Abstrct
The purpose of this study was to evaluate the efficacy and safety of rivaroxaban for the treatment of cancer-associated venous thromboembolism (VTE).
We performed a retrospective chart review of cancer patients with a pulmonary embolism, deep vein thrombosis, or both. Our analysis included all patients who received rivaroxaban from March 2013 to June 2016 at the Hemato-Oncology Division at the Pusan National University Hospital in Korea.
Preliminary results identified 123 patients with a history of cancer that were treated with rivaroxaban. The average duration of rivaroxaban therapy was 95.25 days. While 35 patients had resolved VTE after the initiation of rivaroxaban, only one patient had it recur on rivaroxaban treatment. Major bleeding was observed in 6 (4.9%) patients and minor bleeding in 12 (9.8%) patients. The majority of bleeding events occurred spontaneously and most incidences of bleeding could be treated conservatively. Recurrence and major bleeding events on rivaroxaban were relatively low despite the fact that many patients had metastatic disease. Among 52 patient deaths (42.3%), none were due to VTE or bleeding complications; the cause of death in the majority of cases was cancer progression.
Rivaroxaban is effective and safe for the treatment of cancer-associated VTE.
Keywords: cancer-associated venous thromboembolism, rivaroxaban
1. Introduction
Cancer patients have a significantly higher risk of developing venous thromboembolism (VTE) compared to the general population; it was a leading cause of morbidity and mortality in cancer patients.[1] The management of cancer-associated VTE is challenging due to the relatively high risk of recurrence of VTE and increased risk of major bleeding in this patient population compared to VTE patients without cancer.[2,3] Current guidelines still recommend that the initial treatment for cancer-associated VTE be carried out with low-molecular-weight heparin (LMWH) over a vitamin K antagonist (VKA), dabigatran, rivaroxaban, apixaban, or edoxaban.[4] However, there are many issues with LMWH use, such as high drug cost, impaired quality of life, difficultly of a parenteral injection, and the risk of heparin-induced thrombocytopenia.[5]
Rivaroxaban, a direct oral anticoagulant (DOAC) which inhibits factor Xa, was approved for the treatment of VTE in 2013 based on 2 pivotal trials (EINSTEIN-DVT and EINSTEIN-PE), which demonstrated that rivaroxaban was equally as efficacious as heparin with significantly lower bleeding rates for the initial treatment and secondary prevention of symptomatic VTE.[6,7] However, both of the trials enrolled a limited number of cancer patients and the details of their malignancies were not collected. Rivaroxaban offers an alternative anticoagulant for the patients with cancer, and the benefit of an oral formulation is it does not require monitoring and has less interactions with the patients’ diet and other drugs.[8] The efficacy and safety of rivaroxaban in cancer patients is not well established, which has limited the use of rivaroxaban in clinical practice.
Rivaroxaban is approved for the treatment of VTE in the general population, but there are insufficient guidelines on its safe and effective use for VTE in patients with cancer. Since major clinical trials comparing DOACs to LMWH for cancer-associated VTE are underway, we have evaluated the efficacy and safety of rivaroxaban in patients with cancer-associated VTE.
2. Method
2.1. Patients
We performed a retrospective chart review of active cancer patients over 18 years of age with a pulmonary embolism (PE), deep vein thrombosis (DVT), or both. Our analysis included all patients who received either an outpatient prescription or an inpatient order for rivaroxaban between March 2013 and June 2016 at the Hemato-Oncology Division at the Pusan National University Hospital in Busan, Korea. The usual initial dose of rivaroxaban was 15 mg orally twice daily for 3 weeks followed by 20 mg once daily for 6 months. Active cancer was defined as a cancer diagnosed within 6 months before the index VTE, recently recurrent or progressive cancer or any malignancy that required curative or palliative treatment within the previous 6 months. The malignant disease was grouped as either a solid or hematologic malignancy. VTE was classified as incidental, if VTE was detected on CT scans ordered for reasons other than suspected VTE (during cancer staging, treatment evaluation, or cancer recurrence detection), or symptomatic.
The study protocol was approved by the Institutional Review Board (IRB) of Pusan National University Hospital, Busan, Korea. The need for informed consent was waived by the IRB.
2.2. Clinical data
The medical charts of all eligible patients were reviewed for the following data: demographic details, sex, age, weight, height, diagnosis, cancer type, cancer stage, treatment, date of diagnosis, baseline laboratory results of patients, period of taking rivaroxaban, type of VTE, resolution, recurrence, occurrence of major bleeding or minor bleeding, and date and cause of death. In addition, the following risk factors for VTE were recorded at baseline: immobilization (confinement to bed or chair for >75% of the time for at least 2 weeks before the VTE event), major surgery in the 4 weeks before VTE, active chemotherapy or hormone therapy, and obesity (body mass index >30 kg/m2).
2.3. Outcomes
All patients were retrospectively reviewed. The end point of this study was all-cause mortality.
Recurrent PE was defined as a new intraluminal filling defect on a pulmonary angiography or CT pulmonary angiography. Recurrent DVT was confirmed if a new thrombus was clearly identified by CT angiography or ultrasonography.
Major bleeding was defined as overt bleeding plus a hemoglobin decrease of ≥2 g/dL, transfusion of ≥2 units of packed red blood cells, or intracranial, intraspinal, intraocular, retroperitoneal, pericardial, or fatal bleeding.[9] Minor bleeding was defined as overt bleeding that did not meet criteria for major bleeding.
2.4. Statistical analysis
All statistical analyses were performed using the SPSS software, version 18.0 (SPSS Inc., Chicago, IL). Statistical assessment was carried out using the Student t test for continuous variables and the Fisher exact tests for binomial variables, with a value of P < .05 considered to indicate statistical significance.
3. Results
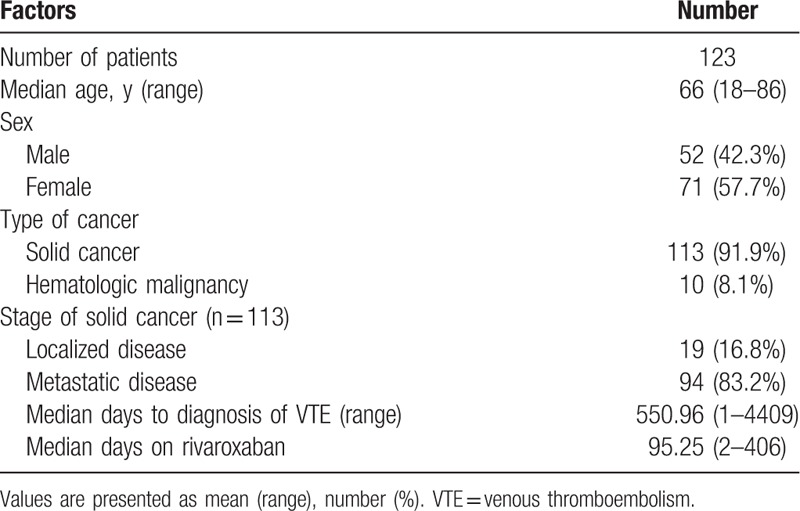
Over the length of this study, a total of 123 patients managed with rivaroxaban for the treatment and secondary prevention of cancer-associated VTE were enrolled. The median age of patients was 66 years (range: 18–86 years old) and 57.7% of patients were female. Of all patients, 91.9% had solid tumors and 83.2% had metastatic disease. The median duration from the diagnosis of cancer to the diagnosis of VTE was 550.96 days (range 1–4409 days). The median duration of treatment with rivaroxaban was 92.25 days (range 2–406 days). The baseline characteristics are shown in Table 1.
Table 1.
Patients’ baseline characteristics.
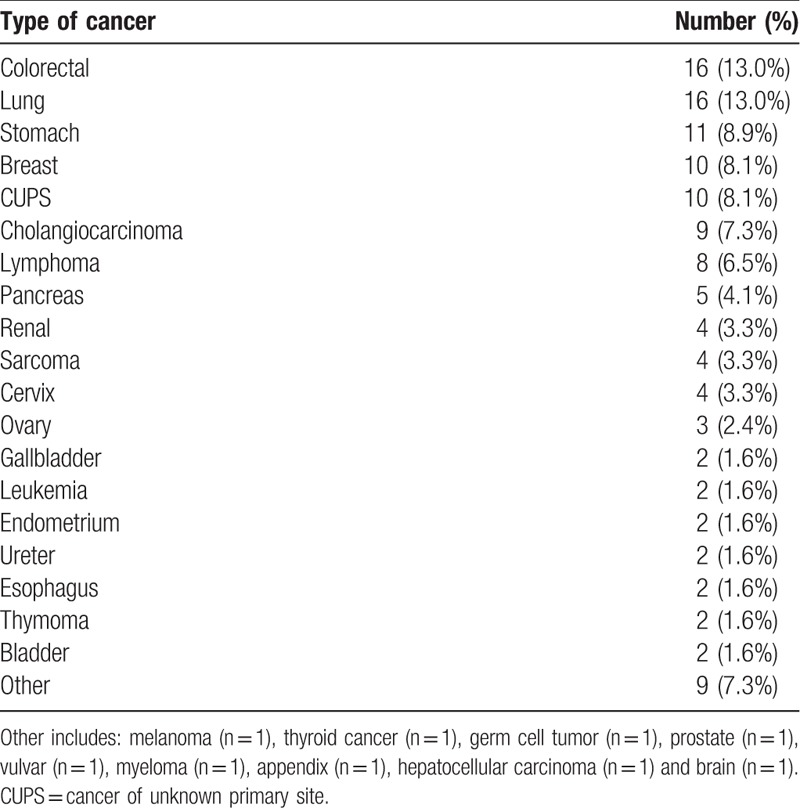
Both colorectal and lung cancer were the most frequent types of cancer (13.0%), followed by breast cancer (8.1%). The type of malignancy of the enrolled patients is shown in Table 2.
Table 2.
Type of malignancy of the patients.
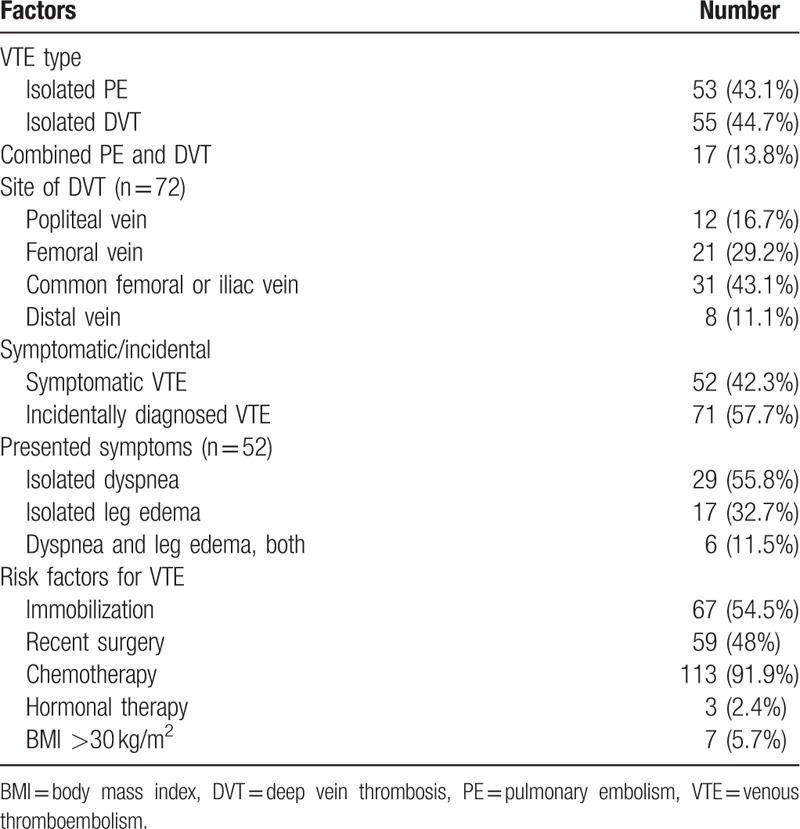
The overall characteristics of patients with VTE are shown in Table 3. A total of 53 patients (43.1%) presented with isolated PE, 55 patients (44.7%) presented with isolated DVT, and 17 patients (13.8%) presented with both. Among the patients with DVT, the most common presenting site was the common femoral or iliac vein (43.1%). Of the 123 patients, 52 (42.3%) were classified as having incidental VTE and 71 (57.7%) as having symptomatic VTE. In patients with symptomatic VTE, the most common presenting symptom was dyspnea. The majority of the incidentally diagnosed DVT were detected by CT scans performed for the diagnosis, staging, or treatment response evaluation of the malignancy. Most patients (91.9%) were receiving chemotherapy at the time of the VTE diagnosis.
Table 3.
Characteristics of patients with VTE.
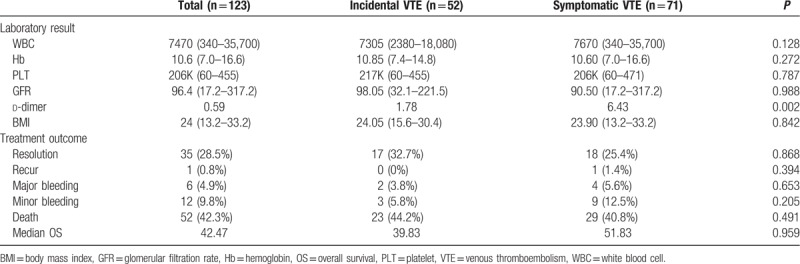
We performed additional analyses to determine the incidence of recurrence, bleeding risk, and mortality according to the presence or absence of symptoms at the time of the VTE diagnosis. The patients were divided into 2 groups: incidental and symptomatic VTE. There were no significant differences between the 2 groups in terms of the variables, except for d-dimer. The d-dimer level was significantly higher in patients with symptomatic VTE (P = .002). During follow-up, symptomatic VTE recurrence was diagnosed in only 1 patient. Two patients with incidental VTE and 4 patients with symptomatic VTE had major bleeding. There were 52 deaths (42.3%) during follow-up, none of which were because of VTE or a bleeding complication. The majority of deaths were a result of cancer progression. The characteristics of patients according to their symptoms at the time of VTE diagnosis are shown in Table 4.
Table 4.
Patients’ characteristics according to symptoms at the time of VTE diagnosis.
Six patients experienced major bleeding events and 12 patients experienced minor bleeding events. The majority of bleeding events occurred spontaneously and most bleeding events could be treated conservatively.
Overall, 52 patients died with the most common cause of death being cancer progression. No deaths were owing to VTE or bleeding complications. Figure 1 shows the Kaplan–Meier cumulative survival curve for patients who were diagnosed with incidental versus symptomatic VTE. In both groups, the median overall survival duration was not significantly different (incidental VTE: 39.83 months, symptomatic VTE: 51.83 months, P = .959). A comparison of the cumulative risk of bleeding according to the symptom status at the time of the VTE diagnosis showed no significant differences between symptomatic and incidental VTE events (Fig. 2).
Figure 1.
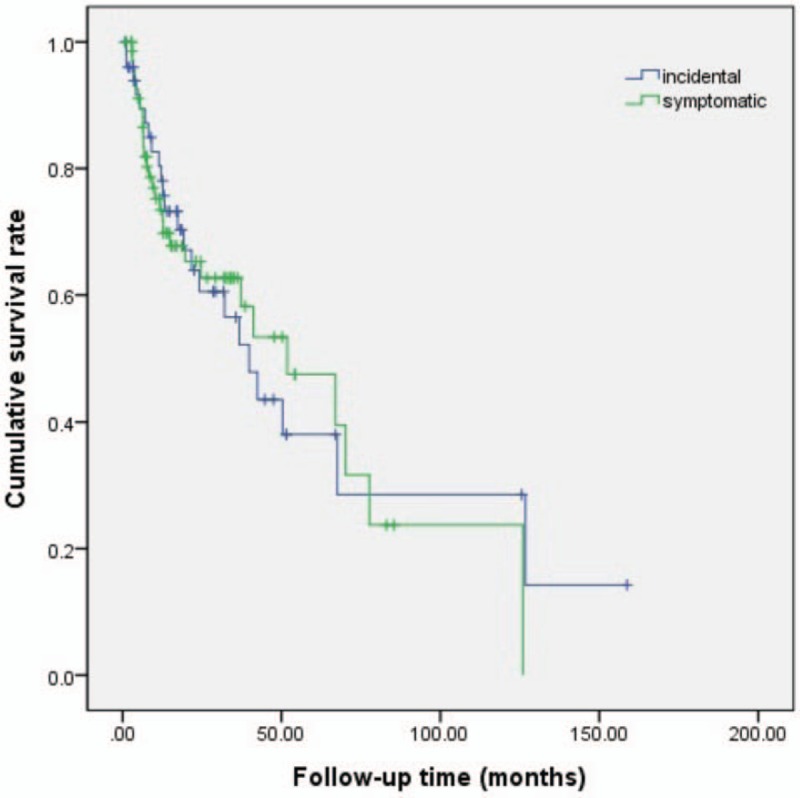
Kaplan–Meier cumulative survival curve until overall death for patients with incidental versus symptomatic venous thromboembolism.
Figure 2.
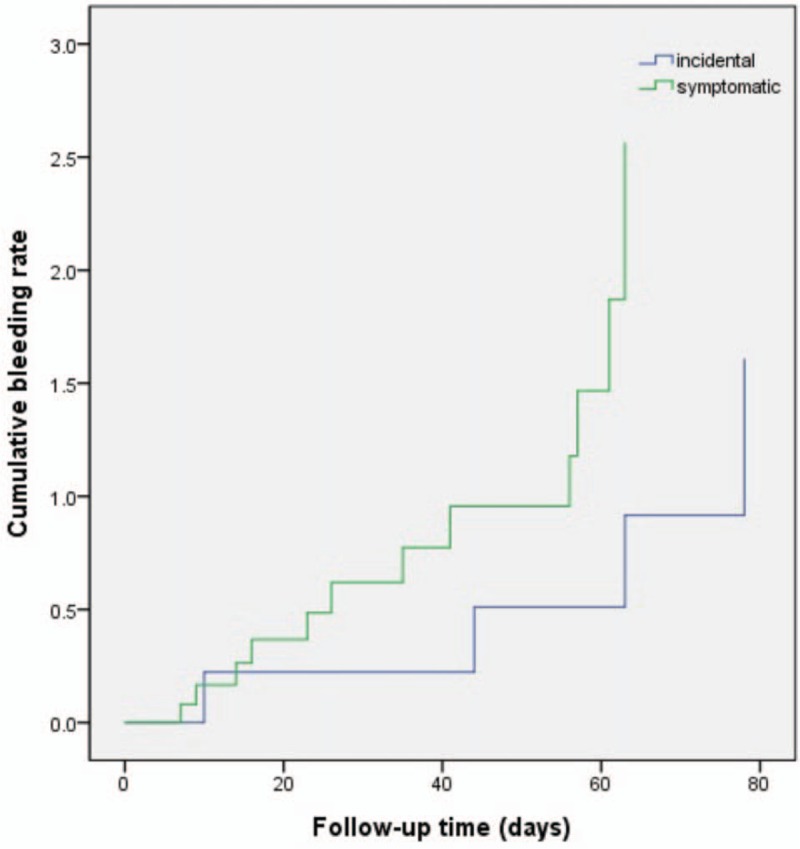
Cumulative risk of bleeding with incidental versus symptomatic venous thromboembolism.
4. Discussion
The results of our study suggest that rivaroxaban may be a safe and effective therapy for the treatment of cancer-associated VTE. In 123 patients, the mean age was 66 years. Of all patients, 83.2% had metastatic disease and approximately 92% of patients were receiving chemotherapy. We observed that the cumulative risk of recurrence after anticoagulation was 1.8% and the rate of a major bleeding complication was 4.9%. The risk of recurrence in this study is promising compared to a rate of approximately 7.2% to 9% observed in active cancer patients treated with LMWH in the CLOT and CATCH trials.[10,11] Another prospective cohort study that evaluated the safety and effectiveness of rivaroxaban for the treatment of cancer-associated VTE reported a similar rate of VTE recurrence (4.4%) and a major bleeding rate of (2.2%).[12] In the Mayo Thrombophilia Clinic direct Oral Anticoagulants Registry, there were no differences in VTE recurrence, major bleeding, or all-cause mortality in patients receiving rivaroxaban or enoxaparin for 3 to 12 months.[13] And 1 meta-analysis data suggest that rivaroxaban is as effective and safe for the prevention of recurrent VTE in patients with cancer compared to enoxaparin.[14]
The rate of major bleeding observed in this study was 4.9% and the gastrointestinal tract was the main site of major bleeding in our study population. Therefore, the use of rivaroxaban in patients with a vulnerable mucosal lesion on unresectable primary tumors of the gastrointestinal tract needs to be avoided.
Clinically, incidental VTE diagnosed on CT scans with higher resolution and sensitivity is a relatively common problem in the oncologic population.[15] Therefore, we conducted a subgroup analysis to estimate the impact of VTE, both symptomatic and incidental, on the survival of patients with cancer. The management of incidental VTE remains controversial.[16] Previously published retrospective studies have demonstrated that incidental VTE is associated with similar rates of mortality and recurrent VTE as symptomatic VTE.[17,18] This finding was in agreement with our study, and based on this, recently published guidelines recommend a minimum of 6 months of LMWH monotherapy for incidental VTE in cancer patients.[19]
There are some limitations of our study. First, this is a retrospective cohort study which may introduce a selection bias and misdiagnosis or information bias. Second, a control group of cancer-associated VTE treated with LMWH was not available for comparison with rivaroxaban to evaluate the impact of risk factors for the recurrence of cancer-associated VTE or the major bleeding rate. Third, the total number of enrolled patients was relatively small. Larger studies will be required to provide more reliable estimates of efficacy, safety, and comparability to LMWH, the current standard of care. However, a direct comparison with LMWH may be difficult when oral medications, such as rivaroxaban, are approved for VTE treatment and are used in the general population. Until the data from a direct comparison become available, our study provides reassurance for the use of rivaroxaban for the treatment of cancer-associated VTE.
In conclusion, the results of our retrospective cohort analysis suggest that rivaroxaban can be considered a convenient option to treat patients with cancer-associated VTE. Additional prospective studies to compare rivaroxaban with LMWH in patients with cancer-associated VTE are warranted.
Author contributions
Conceptualization: Sang-Bo Oh, Young-Jin Choi.
Data curation: Sang-Bo Oh, Hyo-Jeong Kim, Young-Mi Seol, Young-Jin Choi.
Formal analysis: Sang-Bo Oh, Hyo-Jeong Kim, Young-Mi Seol.
Funding acquisition: Young-Jin Choi.
Investigation: Sang-Bo Oh, Hyo-Jeong Kim, Young-Mi Seol, Young-Jin Choi.
Methodology: Sang-Bo Oh, Hyo-Jeong Kim, Young-Mi Seol, Young-Jin Choi.
Supervision: Young-Mi Seol, Young-Jin Choi.
Validation: Young-Mi Seol, Young-Jin Choi.
Visualization: Sang-Bo Oh.
Writing – original draft: Sang-Bo Oh.
Writing – review & editing: Young-Jin Choi.
Footnotes
Abbreviations: DOAC = direct oral anticoagulant, DVT = deep vein thrombosis, IRB = Institutional Review Board, LMWH = low-molecular-weight heparin, PE = pulmonary embolism, VKA = vitamin K antagonist, VTE = venous thromboembolism.
The authors report no conflicts of interest.
References
- [1].Khorana AA, Carrier M, Garcia DA, et al. Guidance for the prevention and treatment of cancer-associated venous thromboembolism. J Thromb Thrombolysis 2016;41:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schulman S, Zondaq M, Linkins L, et al. Recurrent venous thromboembolism in anticoagulated patients with cancer: management and short-term prognosis. J Thromb Haemost 2015;13:1010–8. [DOI] [PubMed] [Google Scholar]
- [3].Trujillo-Santos J, Nieto JA, Ruíz-Gamietea A, et al. Bleeding complications associated with anticoagulant therapy in patients with cancer. Thromb Res 2010;125suppl 2:S58–61. [DOI] [PubMed] [Google Scholar]
- [4].Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- [5].Frere C, Benzidia I, Marjanovic Z, et al. Recent advances in the management of cancer-associated thrombosis: New hopes but new challenges. Cancers 2019;11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510. [DOI] [PubMed] [Google Scholar]
- [7].Buller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97. [DOI] [PubMed] [Google Scholar]
- [8].Short NJ, Connors JM. New oral anticoagulants and the cancer patient. Oncologist 2014;19:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schulman S, Angeras U, Bergqvist D, et al. Definition of major bleeding in clinical investigations of antihemostatic medical products in surgical patients. J Thrombo Haemost 2010;8:202–4. [DOI] [PubMed] [Google Scholar]
- [10].Lee AT, Levine MN, Baker RI, et al. Low-molecular-weight-heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146–53. [DOI] [PubMed] [Google Scholar]
- [11].Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA 2015;18:677–86. [DOI] [PubMed] [Google Scholar]
- [12].Mantha S, Laube E, Miao Y, et al. Safe and effective use of rivaroxaban for treatment of cancer-associated venous thromboembolis disease: a prospective cohort study. J Thromb Thrombolysis 2017;43:166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Simmons B, Wysokinski W, Saadiq RA, et al. Efficacy and safety or rivaroxaban compared to enoxaparin in treatment of cancer-associated venous thromboembolism. Eur J Haematol 2008;101:136–42. [DOI] [PubMed] [Google Scholar]
- [14].Xing J, Yin X, Chen D. Rivaroxaban versus enoxaparin for the prevention of recurrent venous thromboembolism in patients with cancer: A meta-analysis. Medicine (Baltimore) 2018;97:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Browne AM, Cronin CG, English C, et al. Unsuspected pulmonary emboli in oncology patients undergoing routine computed tomography imaging. J Thrac oncol 2010;5:798–803. [DOI] [PubMed] [Google Scholar]
- [16].Di Nisio M, Carrier M. Incidental venous thromboembolism: is anticoagulation indicated? Hematology Am Soc Hematol Educ Program 2017;2017:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].O’Connell C, Razavi P, Ghalichi M, et al. Unsuspected pulmonary emboli adversely impact survival in patients with cancer undergoing routine staging multi-row detector computed tomography scanning. J Thromb Haemost 2011;9:305–11. [DOI] [PubMed] [Google Scholar]
- [18].Den Exter PL, Hooijer J, Dekkers OM, et al. Risk of recurrent venous thromboembolism and mortality in patients with cancer incidentally diagnosed with pulmonary embolism: a comparison with symptomatic patients. J Clin Oncol 2011;29:2405–9. [DOI] [PubMed] [Google Scholar]
- [19].Di Nisio M, Lee AY, Carrier M, et al. Diagnosis and treatment of incidental venous thromboembolism in cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost 2015;13:880–3. [DOI] [PubMed] [Google Scholar]


