Abstract
Background:
Stroke is one of the leading causes of death and disability for adult men and women worldwide, and a number of studies have explored the influences of smoking on stroke. However, few studies have discussed the relationship between stroke and smoking with consideration of the following factors: sex, the number of cigarettes smoked per day, stroke subtype, and the follow-up duration. Consequently, we aimed to extend previous work by using a systematic review to explore the relationship between stroke and cigarette smoking in reference to the above factors.
Methods:
A systematic review was conducted using the PubMed, Embase, and Cochrane Central Register databases and the following search criteria: [“stroke” (MeSH) and “smoking” (MeSH)]. All analyses were conducted with Stata, and funnel plots and Egger regression asymmetry tests were used to assess publication bias.
Results:
The meta-analysis included 14 studies involving 303134 subjects. According to the meta-analysis, smokers had an overall increased risk of stroke compared with nonsmokers, with a pooled odds ratio (OR) of 1.61 (95% confidence interval [CI]: 1.34–1.93, P < .001). A subgroup analysis conducted based on smoking status revealed ORs of 1.92 (95% CI: 1.49–2.48) for current smokers and 1.30 (95% CI: 0.93–1.81) for former smokers. In addition, the relationship between stroke of any type and smoking status was also statistically significant; current smokers had an increased risk of stoke compared with nonsmokers (OR: 1.46, 95% CI: 1.04–2.07, P < .001), which was influenced by sex (men: OR: 1.54, 95% CI: 1.11–2.13, P = .002; women: OR: 1.88, 95% CI: 1.45–2.44, P < .023). From the analysis, we also observed that passive smoking increased the overall risk of stroke by 45% (OR: 1.45, 95% CI: 1.0–2.11, P < .05). Based on the dose-response meta-analysis, the risk of stroke increased by 12% for each increment of 5 cigarettes per day.
Keywords: meta-analysis, smoking, stroke
1. Introduction
Stroke, known as an acute cerebrovascular event closely related to brain tissue injury, is one of the leading causes of death and disability for adult men and women worldwide.[1] According to World Health Organization reports, every year, approximately 15 million people have a stroke,[2] leading to a substantial economic burden. Stroke can be divided into different subtypes: 87% are classified as ischaemic stroke (IS), 10% are classified as intracerebral hemorrhagic stroke (HS), and the remaining 3% are classified as subarachnoid HS.[3] Epidemiological studies have revealed that there are sex differences in stroke and that the burden from stroke in women was on the rise in Eastern Europe (post-socialist countries).[4]
Smoking is prevalent all over the world, especially among youth and in developing countries.[5] Although policies using different measures have successfully encouraged people to quit smoking, the absolute global number of smokers has increased because of population growth.[6] According to the Centers for Disease Control and Prevention (CDC), their most recent data indicated that 20.8% of the US adult population smokes.[4,7] Low nicotine-content cigarettes are generally believed to be relatively safe regarding the health effects.[8] However, smoking is still one of the most important risk factors for stroke, and previous studies have revealed a strong dose-dependent relationship between smoking and the risk of IS.[7,9,10] Other studies have also revealed that smoking has a close relationship with inflammatory factors, which play an important role in stroke pathogenesis.[11–13]
In recent years, a number of studies have been conducted to explore the influences of smoking on stroke;[14,15] however, few studies have been conducted to explore the relationship between stroke and smoking with consideration of the following factors: sex, the number of cigarettes per day, stroke subtype, follow-up duration, and so on. Consequently, we aimed to extend the previous work by using a systematic review to explore the relationship between stroke and cigarette smoking in reference to the above factors.
2. Methods
2.1. Ethics statement
As all analyses were based on previously published studies, and no ethical approval or patient consent was required.
2.2. Search strategy
We conducted a systematic review using the PubMed, Embase, and Cochrane Central Register databases and the following search criteria [“stroke” (MeSH) and “smoking” (MeSH)]. The search was limited to studies published between January 1, 1980, and June 1, 2018. The studies must have included human subjects and must have been published in English. Furthermore, the references of the retrieved articles were manually searched for additional studies.
2.3. Selection criteria and data extraction
To meet the inclusion criteria, the original research should meet the following criteria.
-
1.
The study was a case–control, cross-sectional, cohort or nested case–control design.
-
2.
The study analyzed the relationship between smoking and stroke, and stroke was one of the endpoints of the results.
-
3.
The relative risk ratios (RRs) and 95% confidence intervals (CIs) of the relationship between smoking and stroke were provided in the publication.
-
4.
The overall risk of stroke (all types), as well as the risk of IS or HS, were reported as the outcomes of interest.
We excluded letters, comments, reviews, meta-analyses, ecological studies, and experimental studies.
2.4. Data extraction
For the included studies, we extracted the following information: the first author's name, the publication year, the country of the study population, the average age of the participants, the sample size, the smoking status of the participants, the follow-up duration, the number of stroke events, the odds ratios (ORs) and corresponding 95% CIs of stroke based on smoking status, and the covariates adjusted for in the statistical analysis. Two investigators, XJ and BQP, extracted the data independently; any disagreements they were resolved by discussion with a third investigator (MWQ).
2.5. Statistical analysis
RRs and 95% CIs were considered measures of effect size, and ORs and hazard ratios (HRs) were considered equal to RRs in view of the low incidence rates. Pooled risk estimates were performed based on smoking status. The number of cigarettes was divided into different categories across studies, and the mean or median number of cigarettes in each category was extracted. If the mean or median was not provided, the midpoint of each category was extracted. If the upper boundary of the highest category was not provided, we considered 1.2 times its lower boundary as the midpoint. If the lower boundary of the lowest category was not provided, we considered it to be 0. Heterogeneity was evaluated by the Q-test statistic. We performed a subgroup meta-analysis stratified by age, stroke subtype, and study sample size. Funnel plots and Egger regression asymmetry tests were used to assess publication bias. All analyses were conducted by Stata (version 12.0, StataCorp, College Station, TX). The significance level in this meta-analysis was set as 0.05.
3. Results
3.1. Study selection
In the initial search, a total of 486 studies were identified. After removing the duplicates, 108 studies remained and needed to be further evaluated. Sixty-eight studies were removed after reading the abstracts and titles. We excluded 26 studies for the following reasons: 14 studies did not report the relevant data (such as ORs) on smoking and stroke; 4 studies were commentaries, letters or editorials; and 8 studies did not evaluate the relationship between stroke and smoking status. For the final meta-analysis, 14 studies met the inclusion criterion and were included (Fig. 1).
Figure 1.
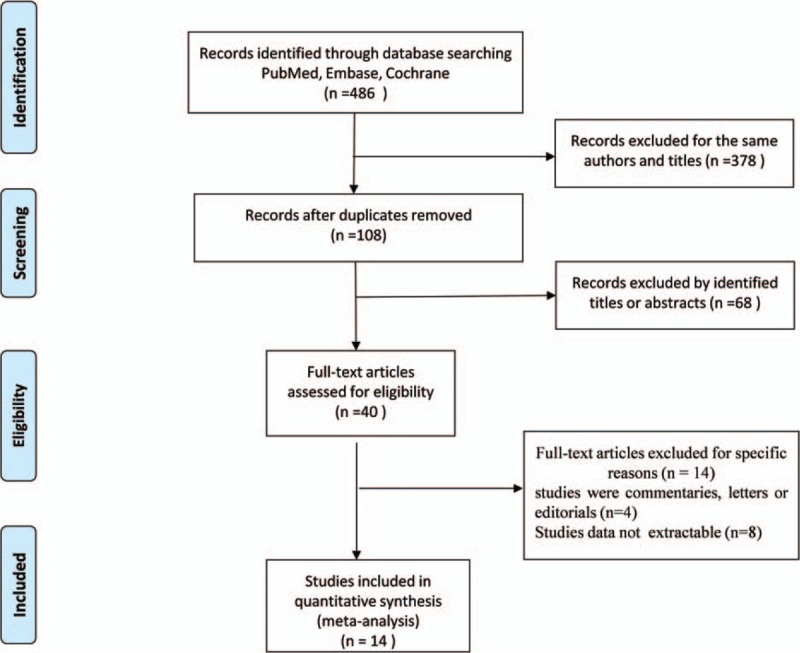
Flow chart for the process of selecting eligible publications.
3.2. Characteristics of the selected studies
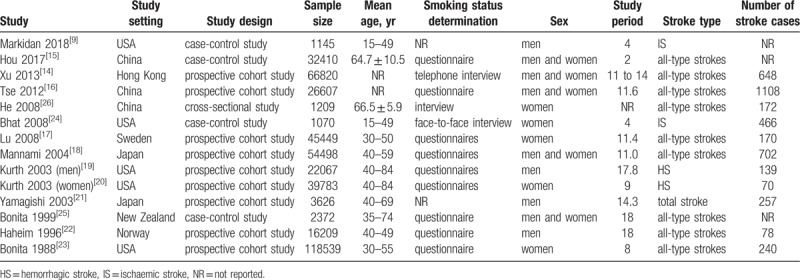
A total of 14 studies including 303134 participants met the inclusion criteria, and 4050 stroke events were identified in the included studies (Table 1). Among the included studies, 9 studies[14,16–23] were prospective cohort studies, 4 studies[9,15,24,25] were case-control studies and 1 study[26] was a cross-sectional study. The publication years of the included studies were between 1988 and 2018. The sample sizes of the selected studies ranged from 1070 to 118539 participants. The average follow-up period ranged from 2 to 18 years. Four studies[9,19,21,22] evaluated only men, 5 studies[17,20,23,24,26] evaluated only women, and 5 studies[17,20,23,24,26] included both men and women. Twelve studies defined former smokers and current smokers, and 2 studies did not refer to former smokers in the publications. In addition, among the included studies, 10 studies[14–18,21–23,25,26] evaluated the relationship between smoking and overall stroke (stroke of any type), 2 studies[19,20] evaluated only IS, and 2 studies[9,24] evaluated only HS. Most of the included studies adjusted for potential confounding factors, including age, race, education, hypertension, alcohol use, and exercise.
Table 1.
The basic characteristics of the included studies.
3.3. Smoking and overall risk of stroke
The pooled OR for the relationship between current smoking and the risk of stroke is shown in Figure 3. Smokers had an increased risk of overall stroke compared with nonsmokers, with a pooled OR of 1.61 (95% CI: 1.34–1.93). A subgroup analysis was conducted based on smoking status: the ORs were 1.92 (95% CI: 1.49–2.48) for current smokers and 1.30 (95% CI: 0.93–1.81) for former smokers. Significant statistical heterogeneity was noted in the studies reporting ORs for overall stroke (I2 = 88.0%, P < .001), and a random-effects model was used in this meta-analysis (Fig. 2).
Figure 3.
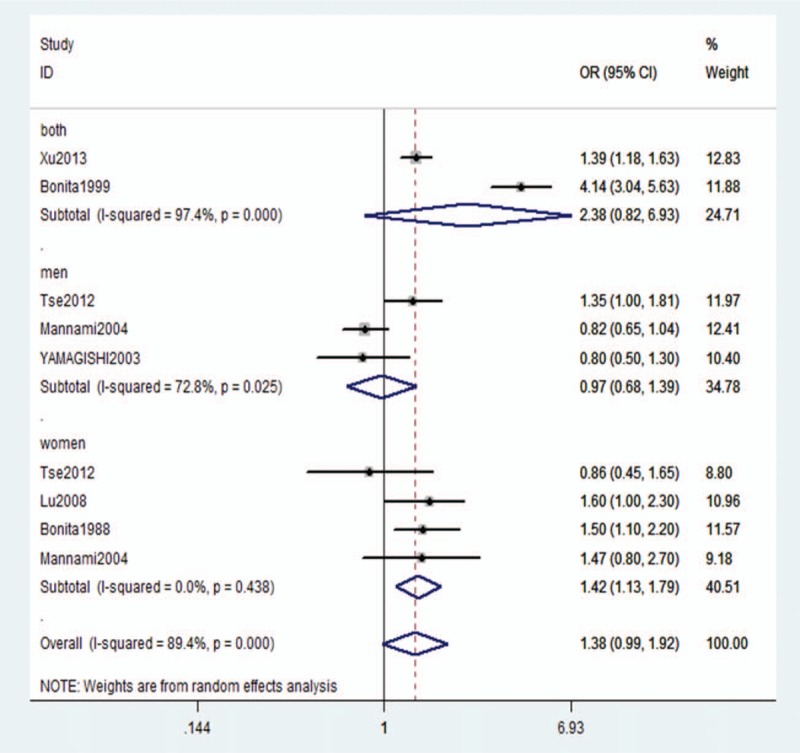
Adjusted relative risk of overall stroke for former smokers compared with nonsmokers.
Figure 2.
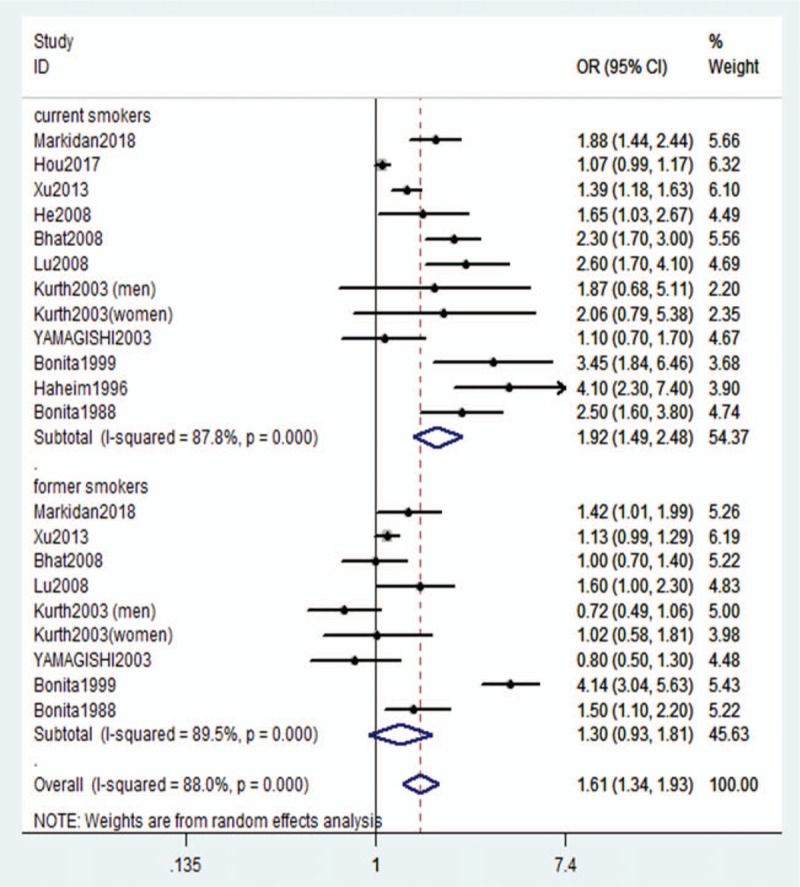
Adjusted relative risk of overall stroke for current smokers compared with former smokers.
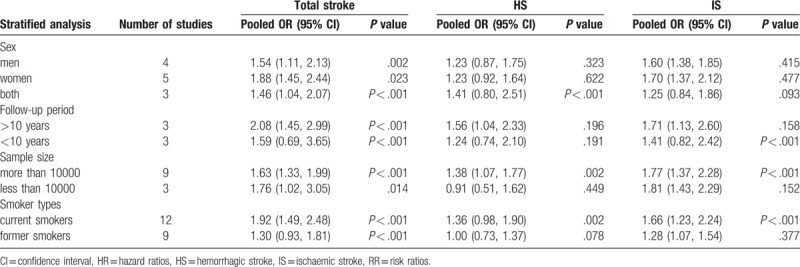
3.4. Stratified analysis
To better explore the sources of heterogeneity between studies and the relationship between smoking and stroke, a stratified analysis was conducted based on effect modifiers or confounding factors. Based on the stratified analysis, the incidence rate of overall stroke increased in all the included subgroups. However, we did not find that sex significantly influenced the relationship between smoking and IS or HS. Furthermore, we found that the follow-up duration can significantly influence the observed relationship between smoking and stroke (Table 2).
Table 2.
Stratified analysis of the pooled odds ratio of stroke or current smokers.
3.5. Former smoking and overall risk of stroke
According to the stratified analysis, the relationship between stroke of any type and current smoking was statistically significant; current smokers had an increased risk of stoke compared with nonsmokers (OR: 1.46, 95% CI: 1.04–2.07, P < .001), which was influenced by sex (men: OR: 1.54, 95% CI: 1.11–2.13, P = .002; women: OR: 1.88, 95% CI: 1.45–2.44, P < .023). However, we did not obtain the same results with overall stroke in former smokers versus nonsmokers (OR: 1.38, 95% CI: 0.99–1.92, P < .001), which did not change with sex. In former smokers, the ORs were 0.97 (95% CI: 0.68–1.39, P = .025) and 1.18 (95% CI: 0.72–1.92) for men and women, respectively (Fig. 3).
3.6. A dose-dependent relationship between current smoking and stroke
Among the included studies, 7 reported the details of the relationship between the number of cigarettes smoked and the incidence of stroke. In the dose-response meta-analysis, the non-linear association between cigarette smoking and stroke was non-significant (P for nonlinearity = .729). A linear elevation in stroke incidence was observed when cigarette consumption increased, and the risk of stroke was increased by 12% for each increment of 5 cigarettes per day (95% CI: 0.01, 0.02, P < .001) (Fig. 4).
Figure 4.
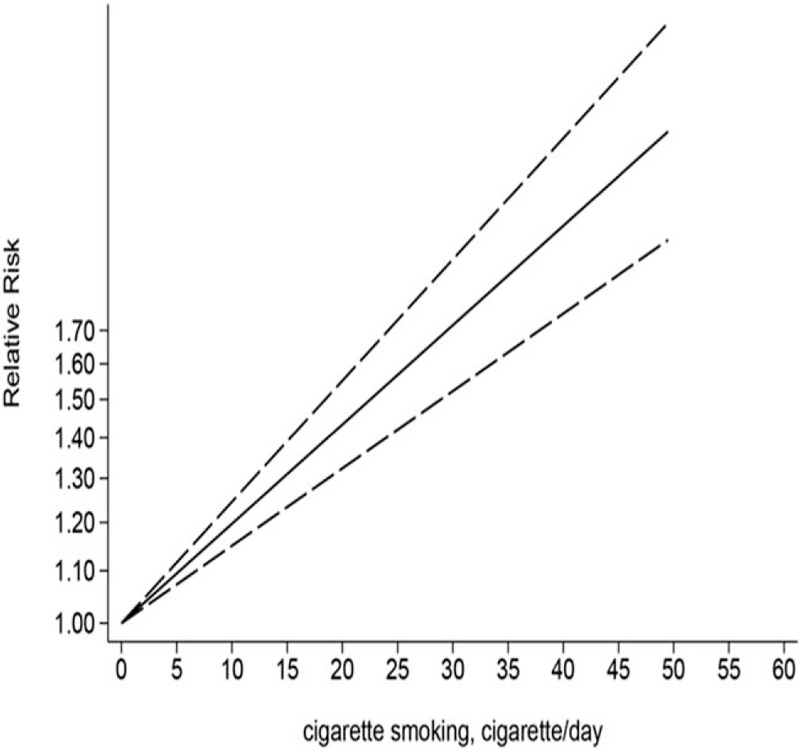
Linear dose-dependent relationship between number of cigarettes smoked per day and the relative risk of stroke among all subjects.
3.7. Passive smoking and risk of stroke
From the analysis, we also observed that passive smoking can increase the overall risk of stroke by 45% (OR: 1.45, 95% CI: 1.0–2.11) compared with no current exposure to passive smoking, and this difference in risk was statistically significant (P < .05).
3.8. Publication bias
To evaluate the publication bias, funnel plots and Egger regression asymmetry tests were used. A funnel diagram did not show symmetry, which meant that publication bias existed in this meta-analysis. Consequently, a trim-and-fill method was conducted to evaluate the sensitivity, which hypothetically imputed 8 negative and unpublished studies that were missing from the initial analysis (Fig. 5).
Figure 5.
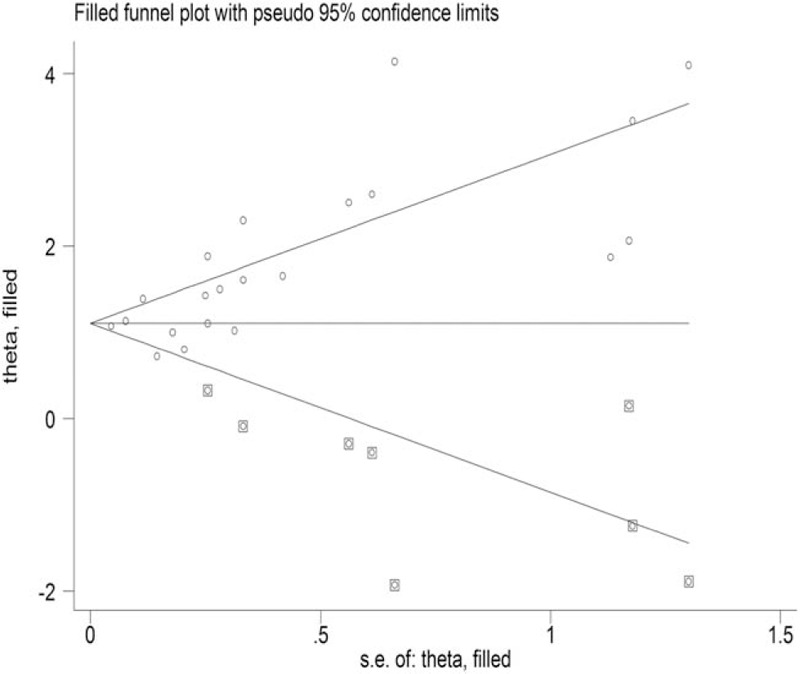
Filled funnel plot for smoking and the overall risk of stroke.
4. Discussion
In this meta-analysis, we found an increased risk of stroke in smokers compared with nonsmokers, which was consistent with previous studies. An increased risk of stroke associated with current smoking was observed in most subgroups. In addition, there was a dose-dependent relationship between current smoking status and the overall risk of stroke, and the risk was increased by 12% for each increment of 5 cigarettes per day. However, we did not obtain the same results for the relationship between overall stroke and former smokers (OR: 1.38, 95% CI: 0.99–1.92, P < .001). According to the stratified analysis, there was a relationship between overall stroke and current smoking status regardless of sex, follow-up duration or study sample size. However, we did not obtain the same results with the HS and IS subgroups. The above factors investigated in this study significantly influenced the results.
Although several meta-analyses have evaluated the relationship between smoking and stroke,[27–29] our meta-analysis provides new information and implications. Compared with the meta-analysis published in 1989, our meta-analysis updated the included studies and performed subgroup analysis based on smoking status.[27] Another meta-analysis published in 2011 only discussed the association between second-hand smoke exposure and stroke,[28] and the meta-analysis published in 2018 mainly discussed the association of low cigarette consumption and the risk of coronary heart disease and stroke.[29] For the previous cohort studies, most of the studies only considered 1 confounding factor.[9,15,24,30] In our meta-analysis, we performed subgroup analysis with consideration of the following factors sex, the number of cigarettes smoked per day, stroke subtype, follow-up duration, and smoking status. We also performed a dose-response analysis between the number of cigarettes per day and the risk of stroke. According to the results of the meta-analysis, we can conclude that current smokers have a relationship with the risk of stroke, which was consistent with finding from previous studies. However, in this meta-analysis, we did not find an association between former smokers and the incidence of stroke, indicating that quitting smoking has a positive effect on the incidence of stroke. This result highlights the benefits of smoking cessation on stoke outcomes.
In this meta-analysis, minor differences were observed between sexes. In the subgroup analysis, we found that there was a greater risk of stroke for women. Earlier studies also revealed a strong dose-dependent relationship between smoking and stroke among women.[23,24] Current use of oral contraceptives and a higher dose of harmful chemicals per unit of body mass may contribute to these results. In addition, we observed that current smoking is related to the occurrence of stroke of any type. The idea that there is a population of ex-smokers who started smoking again and are actually current smokers cannot be neglected. Moreover, passive smoking can also increase the risk of stroke; consequently, people living in an environment with smokers should also be considered.
The mechanism by which passive smoking can increase the risk of stroke has been reported in many studies. Passive smoking can lead to carotid atherosclerosis,[31] and the levels of homocysteine, fibrinogen, and oxidized low-density lipoprotein cholesterol can also be elevated by smoking.[29,32] Epidemiological studies have concluded that smoking can increase the risk of sudden cardiac death.[33] A meta-analysis also revealed that smoking was associated with an increased risk of atrial fibrillation in a dose-dependent manner.[34] Additionally, smoking has also proven to be related to diabetes, high blood pressure, and elevated resting heart rate, which are all risk factors for stroke.[35,30] Furthermore, smoking plays a possible role in some genetic diseases, such as Fabry disease, which can impair the central nervous system and lead to cerebral infarction and transient ischaemic attack.[36,37] The key strength of the present study is that it explored the relationship between stroke and smoking from different aspects. From this meta-analysis, we can conclude that passive smokers and ex-smokers have a higher risk of stroke than nonsmokers do. In addition, the relationship between smoking and stroke can also be influenced by sex.
4.1. Limitations
Limitations also exist in this study. First, heterogeneity was observed in this meta-analysis, resulting from different characteristics of the participants and inconsistent definitions of outcome measures. To decrease the publication bias, stratified analyses were conducted. Second, the trim-and-fill method was used, which can estimate the number of unpublished and grey literature studies and suggested that the association was not affected by unpublished negative studies. Third, stroke can be divided into different subtypes. In this meta-analysis, however, we discussed only IS and HS. HS can be further subdivided into intracerebral HS and subarachnoid HS; however, because of limited information, we did not perform further analyses on the HS subtypes.
4.2. Future directions
Stroke can be divided into subtypes; however, most of the included studies discussed only IS and HS. HS can be subdivided into intracerebral HS and subarachnoid HS, and further analysis may be performed between the subtypes of HS in future studies. In addition, this meta-analysis indicated that former smokers did not show a higher risk of stroke; however, further studies should be conducted among former smokers to determine whether the duration of smoking cessation affects the current risk of stroke.
5. Conclusion
From this meta-analysis, we can conclude that stroke has a dose-dependent relationship with smoking, regardless of status as a current smoker or passive smoker. In addition, there was a relationship between stroke of any type and current smoking status regardless of sex, follow-up duration or study sample size. However, in this meta-analysis, we did not find an association between former smokers and the incidence of stroke, indicating that quitting smoking has a positive effect on the incidence of stroke.
Additionally, there are still some potential confounders due to lack of information; consequently, additional studies with larger sample sizes and more detailed information are needed for further clarification.
Author contributions
Conceptualization: Qiuping Zheng.
Data curation: Xiao Jin, Shaohong Qiu.
Formal analysis: Xiao Jin, Shaohong Qiu.
Funding acquisition: Mingwo Pan.
Methodology: Biqi Pan, Liu Jun, Qiuping Zheng, Mingwo Pan.
Project administration: Biqi Pan.
Resources: Biqi Pan.
Software: Shaohong Qiu.
Writing – original draft: Xiao Jin, Qiuping Zheng, Mingwo Pan.
Writing – review & editing: Liu Jun, Qiuping Zheng, Mingwo Pan.
Footnotes
Abbreviations: CI = confidence interval, HS = hemorrhagic stroke, IS = ischaemic stroke, OR = odds ratio, RR = risk ratio.
BP and XJ contributed equally to this work.
This project was funded by the Project of Guangdong Provincial Department of Finance (No. [2014]157, No. [2018]8), Science and Technology Research Project of Guangdong Provincial Hospital of Chinese Medicine (No. YK2013B2N19, YN2015MS15).
The authors have no conflicts of interest to disclose.
References
- [1].Li WA, Geng X, Ding Y. Stroke is a global epidemic: new developments in clinical and translational cerebrovascular diseases research. Neurol Res 2017;39:475–6. [DOI] [PubMed] [Google Scholar]
- [2].Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2013 update: a report from the American heart association. Circulation 2013;127:e6–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lackland DT, Roccella EJ, Deutsch AF, et al. Factors influencing the decline in stroke mortality: a statement from the American heart association/American stroke association. Stroke 2013;45:315–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Arnao V, Acciarresi M, Cittadini E, et al. Stroke incidence, prevalence and mortality in women worldwide. Int J Stroke 2016;11:287–301. [DOI] [PubMed] [Google Scholar]
- [5].Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA 2014;311:183–92. [DOI] [PubMed] [Google Scholar]
- [6].Sreeramareddy CT, Pradhan PM, Mir IA, et al. Smoking and smokeless tobacco use in nine South and Southeast Asian countries: prevalence estimates and social determinants from Demographic and Health Surveys. Popul Health Metr 2014;12:22–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Feigin VL, Roth GA, Naghavi M, et al. Global burden of stroke and risk factors in 188 countries, during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol 2016;15:913–24. [DOI] [PubMed] [Google Scholar]
- [8].Denlinger-Apte RL, Joel DL, Strasser AA, et al. Low nicotine content descriptors reduce perceived health risks and positive cigarette ratings in participants using very low nicotine content cigarettes. Nicotine Tob Res 2017;19:1149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Markidan J, Cole JW, Cronin CA, et al. Smoking and risk of ischemic stroke in young men. Stroke 2018;49:1276–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thrift AG, Thayabaranathan T, Howard G, et al. Global stroke statistics. Int J Stroke 2017;12:13–32. [DOI] [PubMed] [Google Scholar]
- [11].Kwan JHG, Bryant T. IL-6 is a predictive biomarker for stroke associated infection and future mortality in the elderly after an ischemic stroke. Exp Gerontol 2013;48:960–5. [DOI] [PubMed] [Google Scholar]
- [12].Di Raimondo DTA, Buttà C, Miceli S, et al. Effects of ACE-inhibitors and angiotensin receptor blockers on inflammation. Curr Pharm Des 2012;18:4385–413. [DOI] [PubMed] [Google Scholar]
- [13].Tuttolomondo ADSR, Di Raimondo D, Pedone C, et al. Effects of clinical and laboratory variables and of pretreatment with cardiovascular drugs in acute ischaemic stroke: a retrospective chart review from the GIFA study. Int J Cardiol 2011;151:318–22. [DOI] [PubMed] [Google Scholar]
- [14].Xu L, Schooling CM, Chan WM, et al. Smoking and hemorrhagic stroke mortality in a prospective cohort study of older Chinese. Stroke 2013;44:2144–9. [DOI] [PubMed] [Google Scholar]
- [15].Hou L, Han W, Jiang J, et al. Passive smoking and stroke in men and women: a national population-based case-control study in China. Sci Rep 2017;7:45542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tse LA, Fang XH, Wang WZ, et al. Incidence of ischaemic and haemorrhagic stroke and the association with smoking and smoking cessation: a 10-year multicentre prospective study in China. Public Health 2012;126:960–6. [DOI] [PubMed] [Google Scholar]
- [17].Lu M, Ye W, Adami H-O, et al. Stroke incidence in women under 60 years of age related to alcohol intake and smoking habit. Cerebrovasc Dis 2008;25:517–25. [DOI] [PubMed] [Google Scholar]
- [18].Mannami T, Iso H, Baba S, et al. Cigarette smoking and risk of stroke and its subtypes among middle-aged Japanese men and women: the JPHC study cohort I. Stroke 2004;35:1248–53. [DOI] [PubMed] [Google Scholar]
- [19].Kurth T, Kase CS, Berger K, et al. Smoking and the risk of hemorrhagic stroke in men. Stroke 2003;34:1151–5. [DOI] [PubMed] [Google Scholar]
- [20].Kurth T, Kase CS, Berger K, et al. Smoking and risk of hemorrhagic stroke in women. Stroke 2003;34:2792–5. [DOI] [PubMed] [Google Scholar]
- [21].Kazumasa Yamagishi Hiak, Takeshi Tanigawa Ynss, Tetsuya Ohira, et al. Smoking raises the risk of total and ischemic strokes in hypertensive men. Hypertension Research 2003;26:209–17. [DOI] [PubMed] [Google Scholar]
- [22].Håheim LL, Holme I, Hjermann I, et al. Smoking habits and risk of fatal stroke: 18 years follow up of the Oslo study. J Epidemiol Community Health 1996;50:621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ruth Bonita Meir J. Cigarette smoking and risk of stroke in middle-aged women. New Engl J Med 1988;318:937–41. [DOI] [PubMed] [Google Scholar]
- [24].Bhat VM, Cole JW, Sorkin JD, et al. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke 2008;39:2439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bonita R, Duncan J, Truelsen T, et al. Passive smoking as well as active smoking increases the risk of acute stroke. Tob Control 1999;8:156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].He Y, Lam TH, Jiang B, et al. Passive smoking and risk of peripheral arterial disease and ischemic stroke in Chinese women who never smoked. Circulation 2008;118:1535–40. [DOI] [PubMed] [Google Scholar]
- [27].Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. Br Med J 1989;298:789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Oono IP, Mackay DF, Pell JP. Meta-analysis of the association between secondhand smoke exposure and stroke. J Public Health 2011;33:496–502. [DOI] [PubMed] [Google Scholar]
- [29].Hackshaw A, Morris JK, Boniface S, et al. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ 2018;j5855–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Thomas SB, J Michael G, Julie EB, et al. A prospective study of cigarette smoking and risk of incident hypertension in women. J Am Coll Cardiol 2007;50:2085–92. [DOI] [PubMed] [Google Scholar]
- [31].Rehill N, Beck CR, Yeo KR, et al. The effect of chronic tobacco smoking on arterial stiffness. Br J Clin Pharmacol 2006;61:767–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Al PDBE Effect of exposure to secondhand smoke on markers of inflammation: the ATTICA study. Am J Med 2004;116:145–50. [DOI] [PubMed] [Google Scholar]
- [33].Dagfinn Aune SSTN. Tobacco smoking and the risk of sudden cardiac death: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol 2018;33:509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Aune D, Schlesinger S, Norat T, et al. Tobacco smoking and the risk of atrial fibrillation: a systematic review and meta-analysis of prospective studies. Eur J Prev Cardiol 2018;25:1437–51. [DOI] [PubMed] [Google Scholar]
- [35].Akter S, Goto A, Mizoue T. Smoking and the risk of type 2 diabetes in Japan: a systematic review and meta-analysis. J Epidemiol 2017;27:553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tuttolomondo APRSI, Miceli S, Arnao V, et al. Neurological complications of anderson-fabry disease. Curr Pharm Des 2013;19:6014–30. [DOI] [PubMed] [Google Scholar]
- [37].Tuttolomondo APRSI, Miceli S, Pinto A, et al. Anderson-fabry disease: a multiorgan disease. Curr Pharm Des 2013;19:5974–96. [DOI] [PubMed] [Google Scholar]


