Supplemental Digital Content is available in the text
Keywords: acute exacerbation of chronic obstructive pulmonary disease, antibiotic therapy, meta-analysis, procalcitonin
Abstract
Background:
The benefit of a procalcitonin (PCT)-guided antibiotic strategy in acute exacerbation of chronic obstructive pulmonary disease (AECOPD) remains uncertain.
Objectives:
This updated meta-analysis was performed to reevaluate the therapeutic potential of PCT-guided antibiotic therapy in AECOPD.
Data sources:
We searched PubMed, Embase, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov up to February 2019 to identify randomized controlled trials (RCTs) investigating the role of PCT-guided antibiotic strategies in treating adult patients with AECOPD. Relative risk (RR) or mean differences (MD) with accompanying 95% confidence intervals (CIs) were calculated with a random-effects model.
Results:
Eight RCTs with a total of 1376 participants were included. The results suggested that a PCT-guided antibiotic strategy reduced antibiotic prescriptions (RR: 0.55; 95% CI: 0.39–0.76; P = .0003). However, antibiotic exposure duration (MD: −1.34; 95% CI: −2.83–0.16; P = .08), antibiotic use after discharge (RR: 1.61; 95% CI: 0.61–4.23; P = .34), clinical success (RR: 1.02; 95% CI: 0.96–1.08; P = .47), all-cause mortality (RR: 1.05; 95% CI: 0.72–1.55; P = .79), exacerbation at follow-up (RR: 0.97; 95% CI: 0.80–1.18; P = .78), readmission at follow-up (RR: 1.12; 95% CI: 0.82–1.53; P = .49), length of hospital stay (MD: −0.36; 95% CI: −1.36–0.64; P = .48), and adverse events (RR: 1.33; 95% CI: 0.79–2.23; P = .28) were similar in both groups.
Implications of key findings:
A PCT-guided antibiotic strategy is associated with fewer antibiotic prescriptions, and has similar efficacy and safety compared with standard antibiotic therapy in AECOPD patients.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable, and treatable disease. COPD is characterized by persistent airflow limitation and respiratory symptoms due to airway and/or alveolar abnormalities, and is associated with increasing morbidity and mortality worldwide.[1] Exacerbations of COPD are one of the most common causes of acute medical hospital admissions and death in patients suffering from this clinical condition. Such exacerbations, which represent a significant direct healthcare cost associated with COPD, can be caused by various factors, including viral and/or bacterial respiratory tract infections and air pollution.[2] Although bacteria are considered the major cause of acute exacerbations of COPD (AECOPD), treatment of AECOPD remains difficult because a precise cause cannot be identified in many patients.[3]
The primary approach for COPD patients with a severe exacerbation caused by bacterial infection is antibiotic therapy. A meta-analysis demonstrated that antibiotics reduce the risk of failure in severe AECOPD and reduce mortality in intensive care unit (ICU) patients.[4] Current international guidelines recommend that antibiotics be used only in cases of increased dyspnea (a key symptom of an exacerbation, together with increased sputum purulence and volume, and increased cough and wheeze), or if patients need invasive or noninvasive mechanical ventilation.[1,5] However, signs and symptoms of AECOPD with a bacterial or nonbacterial cause tend to overlap in clinical practice.[6,7]
Evidence indicates that not all AECOPD patients will benefit from antibiotic treatment.[8] Current strategies for treatment of AECOPD may lead to overuse of antibiotics, further increasing drug resistance and side effects. Meanwhile, long-term antibiotic therapy may lead to increased medical costs, prolonged length of hospital stay, and increased hospital mortality, especially for patients with severe AECOPD.[9,10] Therefore, identifying patients with AECOPD who are most likely to benefit from antibiotics is a challenge for clinicians.
As a surrogate marker of the host response to bacterial infections, procalcitonin (PCT) has been approved by the US Food and Drug Administration to guide antibiotic prescribing practice in the context of acute respiratory infections and sepsis.[11] A previous meta-analysis based on limited data regarding this issue indicated that a PCT-guided strategy reduced antibiotic exposure in patients with AECOPD, the role of PCT in the distinction between bacterial, viral, and other causes of AECOPD remains controversial.[12] Hence, we conducted an updated meta-analysis to comprehensively reevaluate the role of PCT-guided antibiotic strategies in the treatment of patients with AECOPD.
2. Methods
2.1. Literature search strategy
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[13] Details of our protocol were registered on the International Prospective Register of Systematic Reviews (PROSPERO) and are available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42019124795. Since this was not a study of human subjects, but a synthesis of previously published literature, it does not require institutional review board approval.
Electronic searches were conducted in PubMed, Embase, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov from inception to February 1, 2019 with no language restriction. We used the following search terms: (Title/Abstract): (Procalcitonin or Calcitonin Precursor Polyprotein or Calcitonin-1 or Calcitonin 1 or Calcitonin Related Polypeptide Alpha or Pro-Calcitonin) and (Chronic Obstructive Pulmonary Disease or COPD or pulmonary disease, chronic obstructive or Chronic Obstructive Airway Disease or Chronic Obstructive Lung Disease or Chronic Airflow Obstruction). We considered all potentially eligible studies for review, and also performed a manual search using the reference lists of key articles published.
2.2. Inclusion and exclusion criteria
We included studies with the following characteristics:
Type of participant: adult AECOPD patients with confirmed or suspected infection;
Type of intervention: patients randomized to PCT-guided antibiotic strategy;
Type of comparison: controls randomized to standard antibiotic therapy;
Type of outcome: primary outcomes including antibiotic exposure (antibiotic prescription, antibiotic exposure duration, and antibiotic use after discharge) and secondary outcomes including clinical success, all-cause mortality, exacerbation at follow-up, readmission at follow-up, length of hospital stay, and adverse events;
Types of studies: randomized controlled trials (RCTs).
The following exclusion criteria were applied: reviews, letters to the editor, case reports, or animal experimental studies; articles with duplicate data or repeated analyses; articles with insufficient data; or no evidence of infection or PCT determination was mentioned in the study.
2.3. Study selection and data extraction
The study was conducted between October 2018 and February 2019. Two independent reviewers (X.Y. and L.Y.) analyzed the article title and abstract to evaluate which should be analyzed as a full paper. Any disagreement was resolved by the adjudicating senior author (B.W.), and a final consensus was reached among all authors. One author (Z.L.) extracted data from included studies with a standardized data collection form and 2 other authors (F.G. and J.M.) checked the extracted data.
The following information was extracted from each study: first author, year of publication, country, study design, sample size, age, gender, antibiotic initiation, antibiotic cessation, method of PCT assay, clinical setting, diagnosis, and follow-up, as well as all outcomes of interest.
2.4. Quality assessment
The quality of each study was independently assessed by two authors (X.Y. and L.Y.) and discrepancies were resolved through discussion, according to Cochrane guidance. Study quality was assessed with the bias risk tool recommended by the Cochrane Collaboration.[14] We assigned a value of high, unclear, or low to the following items: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias.
2.5. Statistical analysis
Before data analysis, we estimated means from medians and standard deviations from interquartile ranges using methods described in a previous study.[15] The results from all relevant studies were combined to estimate the pooled relative risk (RR) with 95% confidence intervals (CIs) for dichotomous data. For continuous data, mean differences (MD) and 95% CIs were estimated.
Pooled RRs, MDs, and their 95% CIs were calculated with a random-effects model. We assessed heterogeneity among individual studies with I2 analysis. Heterogeneity was suggested where P < .10 for the Q test. I2 values of 0% to 24.9%, 25% to 49.9%, 50% to 74.9%, and 75% to 100% were considered zero, low, moderate, and high thresholds for statistical heterogeneity, respectively.[16,17] Sensitivity analysis and random effects meta-regression analysis were also performed by excluding trials that potentially biased the result for the primary outcome (antibiotic prescriptions).
Publication bias was assessed by visually inspecting a funnel plot in which the log RRs were plotted against their standard errors. The presence of publication bias was also evaluated by using the Begg's and Egger's tests.[18,19]P < .05 was considered statistically significant, except where otherwise specified. Trim-and-fill analysis was also performed to detect potential publication bias.[20] All statistical analyses were performed with Review Manager version 5.3 (The Nordic Cochrane Centre, Denmark) and Stata 15.1 (StataCorp, TX).
3. Results
3.1. Included studies
We identified 440 records in the initial search, of which 8 (1376 participants), published between 2004 and 2018, were included in our analysis according to the eligibility criteria.[21–28] In these studies, 681 patients were allocated to a PCT-guided antibiotic strategy group and 695 patients were allocated to a standard antibiotic therapy group. Four studies[23–25,28] were conducted at multiple sites, while the remaining 4 studies[21,22,26,27] were conducted at single center. The majority of studies were from Europe[21–26,28] and only 1 study[27] was conducted in China.
PCT algorithms used in the different trials were similar. Four studies[21,23,24,26] initiated antibiotics when PCT was above 0.25 μg/L, and 3 studies[22,25,28] initiated antibiotics when PCT was above 0.25 μg/L or 0.1 to 0.25 μg/L if the patient's clinical condition was unstable. Only 1 study[27] initiated antibiotics when PCT was below 0.1 μg/L if the patient's clinical condition was unstable. Table 1 presents the main characteristics and outcome data for included studies.
Table 1.
Characteristics of studies included in the meta-analysis.
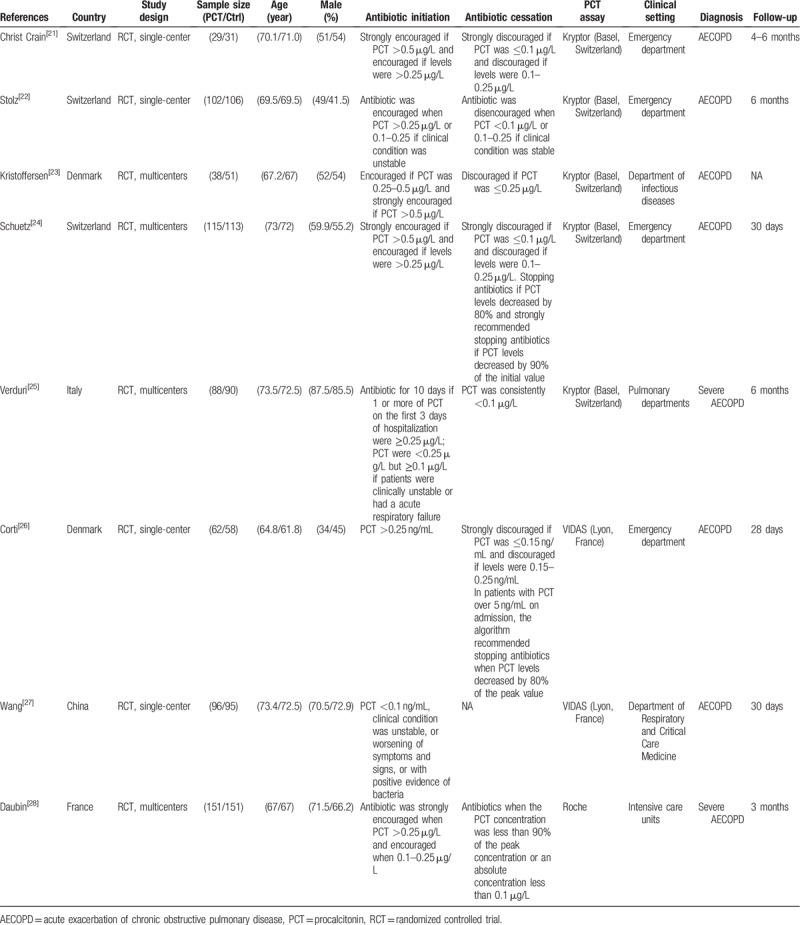
Figure 1 presents the study flow diagram, including reasons for exclusion of studies. Figure 2 illustrates that Cochrane risk of bias scores varied across included studies.
Figure 1.
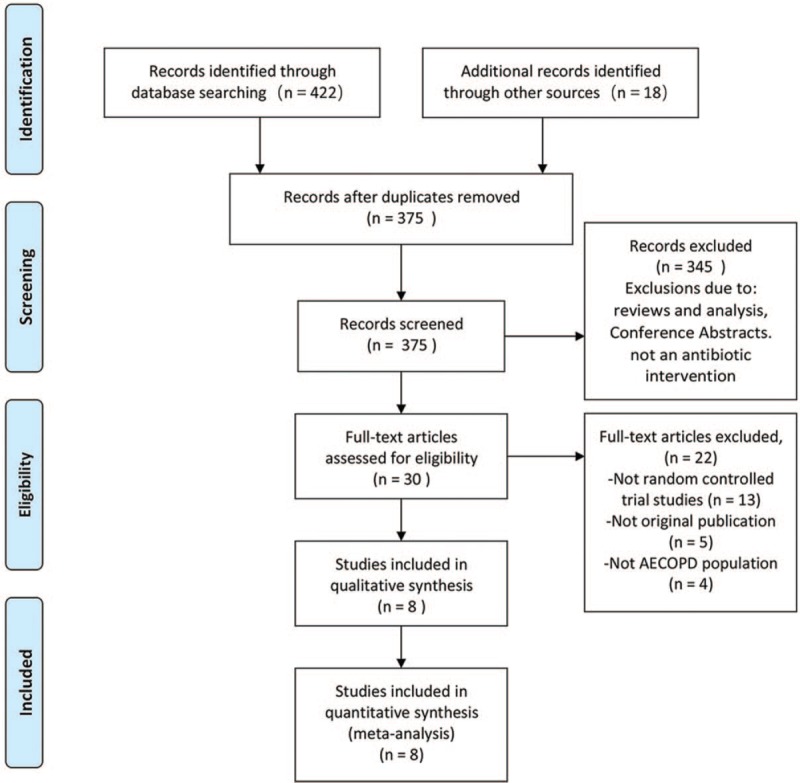
PRISMA flowchart of study selection process in the systematic review. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Figure 2.
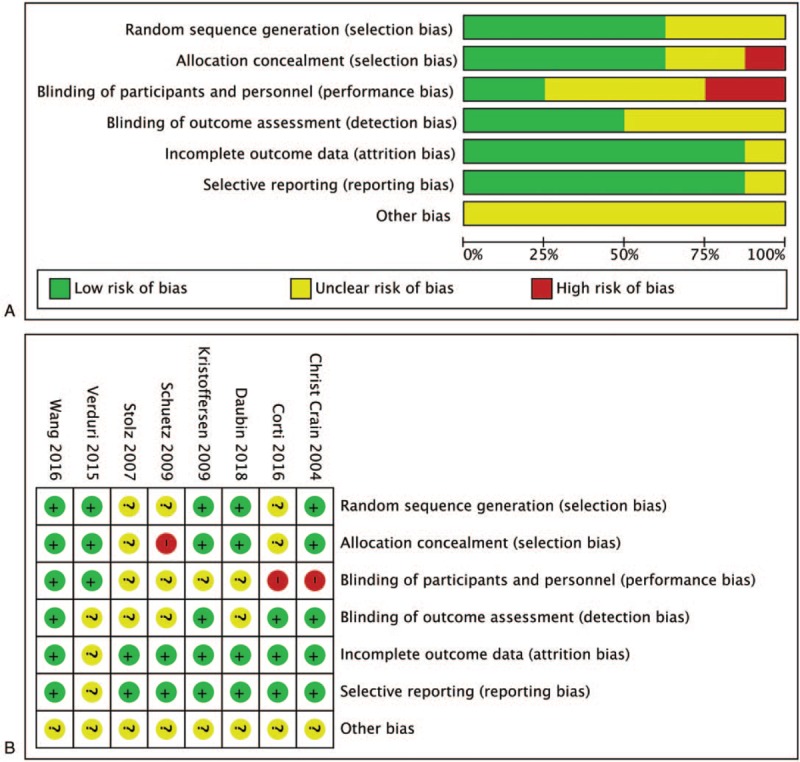
Risk of bias graph (A) and risk of bias summary (B) review authors’ judgments about each risk of bias item for each included study. Green indicates low risk of bias, yellow indicates unclear risk of bias, and red indicates high risk of bias.
3.2. Primary outcome: antibiotic use
Antibiotic prescriptions were examined in 7 studies (n = 1287).[21,22,24–28] A PCT-guided antibiotic strategy was associated with reduced antibiotic prescriptions in patients with AECOPD (RR: 0.55; 95% CI: 0.39–0.76; P = .0003; P for heterogeneity < .00001; I2 = 91%) (Fig. 3A). Four studies (n = 710)[21,24,27,28] provided data suggesting there is no significant difference in antibiotic exposure duration between the 2 groups (MD: −1.34; 95% CI: −2.83–0.16; P = .08; P for heterogeneity = .009; I2 = 74%) (Fig. 3B). Finally, 3 studies (n = 577)[22,25,26] reported antibiotic use after discharge. Aggregated results suggested that antibiotic use after discharge was similar in both groups (RR: 1.61; 95% CI: 0.61–4.23; P = .34; P for heterogeneity = .0009; I2 = 86%) (Fig. 3C).
Figure 3.
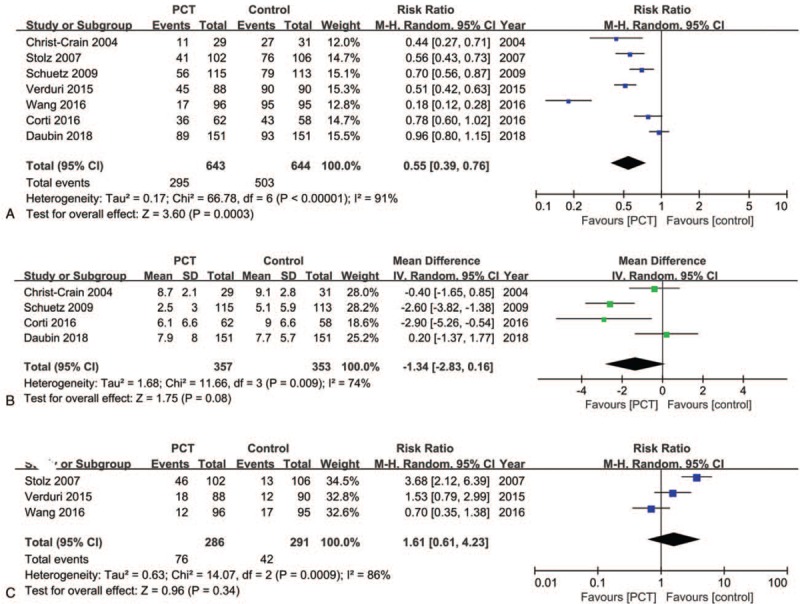
Forest plots of primary outcomes. (A) Effects of PCT-guided antibiotic strategy on antibiotic prescriptions. (B) Effects of PCT-guided antibiotic strategy on antibiotic exposure duration. (C) Effects of PCT-guided antibiotic strategy on antibiotic use after discharge. CIs = confidence intervals, PCT = procalcitonin.
Significant heterogeneity was observed among the studies reporting antibiotic prescriptions (P for heterogeneity < .00001; I2 = 91%). However, sensitivity analysis indicated that the pooled RR was not substantially altered when any single study was excluded, suggesting that the results of the current meta-analysis are robust (Fig. 4). One trial by Wang et al[27] was different from the others in the included population and antibiotic initiation strategy. Nevertheless, heterogeneity decreased from 91% to 81% after exclusion of this trial, and there was no significant change in the results (RR: 0.65; 95% CI: 0.52–0.83; P = .0004; P for heterogeneity < .00001; I2 = 81%).
Figure 4.
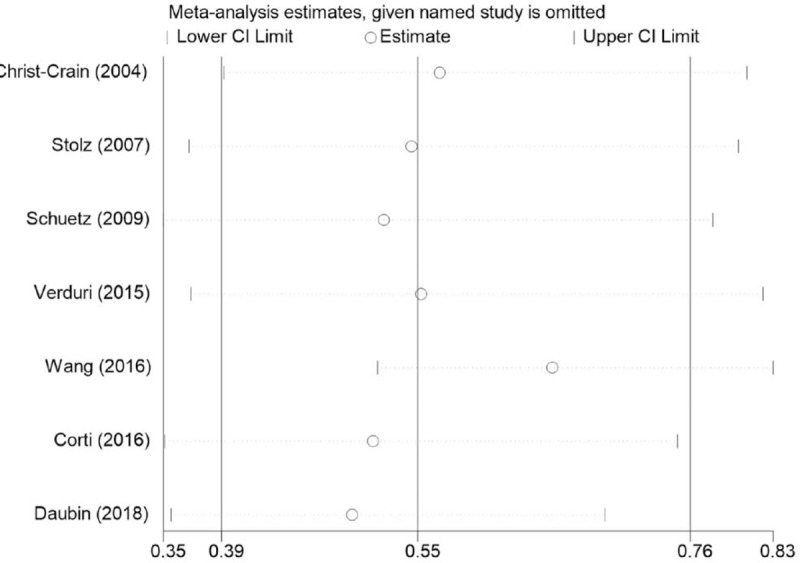
Sensitivity analysis on the relationship between PCT-guided antibiotic strategy and antibiotic prescriptions in patients with AECOPD. AECOPD = acute exacerbation of chronic obstructive pulmonary disease, PCT = procalcitonin.
Finally, random effects meta-regression indicated no significant interaction between antibiotic prescriptions associated with PCT-guided antibiotic strategies and the following variables: clinical setting (P = .111), PCT assay (P = .104), center (P = .461), diagnosis (P = .134), and sample size (P = .178) (Appendix 1).
3.3. Secondary outcomes
Clinical success was reported in 3 studies,[22,25,27] with the aggregated results indicating that there was no difference between the PCT-guided antibiotic strategy and standard antibiotic therapy groups (RR: 1.02; 95% CI: 0.96–1.08; P = .47). The results also indicated that all-cause mortality (RR: 1.05; 95% CI: 0.72–1.55),[21,22,24–28] exacerbation at follow-up (RR: 0.97; 95% CI: 0.80–1.18),[21,22,24–27] readmission at follow-up (RR: 1.12; 95% CI: 0.82–1.53),[21,22,25–27] adverse events (RR: 1.33; 95% CI: 0.79–2.23),[25,26] and length of hospital stay (MD: −0.36; 95% CI: −1.36–0.64)[21–27] were similar in both groups (Table 2).
Table 2.
Secondary outcomes of procalcitonin-guided antibiotic strategy on acute exacerbation of chronic obstructive pulmonary disease patients.
3.4. Publication bias
In the meta-analysis of data regarding the effect of PCT-guided antibiotic strategy on antibiotic prescriptions, the presence of publication bias was not excluded by inspection of the funnel plot (Fig. 5). Begg's and Egger's tests were used to further evaluate publication bias and no evidence was noted (Begg's: P = .176; Egger's: P = .335) (Fig. 6A and B). Trim-and-fill analysis also showed 1 potential missing study on the right side of the funnel plot to ensure symmetry (Fig. 7). The recalculated pooled RR for antibiotic prescription did not significantly change by including 1 unpublished study (RR: 0.492; 95% CI: 0.335–0.723; P < .0001).
Figure 5.
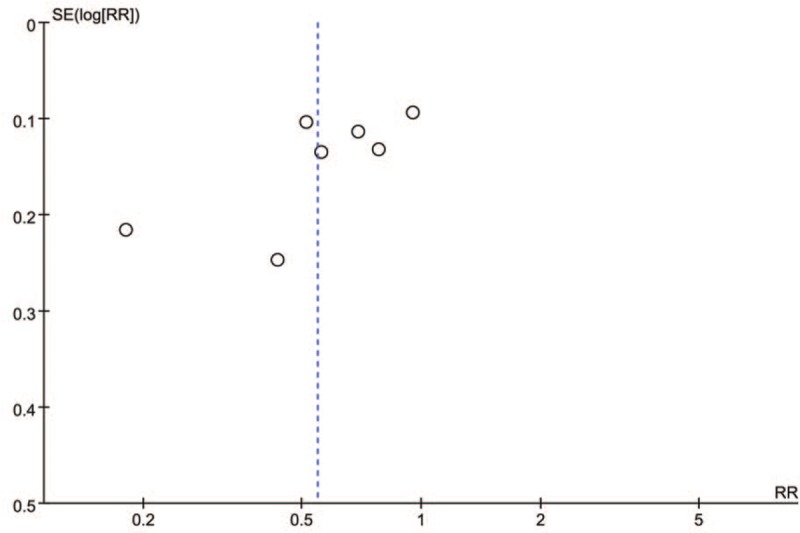
Funnel plot of comparison: antibiotic prescriptions.
Figure 6.
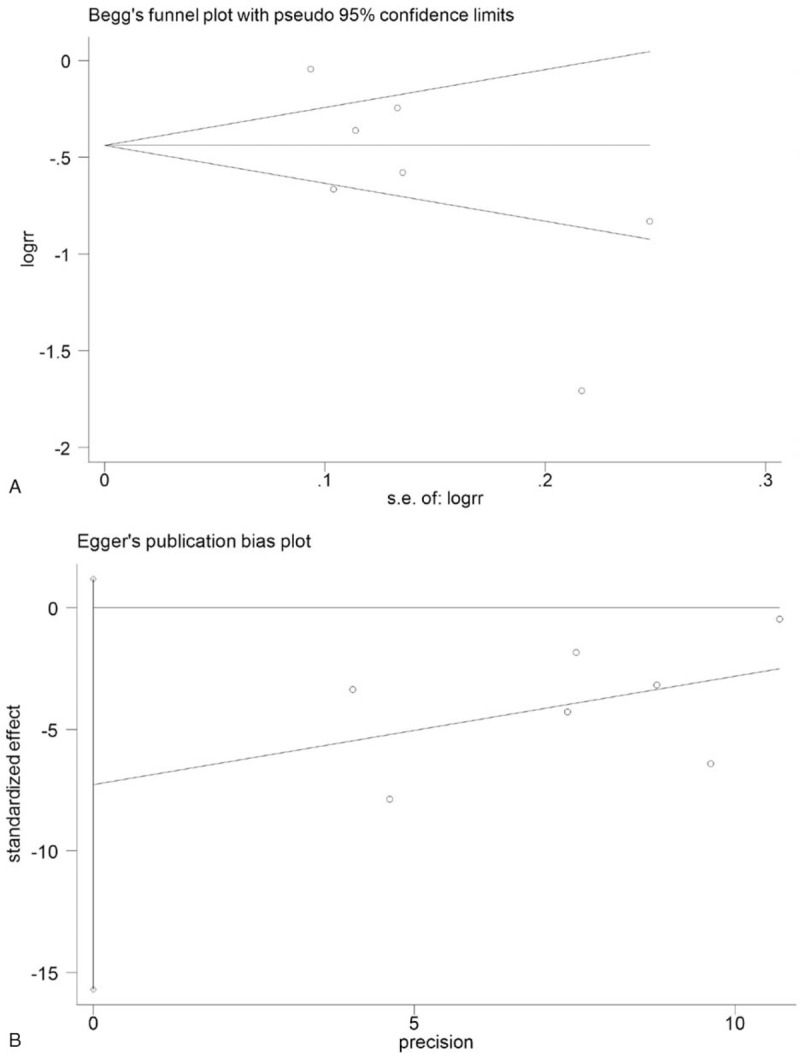
Publication bias plots of Begg's (A) and Egger's tests (B).
Figure 7.
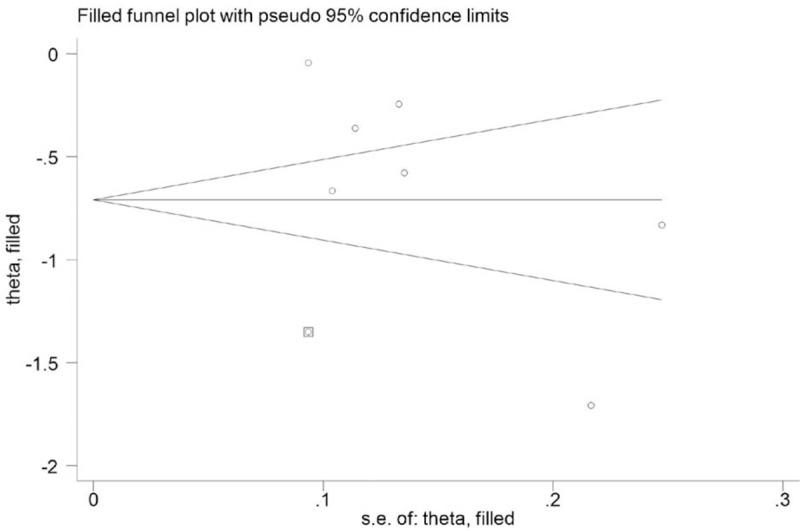
Funnel plot adjusted by trim-and-fill method for antibiotic prescriptions.
4. Discussion
4.1. Main findings
The present meta-analysis identified 8 RCTs investigating the potential value of a PCT-guided antibiotic strategy on antibiotic prescription, antibiotic exposure duration, and antibiotic use after discharge, as well as several secondary outcomes in the treatment of patients with AECOPD versus standard antibiotic therapy. The aggregated results suggest that a PCT-guided antibiotic strategy reduced antibiotic prescriptions, but failed to diminish antibiotic exposure duration or antibiotic use after discharge compared with standard antibiotic therapy (MD: −1.34; 95% CI: −2.83–0.16, and RR: 1.61; 95% CI: 0.61–4.23, respectively). Moreover, clinical success, all-cause mortality, exacerbation and readmission at follow-up, length of hospital stay, and adverse events were similar in both groups.
4.2. Comparison with previous studies
To date, 2 meta-analyses have assessed the value of a PCT-guided antibiotic strategy in AECOPD patients. Our study had several strengths, which provide more robust evidence to support and extend the weak results reported in the previous 2 studies. The first meta-analysis conducted by Lin et al[12] used data only from 4 RCTs involving 679 participants and reported that PCT-guided therapy significantly reduced antibiotic use compared with standard treatment. Our results are consistent with this, although we included 4 additional RCTs with a further 697 participants that have been published more recently. Notably, these studies enabled us to perform an aggregate analysis of antibiotic use after discharge, length of hospital stay, and adverse events, which were lacking in previous meta-analyses.
Another meta-analysis published in early 2017 was conducted by Mathioudakis et al.[29] The paper comprised 8 clinical trials (n = 1062 AECOPD patients), including 1 quasi-randomized trial with low available evidence and 1 conference abstract. The study concluded that PCT-guidance decreased antibiotic prescriptions (RR: 0.56; 95% CI: 0.43–0.73) and total antibiotic exposure (MD: −3.83; 95% CI: −4.32–−3.35). However, Mathioudakis et al[29] incorrectly incorporated data from the included studies regarding antibiotic exposure, all-cause mortality, and clinical success in patients with lower respiratory tract infections rather than AECOPD patients. In brief, the limitations of previous meta-analyses weaken the credibility of their results.
PCT is a precursor of calcitonin, which is synthesized by human thyroid C cells, and is a hormone-free glycoprotein composed of 116 amino acids. It was first proposed as a marker of bacterial infection by Assicot et al[30] in 1993 in the Lancet. Under normal physiological conditions, synthesis of PCT is low, but once infection occurs, PCT levels begin to rise in 2 to 3 hours reaching a peak (induced by cytokines such as endotoxin) in 12 to 48 hours. Typically, PCT increases more in bacterial infections than in viral infections, and the degree of elevation correlates with severity of the infection.[31,32] Over time, decreased PCT is observed with regression of infection. PCT reflects the host response to a bacterial infection and thus provides more accurate adjudicative information than traditional clinical and diagnostic parameters, such as neutrophils and C-reactive protein.[33]
Multiple meta-analyses have assessed the benefits of using serum PCT levels to guide antibiotic stewardship in ICU patients with infections, and these reports indicate that PCT-guided treatment could significantly reduce the use of antibiotics.[34–39] A recent trial conducted in the United States also reported that implementation of PCT guidance as part of a clinical decision-making algorithm was associated with reduced antibiotic exposure without negatively impacting hospital readmission in the management of adult patients admitted with COPD exacerbations.[40] This result differs from our current results.
4.3. Limitations
Our meta-analysis is subject to some limitations. First, across included studies there was significant heterogeneity of RRs for antibiotic prescription (P for heterogeneity < .00001; I2 = 91.0%). Despite the utility of sensitivity analysis and meta-regression, the origin of heterogeneity could not be fully traced.
Second, PCT is a relatively expensive detection method, so cost-effectiveness comparisons are very important when comparing a PCT-guided antibiotic strategy and standard antibiotic therapy. However, only 1 study[21] analyzed cost-effectiveness (antibiotic costs per patient), so a cost-effectiveness analysis was not possible.
Third, antibiotic therapy and types of antibiotics in the control group were not clearly defined in most studies, and it is unclear whether changes in antibiotic strategies (narrow-spectrum antibiotics and broad-spectrum antibiotics) could affect the results of our meta-analysis.
Fourth, due to the included literature being relatively limited, subgroup analysis was not performed. For example, the effect of ward type (general, emergency, and ICU), severity (mild to moderate and severe AECOPD), location (European and Asian), and PCT threshold (PCT < 1.0 vs. PCT > 1.0) were not investigated. In reality, PCT values are usually low in most patients with AECOPD, with approximately 51% to 75% of patients having a PCT <0.1 ng/mL, and only 7% to 20% having a PCT level >0.25 ng/mL.[41] Moreover, it is reported that patients with a PCT level of <0.1 ng/mL benefited from treatment with doxycycline.[42] Therefore, analysis of these subgroups is of great guiding significance in clinical practice.
5. Conclusion
In summary, our results suggest that the use of PCT-guided antibiotic therapy in patients with AECOPD can effectively reduce antibiotic prescriptions and shows similar efficacy and safety for other parameters compared with standard antibiotic therapy. However, it does not diminish antibiotic exposure duration or antibiotic use after discharge.
Further research is required to determine the optimal cutoff value for PCT and to conduct cost-effectiveness comparisons between PCT-guided antibiotic therapy and standard antibiotic therapy. It is also necessary to consider whether our findings can be generalized to other regions, including America and Asia, as well as to patients receiving antibiotic therapy for AECOPD with or without community-acquired pneumonia. Nonetheless, the current findings have substantial clinical and public health implications supporting the use of PCT-guided therapy in AECOPD.
Author contributions
Data curation: Ling Yu.
Formal analysis: Jian Ma.
Resources: Bingyu Wang.
Software: Fengli Gao.
Supervision: Zhuying Li.
Writing – original draft: Xingxing Yuan.
Supplementary Material
Footnotes
Abbreviations: AECOPD = acute exacerbation of chronic obstructive pulmonary disease, CIs = confidence intervals, COPD = chronic obstructive pulmonary disease, ICU = intensive care unit, MD = mean differences, PCT = procalcitonin, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PROSPERO = International Prospective Register of Systematic Reviews, RCTs = randomized controlled trials, RR = relative risk.
ZYL, XXY, and LY contributed equally to this work.
This research was funded by a Doctoral Innovation Fund of Heilongjiang University of Traditional Chinese Medicine 2015 (2015bs09) and Construction Project of Double First-class and Superiority Characteristic Disciplines in Heilongjiang University of Traditional Chinese Medicine (15041180133).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. 2019; Available at: http://www.goldcopd.org/ Accessed January 23, 2019 [Google Scholar]
- [2].Colak A, Yilmaz C, Toprak B, et al. Procalcitonin and CRP as biomarkers in discrimination of community-acquired pneumonia and exacerbation of COPD. J Med Biochem 2017;36:122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Miravitlles M, Anzueto A. Antibiotics for acute and chronic respiratory infection in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;188:1052–7. [DOI] [PubMed] [Google Scholar]
- [4].Vollenweider DJ, Jarrett H, Steurer-Stey CA, et al. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;12:CD010257. [DOI] [PubMed] [Google Scholar]
- [5].Woodhead M. New guidelines for the management of adult lower respiratory tract infections. Eur Respir J 2011;38:1250–1. [DOI] [PubMed] [Google Scholar]
- [6].Soler N, Torres A, Ewig S, et al. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med 1998;157:1498–505. [DOI] [PubMed] [Google Scholar]
- [7].Cameron RJ, de Wit D, Welsh TN, et al. Virus infection in exacerbations of chronic obstructive pulmonary disease requiring ventilation. Intensive Care Med 2006;32:1022–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106:196–204. [DOI] [PubMed] [Google Scholar]
- [9].Evans HL, Lefrak SN, Lyman J, et al. Cost of gram-negative resistance. Crit Care Med 2007;35:89–95. [DOI] [PubMed] [Google Scholar]
- [10].Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. J Am Med Assoc 2009;302:2323–9. [DOI] [PubMed] [Google Scholar]
- [11].Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018;18:95–107. [DOI] [PubMed] [Google Scholar]
- [12].Lin C, Pang Q. Meta-analysis and systematic review of procalcitonin-guided treatment in acute exacerbation of chronic obstructive pulmonary disease. Clin Respir J 2018;12:10–5. [DOI] [PubMed] [Google Scholar]
- [13].Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:735–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [17].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [19].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 2000;95:89–98. [Google Scholar]
- [21].Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet 2004;363:600–7. [DOI] [PubMed] [Google Scholar]
- [22].Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007;131:9–19. [DOI] [PubMed] [Google Scholar]
- [23].Kristoffersen KB, Søgaard OS, Wejse C, et al. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission: a randomized trial. Clin Microbiol Infect 2009;15:481–7. [DOI] [PubMed] [Google Scholar]
- [24].Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009;302:1059–66. [DOI] [PubMed] [Google Scholar]
- [25].Verduri A, Luppi F, D’Amico R, et al. FARM58J2XH Study Group. Antibiotic treatment of severe exacerbations of chronic obstructive pulmonary disease with procalcitonin: a randomized noninferiority trial. PLoS One 2015;10:e0118241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Corti C, Fally M, Fabricius-Bjerre A, et al. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis 2016;11:1381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang JX, Zhang SM, Li XH, et al. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: a prospective randomized controlled trial. Int J Infect Dis 2016;48:40–5. [DOI] [PubMed] [Google Scholar]
- [28].Daubin C, Valette X, Thiollière F, et al. Procalcitonin algorithm to guide initial antibiotic therapy in acute exacerbations of COPD admitted to the ICU: a randomized multicenter study. Intensive Care Med 2018;44:428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, et al. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev 2017;26:160073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Assicot M, Gendrel D, Carsin H, et al. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993;341:515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Menchine MD, Barrett TW, Schriger DL. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med 2008;52:59–60. [DOI] [PubMed] [Google Scholar]
- [32].Müller B, Harbarth S, Stolz D, et al. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC Infect Dis 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mitsuma SF, Mansour MK, Dekker JP, et al. Promising new assays and technologies for the diagnosis and management of infectious diseases. Clin Infect Dis 2013;56:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kopterides P, Siempos II, Tsangaris I, et al. Procalcitonin-guided algorithms of antibiotic therapy in the intensive care unit: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med 2010;38:2229–41. [DOI] [PubMed] [Google Scholar]
- [35].Heyland DK, Johnson AP, Reynolds SC, et al. Procalcitonin for reduced antibiotic exposure in the critical care setting: a systematic review and an economic evaluation. Crit Care Med 2011;39:1792–9. [DOI] [PubMed] [Google Scholar]
- [36].Agarwal R, Schwartz DN. Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin Infect Dis 2011;53:379–87. [DOI] [PubMed] [Google Scholar]
- [37].Schuetz P, Chiappa V, Briel M, et al. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med 2011;171:1322–31. [DOI] [PubMed] [Google Scholar]
- [38].Matthaiou DK, Ntani G, Kontogiorgi M, et al. An ESICM systematic review and meta-analysis of procalcitonin-guided antibiotic therapy algorithms in adult critically ill patients. Intensive Care Med 2012;38:940–9. [DOI] [PubMed] [Google Scholar]
- [39].Tang H, Huang T, Jing J, et al. Effect of procalcitonin-guided treatment in patients with infections: a systematic review and meta-analysis. Infection 2009;37:497–507. [DOI] [PubMed] [Google Scholar]
- [40].Bremmer DN, DiSilvio BE, Hammer C, et al. Impact of procalcitonin guidance on management of adults hospitalized with chronic obstructive pulmonary disease exacerbations. J Gen Intern Med 2018;33:692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Miravitlles M, Moragas A, Hernández S, et al. Is it possible to identify exacerbations of mild to moderate COPD that do not require antibiotic treatment? Chest 2013;144:1571–7. [DOI] [PubMed] [Google Scholar]
- [42].Daniels JM, Schoorl M, Snijders D, et al. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest 2010;138:1108–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


