE, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol 2013;47:861–70. [DOI] [PubMed] [Google Scholar]
- [12].Moreau R. Acute-on-chronic liver failure: a new syndrome in cirrhosis. Clin Mol Hepatol 2016;22:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ohki T, Tateishi R, Shiina S, et al. Visceral fat accumulation is an independent risk factor of hepatocellular carcinoma recurrence after curative treatment in NASH-suspected patients. Gut 2009;58:839–44. [DOI] [PubMed] [Google Scholar]
- [14].Zhao J, Zhao Y, Wang H, et al. Association between metabolic abnormalities and HBV related hepatocelluar carcinoma in Chinese: a cross-sectional study. Nutr J 2011;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Despres J. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition 1993;9:452–9. [PubMed] [Google Scholar]
- [16].Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med 2005;165:777–83. [DOI] [PubMed] [Google Scholar]
- [17].Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 2003;26:372–9. [DOI] [PubMed] [Google Scholar]
- [18].Johnson KT, Harmsen WS, Limburg PJ, et al. Visceral fat analysis at CT colonography. Acad Radiol 2006;13:963–8. [DOI] [PubMed] [Google Scholar]
- [19].Lemieux S, Lesage M, Bergeron J, et al. Comparison of two techniques for measurement of visceral adipose tissue cross-sectional areas by computed tomography. Am J Hum Biol 1999;11:61–8. [DOI] [PubMed] [Google Scholar]
- [20].Pascot A, Lemieux S, Lemieux I, et al. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care 1999;22:1471–8. [DOI] [PubMed] [Google Scholar]
- [21].Heymsfield SB. Development of imaging methods to assess adiposity and metabolism. Int J Obes 2009;32:S76–82. [DOI] [PubMed] [Google Scholar]
- [22].Irving BA, Weltman JY, Brock DW, et al. NIH ImageJ and Slice-O-Matic computed tomography imaging software to quantify soft tissue. Obesity 2007;15:370–6. [DOI] [PubMed] [Google Scholar]
- [23].Nemoto M, Yeernuer T, Masutani Y, et al. Development of automatic visceral fat volume calculation software for CT volume data. J Obes 2014;495084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Weston AD, Korfiatis P, Kline TL, et al. Automated abdominal segmentation of CT scans for body composition analysis using deep learning. Radiology 2019;290:669–79. [DOI] [PubMed] [Google Scholar]
- [25].Chen J, Zhang H, Zhang W, et al. Correlated regression feature learning for automated right ventricle segmentation. IEEE J Transl Eng Health Med 2018;6:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee H, Troschel FM, Tajmir S, et al. Pixel-level deep segmentation: artificial intelligence quantifies muscle on computed tomography for body morphometric analysis. J Digit Imaging 2017;30:487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang Y, Qiu Y, Thai T, et al. A two-step convolutional neural network based computer-aided detection scheme for automatically segmenting adipose tissue volume depicting on CT images. Comput Methods Programs Biomed 2017;144:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Han J, Zhang D, Cheng G, et al. Object detection in optical remote sensing images based on weakly supervised learning and high-level feature learning. IEEE Trans Geosci Remote Sens 2015;53:3325–37. [Google Scholar]
- [29].Xu C, Prince JL. Snakes, shapes, and gradient vector flow. IEEE Trans Image Process 1998;7:359–69. [DOI] [PubMed] [Google Scholar]
- [30].Lee J, Koh D, Ong C. Statistical evaluation of agreement between two methods for measuring a quantitative variable. Comput Biol Med 1989;19:61–70. [DOI] [PubMed] [Google Scholar]
- [31].Rousselet MC, Michalak S, Dupré F, et al. Sources of variability in histological scoring of chronic viral hepatitis. Hepatology 2005;41:257–64. [DOI] [PubMed] [Google Scholar]
- [32].Mitsiopoulos N, Baumgartner R, Heymsfield S, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 1998;85:115–22. [DOI] [PubMed] [Google Scholar]
- [33].Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 2004;97:2333–8. [DOI] [PubMed] [Google Scholar]
- [34].Ohkawa S, Odamaki M, Yoneyama T, et al. Standardized thigh muscle area measured by computed axial tomography as an alternate muscle mass index for nutritional assessment of hemodialysis patients. Am J Clin Nutr 2000;71:485–90. [DOI] [PubMed] [Google Scholar]
- [35].Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- [36].Tandon P, Ney M, Irwin I, et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl 2012;18:1209–16. [DOI] [PubMed] [Google Scholar]
- [37].Kang SH, Jeong WK, Baik SK, et al. Impact of sarcopenia on prognostic value of cirrhosis: going beyond the hepatic venous pressure gradient and MELD score. J Cachexia Sarcopenia Muscle 2018;9:860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yoshizumi T, Nakamura T, Yamane M, et al. Abdominal fat: standardized technique for measurement at CT. Radiology 1999;211:283–6. [DOI] [PubMed] [Google Scholar]
- [39].Carvalho L, Parise ER. Evaluation of nutritional status of nonhospitalized patients with liver cirrhosis. Arq gastroenterol 2006;43:269–74. [DOI] [PubMed] [Google Scholar]
- [40].Riggio O, Angeloni S, Ciuffa L, et al. Malnutrition is not related to alterations in energy balance in patients with stable liver cirrhosis. Clin Nutr 2003;22:553–9. [DOI] [PubMed] [Google Scholar]
- [41].Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis 2012;16:95–131. [DOI] [PMC free article] [PubMed] [Google Scholar]


