Abstract
In mammals, ejaculated sperm acquire their fertilizing ability during migration through the female reproductive tract, which secretes several factors that contribute to sperm capacitation. Gamma-aminobutyric acid (GABA) is a well-known neurotransmitter in the central nervous system, but additionally enhances the sperm acrosome reaction in the rat and cow. However, the detailed effects of GABA concentration on sperm function remain unclear. In this study, we detected the presence of the GABA type A receptor (GABA A) in mouse epididymal sperm by western blot analysis and in the sperm acrosome by immunocytochemistry. We also investigated the effects of GABA on sperm fertilizing ability. We found that GABA facilitated the tyrosine phosphorylation of sperm proteins, which is an index of sperm capacitation. GABA also promoted the acrosome reaction, which was suppressed by a selective GABA A receptor antagonist. We then found that the effective GABA concentration for the acrosome reaction corresponds to sperm concentration, but we did not detect any marked effect of GABA on sperm motility using a computer-assisted sperm analysis system. Using immunohistochemistry, we also detected GABA expression in the epithelia of the mouse uterus and oviduct. Furthermore, we found that the mRNA levels of glutamate decarboxylase (Gad), which generates GABA from L-glutamate, were higher in the oviduct than in the uterus, and that Gad mRNA levels were higher at estrus than at the diestrus stage. These results indicate that the GABA concentration can act as a modulator of the acrosome reaction and sperm capacitation in the female reproductive tract.
Keywords: Acrosome reaction, Capacitation, Gamma-aminobutyric acid (GABA), Sperm
Mammalian sperm are unable to fertilize an egg immediately after ejaculation and must first acquire fertilizing ability by capacitation when migrating through the female reproductive tract toward the area of fertilization [1]. During migration, sperm are exposed to various factors such as hormones, signal transducing molecules, enzymes, ions, and lipids secreted from female tissues [2]. These factors accelerate sperm tyrosine phosphorylation and remove cholesterol from the sperm plasma membrane [3, 4]. Many factors secreted from the female reproductive tract are involved in this complex process.
Several reports have provided evidence for the presence of neurotransmitter receptors in mammalian sperm, including those for dopamine [5], serotonin [6], and neurotensin [7, 8]. Some of these receptors can be stimulated by the corresponding ligand to regulate sperm capacitation or the acrosome reaction [5,6,7,8].
Among the neurotransmitters, gamma-aminobutyric acid (GABA) has emerged as a putative modulator of sperm function. GABA is the most widely distributed inhibitory neurotransmitter in the vertebrate central nervous system, and it is estimated that 20–50% of all synapses in the mammalian brain are GABAergic [9,10,11].
GABAergic signals are transduced into the cell via receptors located in the membranes of neuronal cells. In mammals, at least three types of GABA receptors have been identified, namely, types A, B, and C [12]. The GABA A receptor is a supramolecular receptor complex linked to a Cl− channel, the activation of which produces a transmembrane Cl− ion flux that is antagonized by bicuculline [13]. The GABA B receptor belongs to the G protein-coupled receptor superfamily [14]. While inhibition of the GABA B receptor is mediated by the indirect gating of either potassium or calcium channels, the GABA B receptor is activated by baclofen and antagonized by phaclofen [15]. Like GABA A, the GABA C receptor, the third member of the GABA receptor family [16], is also a ligand-gated chloride channel. However, pharmacologically, the GABA C receptor is insensitive to bicuculline and baclofen and is not regulated by many GABA A modulators, including benzodiazepines and barbiturates [17].
The expression of GABA and the components of GABAergic systems has been identified in many peripheral tissues, including the gastrointestinal tract and kidney [18,19,20]. GABA is generated from L-glutamate by glutamate decarboxylase (GAD). GAD has two isoforms, GAD67 (GAD1) and GAD65 (GAD2). GAD67 is present in the testes and GAD65 in the oviduct [21, 22]. GABA expression has also been investigated in the genital tracts of male and female rats, as well as in human sperm and seminal fluid [23,24,25]. Immunohistochemical studies indicate that GABA is localized to the mucosal layer of the rat oviduct [24].
There is some evidence that the GABA A receptor is expressed in human sperm and that GABA is secreted from the rat reproductive tract [24]. Furthermore, GABA has been shown to enhance the sperm acrosome reaction in both cow and rat [26, 27]. Although GABA concentration in the rat oviduct is regulated during the estrus cycle [28], the effect of GABA concentration on sperm remains unknown. Therefore, we investigated the expression of GABA receptors and examined the effects of GABA concentration on mouse sperm. Additionally, we also analyzed GABA expression and regulation in the reproductive organs of female mice.
Materials and Methods
Mouse sperm preparation
C57BL/6N mice were purchased from Japan SLC (Shizuoka, Japan). Cauda epididymal sperm were collected from male mice (aged between 12 and 52 weeks) euthanized in accordance with the Guide for the Care and Use of Laboratory Animals published by Tohoku University. Dissected cauda epididymis was punctured with needle and exposed sperm cells were transferred into 500 µl of human tubal fluid (HTF) medium [23], consisting of 101.6 mM NaCl, 4.7 mM KCl, 0.37 mM K2PO4, 0.2 mM MgSO4 7H2O, 2 mM CaCl2, 25 mM NaHCO3, 2.78 mM glucose, 0.33 mM pyruvate, 21.4 mM sodium lactate, 286 mg/l penicillin G, 228 mg/l streptomycin, and 5 mg/ml fatty acid-free bovine serum albumin (BSA). For capacitation, the medium containing sperm cells was incubated at 37°C for 90 min under a humidified atmosphere containing 5% CO2. After diffusion for 10 min, the sperm concentration was adjusted as necessary for subsequent experiments. All the experiments were performed using pooled semen samples from three mice.
Western blotting
Sperm suspended in HTF medium were collected by centrifugation (8000 × g, 5 min, 4°C). RIPA buffer (50 mM Tris-HCl, pH 7.6, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 1% protease inhibitor) (Nacalai Tesque, Kyoto, Japan) was added to the sperm pellet followed by sonication to extract proteins. Samples of mouse brain or retina were used as positive controls. The samples were centrifuged (8000 × g, 5 min, 4°C), and the supernatants collected. The supernatant was resuspended in the same volume of 2 × sample buffer (Nacalai Tesque) and boiled for 5 min. Proteins were separated by 10% SDS–PAGE and transferred to polyvinylidene difluoride membranes. The membranes were blocked with Blocking One (Nacalai Tesque) for 60 min at room temperature. After three washes with PBS-T (PBS containing 0.1% Tween 20), the membranes were incubated overnight at 4°C with anti-GABA A receptor alpha 1 (ab33299; 1:2,000; Abcam), anti-GABA B receptor 1 (ab131417; 1:2,000; Abcam), or anti-GABA C receptor rho 1 (sc-21336; 1:2,000; Santa Cruz Biotechnology) primary antibodies; or with a rabbit monoclonal anti-alpha-tubulin antibody (ab52866, 1:5,000; Abcam) as a internal control. After washing three times with PBS-T, the membranes were incubated for 1 h at room temperature with a horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody (1:2,000; Promega, Madison, WI, USA) for GABA A receptor alpha 1, GABA B receptor 1, and alpha-tubulin; and with horseradish peroxidase (HRP)-conjugated anti-goat IgG antibody (1:2,000; Promega) for GABA C receptor rho 1. After two washes, the membranes were reacted with Chemilumi One (Nacalai Tesque), and images were obtained using the LAS-3000-mini Lumino Image Analyzer (Fujifilm, Tokyo, Japan).
Tyrosine phosphorylation of sperm proteins
To detect tyrosine phosphorylation of sperm proteins, the sperm concentration was adjusted to 5 × 106 cells/ml for each treatment group and equal volumes of suspension in HTF medium, with or without BSA, were divided into 1.5 ml microtubes. PBS (control) or GABA (Wako Pure Chemical Industries, Osaka, Japan), dissolved in saline to a final concentration of 0.1, 1, and 10 µM, were added to the samples. The suspensions were cultured for 90 min at 37°C under a humidified atmosphere containing 5% CO2. After incubation, sperm pellets were collected by centrifugation at 8000 × g for 5 min at 4°C. After washing the obtained pellets with PBS, the supernatants were discarded and RIPA buffer and 2 × sample buffer was added to extract the proteins. The obtained proteins were separated by 12% SDS–PAGE and transferred to polyvinylidene membranes. After blocking for 60 min with 1% BSA in Tris-buffered saline containing 0.1% Tween 20 (TBS-T, pH 7.5–7.8), the membranes were treated overnight at 4°C either with a mouse monoclonal anti-phosphotyrosine 4G10 antibody (#05-321, 1:20,000; Merck Millipore, Darmstadt, Germany) or a rabbit monoclonal anti-alpha-tubulin antibody(ab52866) as an internal control. After three washes with TBS-T, the membranes were treated with HRP-conjugated anti-mouse or anti-rabbit IgG antibodies (1:2,000) for 2 h at room temperature. Detection was performed with Chemilumi One and images were obtained using the LAS-3000-mini Lumino Image Analyzer (Fujifilm). Densitometric analyses were performed using Image Gauge v4.22 analysis software (Fujifilm).
Immunocytochemistry
Immunocytochemistry was performed to investigate GABA A receptor localization in mouse sperm. Cauda epididymal sperm were suspended in HTF medium and collected by centrifugation at 2,000 × g for 5 min. Sperm were then fixed with 2% paraformaldehyde (PFA) and permeabilized with 1% Triton X-100 in PBS for 15 min at room temperature. The sperm were then washed twice with PBS and blocked with Blocking One for 60 min at room temperature. The suspensions were incubated with a primary antibody (anti-GABA A receptor alpha 1; 1:100) overnight at 4°C. After being washed three times with PBS, the suspensions were incubated for 2 h at room temperature with anti-rabbit IgG-Alexa Fluor 488 or 555 (1:500; Thermo Fisher Scientific, Waltham, MA, USA), fluorescein isothiocyanate-conjugated peanut agglutinin (FITC–PNA) (J Oil Mills, Tokyo, Japan) [29], and Hoechst 33342 (1:5,000; Thermo Fisher Scientific). Finally, treated samples were washed with PBS, mounted on glass slides, and covered with a glass coverslip. Stained cells were visualized using a fluorescence microscope (BZ-X710; KEYENCE, Osaka, Japan) and counted.
Acrosome reaction assay
Sperm were capacitated in HTF medium for 90 min and equal volumes of the suspension were then divided into microtubes. GABA stock solution was added to each suspension at final concentrations of 0.1, 1, 10, and 100 µM and the samples incubated for 30 min at 37°C under a humidified atmosphere containing 5% CO2. Subsequently, each sample was smeared onto glass slides and air-dried. After 60 min of blocking using Blocking One, the acrosome reaction was assessed by staining with FITC–PNA [29] diluted 1:500 in a light-shielded humidity chamber. After washing with PBS, the slides were covered with mounting medium and glass coverslips. Bicuculline, a GABA A receptor antagonist, was dissolved in DMSO and cultured with sperm suspensions in the presence of either saline or GABA (1 µM). All the groups contained 0.1% DMSO. Sperm acrosomal disappearance rates were evaluated by calculating the number of PNA-negative sperm among total sperm. The acrosome-reacted sperm were counted using a confocal laser scanning fluorescence microscope (LSM700; Carl Zeiss, Jena, Germany). Duplicate counting of at least 100 sperm cells was performed. The percentage of sperm with no fluorescence in the acrosomal region was calculated as the number of PNA-negative sperm cells per total counted sperm.
Sperm motility assay
After incubation for 90 min to allow capacitation, GABA was added to each suspension at the final concentrations of 1, 10, and 100 µM. After 0, 10, 30, and 60 min, 4-µl samples were placed onto four-chamber slides, 12 μm deep (SC-20-01-04-B; Leja, Nieuw-Vennep, Netherlands). Sperm cells in three fields of a chamber were divided into motile and dead sperm, and both the percentage of motile sperm and sperm motility parameters were evaluated using a computer-assisted sperm analysis (CASA) system (SMAS, DITECT, Tokyo, Japan). Films were recorded for 1 sec, with images captured at intervals of 1/60 sec. The sperm motility parameters evaluated were straight-line velocity (VSL, μm/sec), curvilinear velocity (VCL, μm/sec), linearity (LIN = VSL/VCL × 100, %), amplitude of lateral head displacement (ALH, μm), and beat-cross frequency (BCF, Hz). Sperm trajectories were automatically extracted from the movie and overlaid on the last frame using the CASA system.
Immunohistochemical staining of oviduct and uterus
Sexually matured 7- or 8-week-old female mice were primed with 5 IU of equine chorionic gonadotropin (PMSG) for 48 h and 5 IU of human chorionic gonadotropin (hCG) for 14 h. After cervical dislocation, the oviduct and uterus were dissected and fixed in 4% PFA in PBS overnight at 4°C. The fixed tissues were dehydrated and embedded in paraffin, following which 4-µm sections were cut and mounted on glass slides. After deparaffinization and rehydration, antigens were retrieved in HistoVT One (Nacalai Tesque) for 30 min at 90°C. The slides were then blocked with 1% BSA-PBS for 60 min and incubated overnight at 4°C with a rabbit polyclonal primary antibody against GABA (#ab8891, 1:100; Abcam). For an antibody preabsorption test, the anti-GABA antibody was preincubated with a 50-fold excess of antigen-blocking peptide (GABA) overnight at 4°C and used as a negative control. After washing in TBS-T for 10 min, the slides were incubated with Alexa Fluor 488-labeled anti-rabbit IgG antibody diluted 1:1,000 with PBS and propidium iodide (PI) (1:5,000) for 60 min at room temperature. After washing with TBS-T, the slides were covered with glass coverslips. Stained tissues were visualized using a confocal laser scanning fluorescence microscope (LSM700; Carl Zeiss).
Quantitative analysis of Gad mRNA in female reproductive tissues by quantitative RT-PCR
Total RNA was extracted from tissues dissected from 7- or 8-week-old female mice in estrus primed with 5 IU of eCG for 48 h, followed by hCG for 14 h. Diestrus samples were confirmed by the vaginal smear method before dissection. Uterine horns and oviducts were separated and immediately frozen in liquid nitrogen and kept at −80°C until RNA extraction. Extraction of RNA was performed using ISOGEN (Nippon Gene, Tokyo, Japan). Total RNA from tissues was reverse transcribed using ReverTra-Ace (Nacalai Tesque). The gene-specific primer sequences were as follows (5′–3′): GAD67 (Gad1) (forward: CAGTCACCTGGAACCCTCAC; reverse: TGCTTGTCTGGCTGGAAGAG; product size: 120 bp); GAD65 (Gad2) (forward: CAGCTGGAACCACCGTGTAT; reverse: TCAGTAACCCTCCACCCCAA; product size: 114 bp); and beta-actin (forward: AGCCTTCCTTCTTGGGTAT; reverse: TGGCATAGAGGTCTTTACGGATG; product size: 99 bp) as an internal control. The PCR conditions consisted of 44 cycles of denaturation at 94°C for 5 sec, annealing at 61°C for 20 sec, and extension at 72°C for 15 sec, with a final extension at 72°C for 5 min. To confirm that the correct gene was amplified, the amplicons were loaded onto 2% agarose gels and electrophoresed. The GAD67 (Gad1) and GAD65 (Gad2) mRNA levels were normalized to that of beta-actin. The mean sample and internal control threshold cycles (Ct) for each sample were calculated using the 2ΔΔCt method.
Statistical analysis
All experiments were replicated at least three times. Data are presented as the means ± standard error (SE). Statistical analyses were carried out using the Student’s t-test, paired t-test, Fisher’s exact test, analysis of variance (ANOVA), and the Tukey–Kramer test. A P value < 0.05 was considered to indicate significant differences (** P < 0.01, * P < 0.05).
Results
Expression of the GABA A receptor in sperm
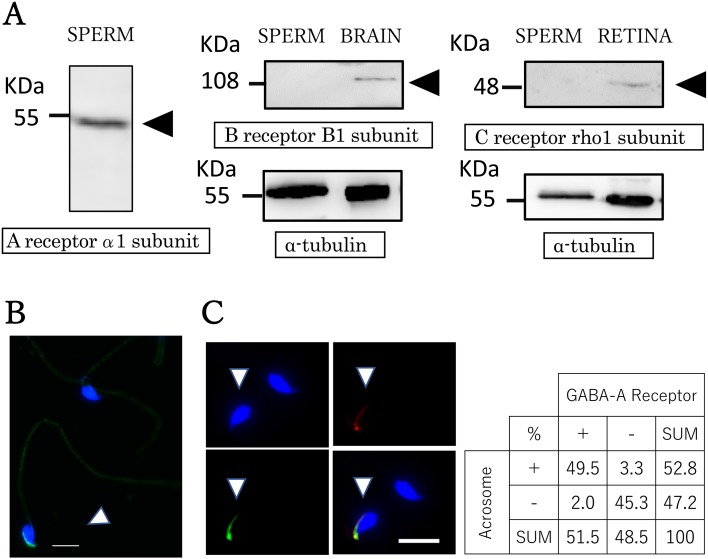
Western blot analysis and immunostaining were performed to determine the expression and localization of GABA receptors in sperm. Western blot analysis revealed that a GABA A receptor-specific band was detected around 52 kDa (Fig. 1A), coinciding with the molecular size of the alpha 1 subunit. We also used antibodies against GABA B and GABA C receptors, but neither could be detected (Fig. 1A). Immunoreactivity was observed in the acrosomal region of the sperm (Fig. 1B). The proportion of sperm showing a positive signal for the GABA A receptor was approximately 50%. We evaluated sperm acrosomal staining patterns by double staining with FITC–PNA and a GABA A receptor antibody (Fig. 1C). Fisher’s exact test indicated a statistically significant correlation between the sperm acrosome and GABA A receptor (P < 0.01).
Fig. 1.
GABA receptor in mice sperm. Detection of GABA A receptors in sperm. (A) GABA receptor expression was analyzed by western blot and immunocytochemistry. Protein extracts from sperm and positive control samples (8 µg) were separated by 10% SDS–PAGE followed by detection with specific antibodies. Arrowheads indicate the specific bands corresponding to GABA receptor subunits at the predicted size; alpha-tubulin was used as a internal control. (B) Representative immunocytochemical image showing GABA A receptor alpha 1 subunit localization in mouse sperm. Note the immunoreactivity detected in the acrosomal region (arrowhead). Blue: nucleus (Hoechst 33342); green: GABA A receptor alpha 1 subunit. Scale bar = 10 µm. (C) Acrosome staining patterns are visible with double staining. Acrosome labeled with FITC–PNA (green) and a GABA A receptor antibody (red). The panel shows the proportion of the GABA A receptor and acrosome in mouse sperm. Blue: nucleus (Hoechst 33342); green: acrosome (FITC–PNA); red: GABA A receptor alpha 1 subunit. Scale bar = 10 µm.
Effects of GABA on tyrosine phosphorylation of sperm proteins
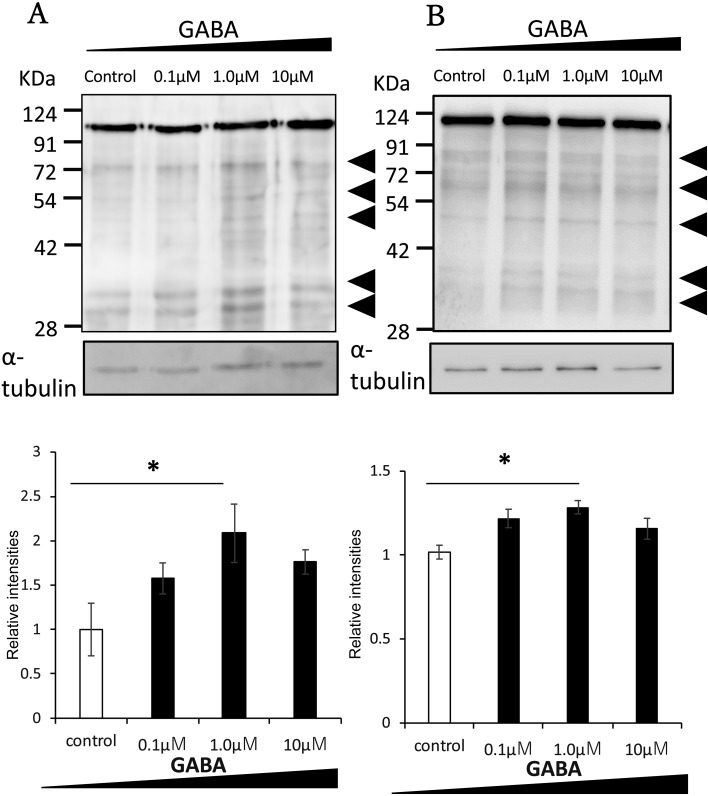
We investigated whether GABA affected the tyrosine phosphorylation of sperm proteins, the major indicator of sperm capacitation. Sperm were cultured with GABA at various concentrations (0–10 μM) and analyzed by western blotting. As shown in Fig. 2A, tyrosine phosphorylation of sperm proteins of around 30, 50, and 70 kDa increased following incubation with 1 µM GABA for 90 min. The protein at 110 kDa was not affected by capacitation [3, 30]. The total amount of tyrosine phosphorylated sperm proteins incubated in HTF medium with BSA (Fig. 2A) and without BSA (Fig. 2B) was increased by GABA application. These results suggest that exposing sperm to GABA enhances capacitation, likely through interaction between GABA and the GABA A receptor.
Fig. 2.
Effect of GABA on tyrosine phosphorylation of sperm proteins. Mouse epididymal sperm were incubated in various GABA concentrations for 90 min. The panels show the quantification and comparison of the total amount of tyrosine phosphorylated proteins between control and GABA-treated sperm incubated in HTF medium with BSA (A) or without BSA (B). Note the increased relative intensities of the bands at around 30, 50, and 70 kDa with the addition of 1 µM GABA. The protein at 110 kDa was not affected by capacitation. The experiment was replicated at least four times with independent sample preparations. The relative intensities of the control protein were normalized to 1. Data are shown as the means ± SE (* P < 0.05).
Effect of GABA on the acrosome reaction
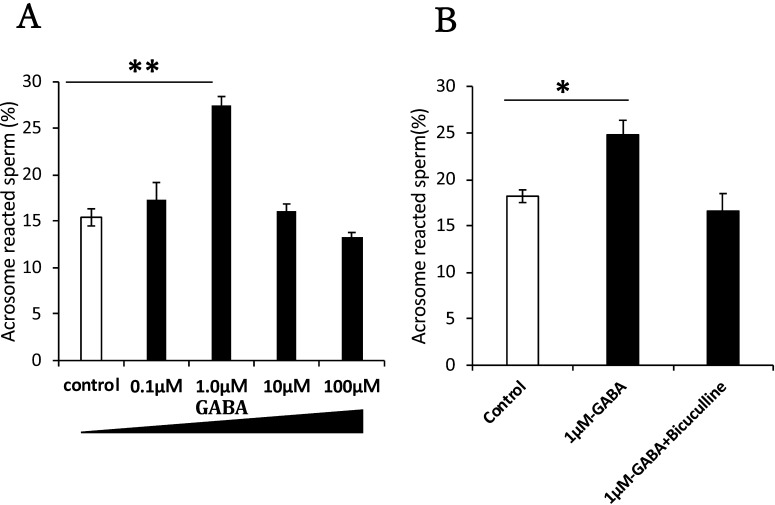
We also evaluated the effect of GABA on the acrosome reaction after incubation for 90 min. Sperm were capacitated and further cultured in the presence of various concentrations of GABA. We observed sperm with no fluorescence over the acrosomal region with FITC–PNA staining [29] (Fig. 1C). At 1 µM, GABA treatment significantly enhanced the acrosome reaction (Fig. 3A). When bicuculline, a GABA A receptor-specific antagonist, was added, the GABA-induced acrosome reaction was blocked, indicating the GABA A receptor was functionally expressed on sperm cells and contributed to the acrosome reaction (Fig. 3B).
Fig. 3.
Effect of GABA on the acrosome reaction. Sperm were treated with GABA and GABA antagonists. GABA administration enhanced the acrosome reaction (A). Mouse sperm were capacitated in HTF medium for 90 min. Saline or various concentrations of GABA (0.1, 1, 10, and 100 µM) dissolved in saline were added to equivalent aliquots of the suspension (100 µl: 5 × 106 cells/ml) and cultured for 30 min. (B) The effect of bicuculline, a GABA A receptor-specific antagonist. A sperm suspension was cultured with bicuculline dissolved in DMSO in the presence of saline or GABA (1 µM). All the groups contained 0.1% DMSO. Sperm acrosomal disappearance rates were evaluated by calculating the number of PNA-negative sperm among total sperm. Each experiment was replicated at least three times. Data are shown as the means ± SE (* P < 0.05, ** P < 0.01).
Effect of GABA on sperm motility
We evaluated the effects of GABA treatment on sperm motility, which is critical for fertilization. When sperm movement shifts to hyperactivated motility, the flagellar bend and beat patterns change to asymmetric and acquire a higher amplitude; this is reflected in the motility parameters, namely, increased VCL and ALH, and decreased LIN [8]. Using the CASA system, we observed increasing tendencies in VSL and VCL with 1 μM GABA treatment for 60 min. However, no significant difference was recorded with any GABA concentration at any time point (Table 1). These results suggest that GABA does not affect sperm motility.
Table 1. CASA measurements of the effects of GABA on mouse sperm motility.
| Sperm motility parameters | Control | GABA |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 µM | 1 µM | 10 µM | 100 µM | |||||||||||||
| 0 min | Motile (%) | 54.66 | ± | 4.59 | 51.77 | ± | 3.75 | 60.13 | ± | 1.20 | 47.67 | ± | 3.67 | 52.06 | ± | 3.14 |
| VSL (μm/sec) | 28.30 | ± | 2.80 | 20.30 | ± | 2.00 | 19.33 | ± | 1.79 | 21.79 | ± | 2.21 | 22.77 | ± | 3.62 | |
| VCL (μm/sec) | 108.84 | ± | 6.18 | 71.19 | ± | 9.15 | 72.86 | ± | 4.27 | 83.98 | ± | 8.91 | 92.09 | ± | 15.86 | |
| LIN (%) | 0.30 | ± | 0.04 | 0.35 | ± | 0.05 | 0.34 | ± | 0.04 | 0.31 | ± | 0.03 | 0.30 | ± | 0.03 | |
| ALH (μm) | 3.53 | ± | 0.15 | 2.65 | ± | 0.42 | 2.46 | ± | 0.12 | 2.87 | ± | 0.20 | 2.95 | ± | 0.46 | |
| BCF (Hz) | 11.90 | ± | 0.26 | 12.21 | ± | 0.24 | 12.36 | ± | 0.09 | 11.85 | ± | 0.40 | 11.99 | ± | 0.62 | |
| 10 min | Motile (%) | 60.95 | ± | 4.83 | 54.63 | ± | 3.66 | 60.48 | ± | 4.65 | 54.78 | ± | 2.12 | 58.50 | ± | 3.00 |
| VSL (μm/sec) | 24.39 | ± | 4.93 | 25.01 | ± | 1.54 | 21.80 | ± | 2.35 | 21.13 | ± | 1.31 | 25.81 | ± | 2.37 | |
| VCL (μm/sec) | 87.93 | ± | 8.25 | 105.03 | ± | 5.28 | 78.48 | ± | 8.68 | 86.16 | ± | 8.78 | 95.21 | ± | 5.20 | |
| LIN (%) | 0.32 | ± | 0.05 | 0.28 | ± | 0.04 | 0.32 | ± | 0.04 | 0.31 | ± | 0.02 | 0.32 | ± | 0.04 | |
| ALH (μm) | 3.00 | ± | 0.18 | 3.39 | ± | 0.18 | 2.80 | ± | 0.22 | 3.06 | ± | 0.35 | 3.15 | ± | 0.19 | |
| BCF (Hz) | 12.74 | ± | 0.05 | 12.09 | ± | 0.32 | 12.01 | ± | 0.16 | 12.41 | ± | 0.23 | 12.03 | ± | 0.16 | |
| 30 min | Motile (%) | 57.75 | ± | 5.23 | 60.12 | ± | 2.80 | 52.76 | ± | 2.11 | 54.50 | ± | 2.75 | 57.58 | ± | 2.22 |
| VSL (μm/sec) | 21.75 | ± | 1.89 | 21.68 | ± | 1.43 | 18.76 | ± | 1.43 | 25.55 | ± | 2.80 | 28.08 | ± | 3.01 | |
| VCL (μm/sec) | 85.74 | ± | 4.72 | 81.60 | ± | 3.08 | 74.58 | ± | 6.02 | 92.06 | ± | 9.79 | 105.36 | ± | 14.60 | |
| LIN (%) | 0.34 | ± | 0.04 | 0.33 | ± | 0.02 | 0.34 | ± | 0.05 | 0.29 | ± | 0.02 | 0.31 | ± | 0.05 | |
| ALH (μm) | 2.92 | ± | 0.12 | 2.88 | ± | 0.20 | 2.59 | ± | 0.18 | 3.04 | ± | 0.27 | 3.46 | ± | 0.41 | |
| BCF (Hz) | 12.83 | ± | 0.32 | 12.12 | ± | 0.18 | 12.27 | ± | 0.30 | 12.17 | ± | 0.33 | 12.11 | ± | 0.16 | |
| 60 min | Motile (%) | 50.38 | ± | 3.32 | 45.40 | ± | 7.00 | 43.35 | ± | 6.32 | 50.64 | ± | 4.01 | 45.64 | ± | 6.26 |
| VSL (μm/sec) | 25.81 | ± | 1.74 | 37.66 | ± | 13.95 | 39.46 | ± | 12.41 | 32.18 | ± | 9.38 | 29.68 | ± | 5.65 | |
| VCL (μm/sec) | 98.94 | ± | 8.35 | 125.73 | ± | 39.37 | 131.70 | ± | 34.62 | 118.41 | ± | 23.48 | 125.17 | ± | 18.43 | |
| LIN (%) | 0.28 | ± | 0.03 | 0.32 | ± | 0.02 | 0.30 | ± | 0.02 | 0.28 | ± | 0.03 | 0.24 | ± | 0.02 | |
| ALH (μm) | 3.39 | ± | 0.31 | 4.44 | ± | 1.46 | 4.86 | ± | 1.69 | 4.30 | ± | 0.89 | 4.48 | ± | 0.93 | |
| BCF (Hz) | 12.16 | ± | 0.23 | 12.50 | ± | 0.11 | 11.81 | ± | 0.34 | 12.42 | ± | 0.27 | 11.69 | ± | 0.41 | |
Data are shown as the means ± SE. VSL, straight line velocity; VCL, curvilinear velocity, LIN, linearity; ALH, amplitude of lateral head displacement; BCF, beat cross frequency.
Acrosome reaction rates for high and low sperm concentrations
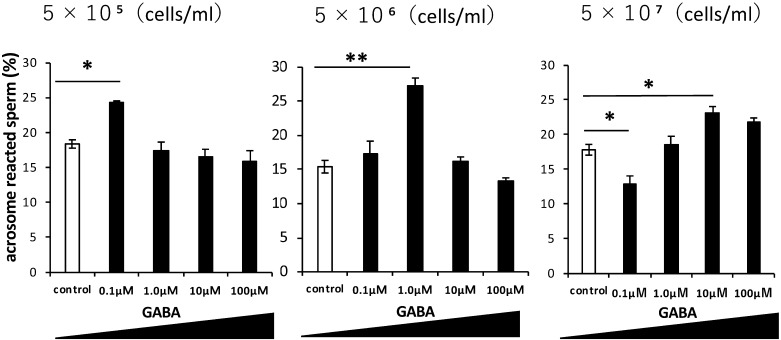
To determine the relationship between GABA and sperm concentrations, GABA solutions were added to different sperm concentrations (Fig. 4). When the sperm concentration was low (5 × 105 cells/ml), the effective GABA concentration was 0.1 µM. When the sperm concentrations increased, the effective GABA concentration was shifted higher. While GABA enhanced the sperm acrosome reaction at a specific concentration, higher concentrations had no effect. These results suggest that there is an optimal concentration range for the induction of the acrosome reaction in sperm cells (Fig. 4).
Fig. 4.
Effect of GABA on the acrosome reaction at three sperm concentrations. Rates of acrosome-reacted (PNA-negative) sperm treated with various GABA concentrations (0, 0.1, 1, 10, 100 µM) at three sperm concentrations (5 × 105, 5 × 106, and 5 × 107 cells/ml). Sperm acrosomal disappearance rates were evaluated by calculating the number of PNA-negative sperm among total sperm. Each experiment was replicated three times. Data are shown as the means ± SE (* P < 0.05, ** P < 0.01).
GABA expression in the uterus and oviduct
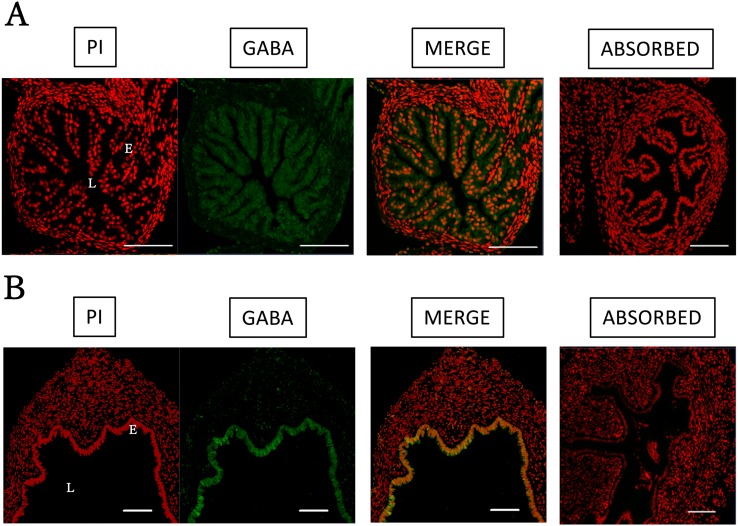
GABA immunoreactivity was detected in luminal epithelial cells of the oviduct. This reactivity was observed throughout the epithelium (Fig. 5A), suggesting that this was the source of GABA secretion. In the uterus, immunoreactivity was also detected in epithelial cells (Fig. 5B). Negative control sections incubated with GABA antibody and epitope-blocking peptide exhibited no positive staining.
Fig. 5.
Immunofluorescence detection of GABA in mouse uterus and oviduct. Immunofluorescence detection of GABA in the mouse uterus and oviduct. Tissues were collected from mature mice, treated with eCG for 48 h followed by hCG treatment for 14 h. The tissues were fixed in 4% PFA. The samples were embedded in paraffin and 4-µm sections were prepared. (A) The oviduct and (B) uterus were stained. Immunoreactivity was abolished by the absorption test. L = lumen; E = epithelium. Green = GABA; red = nuclear stain (PI). Scale bars = 100 µm.
GAD67 (Gad1) and GAD65 (Gad2) mRNA levels in the oviduct and uterus
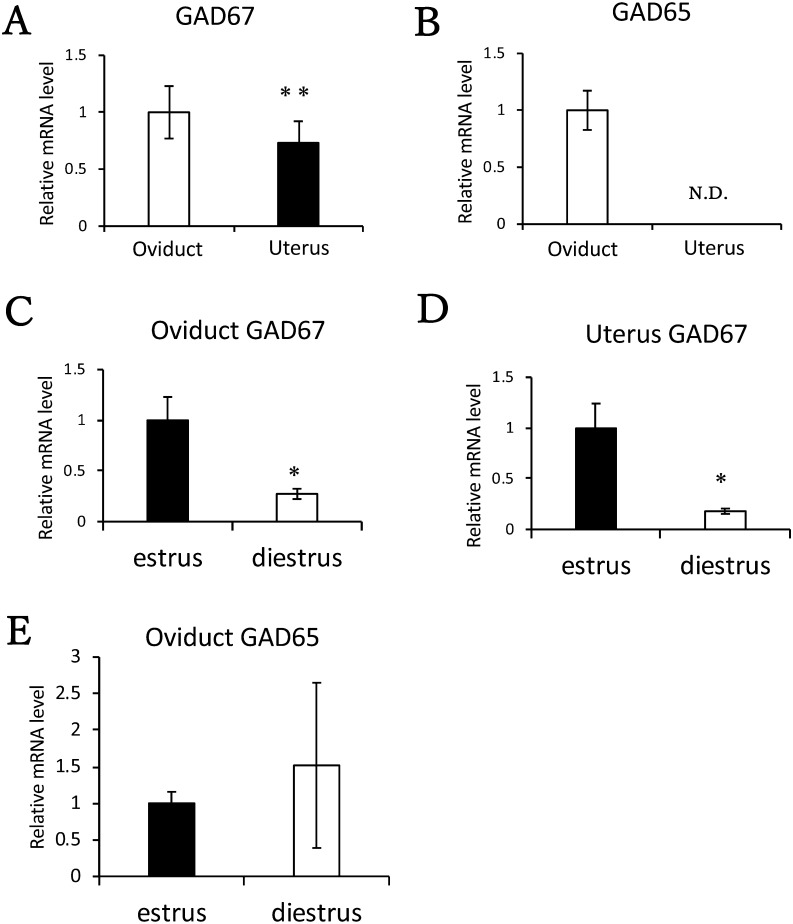
To determine which reproductive tissue might regulate GABA release, we performed quantitative RT-PCR. GAD67 (Gad1) mRNA was detected in the oviduct and uterus, with higher levels in the oviduct (Fig. 6A). However, GAD65 (Gad2) mRNA was not detected in the uterus (Fig. 6B). We also compared Gad mRNA levels in the oviduct and uterus at the estrus and diestrus stages. GAD67 (Gad1) mRNA was expressed at both stages in the oviduct and uterus. Additionally, at the estrus stage, GAD67 (Gad1) mRNA levels were significantly higher than at the diestrus stage (Fig. 6C and 6D). However, no difference was detected in GAD65 (Gad2) mRNA levels between estrus and diestrus stages in the oviduct (Fig. 6E).
Fig. 6.
GAD mRNA levels in the mouse uterus and oviduct. Comparison of (A) GAD67 (Gad1) and (B) GAD65 (Gad2) mRNA levels in female reproductive tissues. The mRNA levels of Gad in the uterus and oviduct were measured using quantitative RT-PCR. The tissues from four or five mice were collected for each experiment. Mice were primed with eCG for 48 h and with hCG for 14 h before tissue collection. The mRNA level in the oviduct was normalized to 1. Comparison of (C) oviduct and (D) uterus GAD67 (Gad1) mRNA levels at the estrus and diestrus stages. Comparison of (E) oviduct GAD65 (Gad2) mRNA levels at the estrus and diestrus stages. The mRNA expression levels at the estrus stage were normalized to 1. Data are shown as the means ± SE (** P < 0.01, * P < 0.05).
Discussion
Visconti et al. [30] showed that tyrosine phosphorylation of several proteins was induced during capacitation. Tyrosine phosphorylation of sperm proteins is induced after PKA activation following an increase in the intracytoplasmic cAMP level [3,31]. We showed that tyrosine phosphorylation of sperm proteins was enhanced by treatment with 1 µM GABA in HTF medium, both with BSA (Fig. 2A) and without BSA (Fig. 2B). Therefore, we hypothesized that GABA may enhance capacitation because tyrosine phosphorylation of sperm proteins was increased by incubation in HTF medium without BSA. We also showed that the acrosome reaction in capacitated sperm (5 × 106 cells/ml) was facilitated by treatment with 1 µM GABA (Fig. 3A) and that the acrosome reaction was blocked by bicuculline, a GABA A receptor-specific antagonist (Fig. 3B). In contrast, GABA did not exert any significant effect on sperm motility (Table 1). GABA was reported to suppress progesterone-enhanced hyperactivation in hamsters, but, in the absence of progesterone, GABA did not affect motility and hyperactivation rates [32]. This suggests that other factors besides GABA may be required to affect these parameters in the mouse. Overall, both tyrosine phosphorylation and the acrosome reaction were highly induced by 1 µM GABA treatment at a sperm concentration of 5 × 106 cells/ml.
The present results demonstrated that GABA induces the acrosome reaction in capacitated mice sperm via the GABA A receptor, as previously described for cows [26]. In addition, results from previous studies indicate that the effects of GABA on sperm are linked to Ca2+, Cl−, and HCO3− influx via GABA A receptors [27]. However, in mammals, GABA A receptor localization in sperm has been shown only for humans. In this study, immunocytochemistry analysis revealed that GABA A receptor expression is restricted to the acrosomal cap of mouse sperm (Fig. 1B), and that a correlation exists between the sperm acrosome and GABA A receptor localization (Fig. 1C). This indicates that the GABA signal is transduced into the sperm via the GABA A receptor on the acrosome. GABA and its receptor-mediated signal transduction system is thought to contribute to sperm capacitation. In human sperm, the GABA A receptor was detected in the equatorial region [33], while in this study the GABA A receptor was detected in the acrosomal region of mouse sperm. The expression patterns of the dopamine type 2 receptor (DRD2) reportedly also differ between human and mouse sperm [34], suggesting that interspecies differences in sperm morphology may be responsible for the different receptor expression patterns. However, GABA also induces the acrosome reaction in human sperm [35], indicating that the influence of GABA on sperm function may be partly shared between human and mouse sperm, as the GABA A receptor was also identified in the head region.
GABA is known to be produced in epididymal epithelial cells and the GABA A receptor has been detected in mouse epididymal sperm [36]. However, the epididymal GABA concentration is not known. In this study, we revealed that sperm capacitation was affected by GABA concentration, implying that GABA concentration in the epididymis may not be effective for sperm capacitation.
We found that the effective GABA concentration for the acrosome reaction changed according to the sperm concentration. At a sperm concentration of 5 × 105 cells/ml, the acrosome reaction rate was highest with 0.1 µM GABA, while at a sperm concentration of 5 × 107 cells/ml, the acrosome reaction rate was highest with 10 µM GABA. This shows that a low GABA concentration is needed to enhance the acrosome reaction rate at low sperm concentrations and vice versa. Therefore, we consider that GABA concentration to enhance the acrosome reaction may correspond to sperm concentration, and we also suggest that GABA does not promote sperm function in a dose-dependent manner. Instead, GABA may promote sperm function only within a specific concentration range. However, the relationship between sperm concentration and the effective GABA concentration must be investigated for in vitro fertilization applications.
GABA and its receptor-mediated signal transduction are thought to contribute to fertility at the sites where GABA is secreted. Therefore, we investigated the localization of GABA in the uterus and oviduct, important points in the sperm route during the fertilization process. Immunoreactivity against GABA was detected in the endometrium epithelium of the uterus and epithelial cells of the oviduct, indicative of GABA secretion (Fig. 5A and 5B). In addition, we also found that the GAD67 (Gad1) mRNA level in the oviduct was significantly higher than that in the uterus (Fig. 6A) and that the GAD67 (Gad1) mRNA level at estrus was higher than that at diestrus (Fig. 6C and 6D). Interestingly, GAD65 (Gad2) mRNA was not detected in the uterus (Fig. 6B) and no significant difference in GAD65 (Gad2) mRNA levels was detected between the estrus and diestrus stages in the oviduct (Fig. 6E). These results indicate that GABA secreted from the female reproductive tract may enhance fertility at the estrus stage. Therefore, because the acrosome reaction occurs in the oviduct, we consider that the high GAD expression in the oviduct strictly regulates GABA concentrations. Moreover, the effective GABA concentration for sperm capacitation may correspond to sperm concentration. Further studies are needed to clarify the working molecular mechanism for GABA signaling in mouse sperm.
Collectively, our results showing an enhanced acrosome reaction are consistent with previous reports in rat and cow [26, 27]. However, this study is the first to report a correlation between GABA and sperm concentrations to understand the potential usefulness of GABA. There are various anatomical and physiological differences between rat and cow, such as oviduct length and the time required for fertilization after ejaculation. Consequently, further elucidation of the physiology of GABA secretion in females will help in the understanding of these interspecies differences. GABA-mediated signaling may have great potential for the control of sperm functions.
References
- 1.Yanagimachi R, Chang MC. Fertilization of hamster eggs in vitro. Nature 1963; 200: 281–282. [DOI] [PubMed] [Google Scholar]
- 2.Ghersevich S, Massa E, Zumoffen C. Oviductal secretion and gamete interaction. Reproduction 2015; 149: R1–R14. [DOI] [PubMed] [Google Scholar]
- 3.Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 1995; 121: 1139–1150. [DOI] [PubMed] [Google Scholar]
- 4.Visconti PE, Ning X, Fornés MW, Alvarez JG, Stein P, Connors SA, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol 1999; 214: 429–443. [DOI] [PubMed] [Google Scholar]
- 5.Ramírez AR, Castro MA, Angulo C, Ramió L, Rivera MM, Torres M, Rigau T, Rodríguez-Gil JE, Concha II. The presence and function of dopamine type 2 receptors in boar sperm: a possible role for dopamine in viability, capacitation, and modulation of sperm motility. Biol Reprod 2009; 80: 753–761. [DOI] [PubMed] [Google Scholar]
- 6.Meizel S, Turner KO. Serotonin or its agonist 5-methoxytryptamine can stimulate hamster sperm acrosome reactions in a more direct manner than catecholamines. J Exp Zool 1983; 226: 171–174. [DOI] [PubMed] [Google Scholar]
- 7.Hiradate Y, Inoue H, Kobayashi N, Shirakata Y, Suzuki Y, Gotoh A, Roh SG, Uchida T, Katoh K, Yoshida M, Sato E, Tanemura K. Neurotensin enhances sperm capacitation and acrosome reaction in mice. Biol Reprod 2014; 91: 53. [DOI] [PubMed] [Google Scholar]
- 8.Umezu K, Hiradate Y, Oikawa T, Ishiguro H, Numabe T, Hara K, Tanemura K. Exogenous neurotensin modulates sperm function in Japanese Black cattle. J Reprod Dev 2016; 62: 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts E, Frankel S. Gamma-Aminobutyric acid in brain: its formation from glutamic acid. J Biol Chem 1950; 187: 55–63. [PubMed] [Google Scholar]
- 10.Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci 1988; 11: 112–116. [DOI] [PubMed] [Google Scholar]
- 11.Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev 1995; 47: 181–234. [PubMed] [Google Scholar]
- 12.Martin IL, Dunn SMJ. GABA receptors. In: Manley S, Barker N (eds.), Tocris Reviews 20. Bristol, UK: Tocris Cookson; 2002: 1–8. [Google Scholar]
- 13.Seutin V, Johnson SW. Recent advances in the pharmacology of quaternary salts of bicuculline. Trends Pharmacol Sci 1999; 20: 268–270. [DOI] [PubMed] [Google Scholar]
- 14.Bowery NG, Hill DR, Hudson AL. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. Br J Pharmacol 1983; 78: 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr DI, Ong J, Prager RH, Gynther BD, Curtis DR. Phaclofen: a peripheral and central baclofen antagonist. Brain Res 1987; 405: 150–154. [DOI] [PubMed] [Google Scholar]
- 16.Johnston GA. Multiplicity of GABA receptors. In: Olsen RW, Venter JC (eds.), Receptor Biochemistry and Methodology. Vol 5. Alan R. Liss, Inc.; 1986; 57–71. [Google Scholar]
- 17.Johnston GA. GABAc receptors: relatively simple transmitter-gated ion channels? Trends Pharmacol Sci 1996; 17: 319–323. [PubMed] [Google Scholar]
- 18.Taniyama K, Kusunoki M, Saito N, Tanaka T. GABA-evoked acetylcholine release from isolated guinea pig ileum. Life Sci 1983; 32: 2349–2353. [DOI] [PubMed] [Google Scholar]
- 19.Erdö SL, Wolff JR. Gamma-Aminobutyric acid outside the mammalian brain. J Neurochem 1990; 54: 363–372. [DOI] [PubMed] [Google Scholar]
- 20.Erdö SL, Kiss B. Presence of GABA, glutamate decarboxilase and GABA transaminase in peripheral tissues: A collection of quantitative data. In: Erdö SL, Bowery N (eds.), GABAergic Mechanisms in the Mammalian Periphery. New York: Raven Press. 1986; 5–17. [Google Scholar]
- 21.Tillakaratne NJ, Erlander MG, Collard MW, Greif KF, Tobin AJ. Glutamate decarboxylases in nonneural cells of rat testis and oviduct: differential expression of GAD65 and GAD67. J Neurochem 1992; 58: 618–627. [DOI] [PubMed] [Google Scholar]
- 22.Tillakaratne NJ, Medina-Kauwe L, Gibson KM. Gamma-aminobutyric acid (GABA) metabolism in mammalian neural and nonneural tissues. Comp Biochem Physiol A Physiol 1995; 112: 247–263. [DOI] [PubMed] [Google Scholar]
- 23.Ritta MN, Calandra RS. Occurrence of GABA in rat testis and its effect on androgen production. In: Racagni G, Donoso A (eds.), GABA and Endocrine Function, Advances in Biochemical Psychopharmacology. New York: Raven Press. 1986; 291–297. [PubMed] [Google Scholar]
- 24.Orensanz LM, Fernández I, Martín del Río R, Storm-Mathisen J. Gamma-aminobutyric acid in the rat oviduct. Adv Biochem Psychopharmacol 1986; 42: 265–274. [PubMed] [Google Scholar]
- 25.Ritta MN, Calamera JC, Bas DE. Occurrence of GABA and GABA receptors in human spermatozoa. Mol Hum Reprod 1998; 4: 769–773. [DOI] [PubMed] [Google Scholar]
- 26.Puente MA, Tartaglione CM, Ritta MN. Bull sperm acrosome reaction induced by gamma-aminobutyric acid (GABA) is mediated by GABAergic receptors type A. Anim Reprod Sci 2011; 127: 31–37. [DOI] [PubMed] [Google Scholar]
- 27.Jin JY, Chen WY, Zhou CX, Chen ZH, Yu-Ying Y, Ni Y, Chan HC, Shi QX. Activation of GABAA receptor/Cl- channel and capacitation in rat spermatozoa: HCO3- and Cl- are essential. Syst Biol Reprod Med 2009; 55: 97–108. [DOI] [PubMed] [Google Scholar]
- 28.Martín del Rio R. Gamma-aminobutyric acid system in rat oviduct. J Biol Chem 1981; 256: 9816–9819. [PubMed] [Google Scholar]
- 29.Lybaert P, Danguy A, Leleux F, Meuris S, Lebrun P. Improved methodology for the detection and quantification of the acrosome reaction in mouse spermatozoa. Histol Histopathol 2009; 24: 999–1007. [DOI] [PubMed] [Google Scholar]
- 30.Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995; 121: 1129–1137. [DOI] [PubMed] [Google Scholar]
- 31.Breitbart H. Signaling pathways in sperm capacitation and acrosome reaction. Cell Mol Biol 2003; 49: 321–327. [PubMed] [Google Scholar]
- 32.Kon H, Takei GL, Fujinoki M, Shinoda M. Suppression of progesterone-enhanced hyperactivation in hamster spermatozoa by γ-aminobutyric acid. J Reprod Dev 2014; 60: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wistrom CA, Meizel S. Evidence suggesting involvement of a unique human sperm steroid receptor/Cl- channel complex in the progesterone-initiated acrosome reaction. Dev Biol 1993; 159: 679–690. [DOI] [PubMed] [Google Scholar]
- 34.Otth C, Torres M, Ramírez A, Fernandez JC, Castro M, Rauch MC, Brito M, Yañez AJ, Rodríguez-Gil JE, Slebe JC, Concha II. Novel identification of peripheral dopaminergic D2 receptor in male germ cells. J Cell Biochem 2007; 100: 141–150. [DOI] [PubMed] [Google Scholar]
- 35.Shi QX, Yuan YY, Roldan ER. γ-Aminobutyric acid (GABA) induces the acrosome reaction in human spermatozoa. Mol Hum Reprod 1997; 3: 677–683. [DOI] [PubMed] [Google Scholar]
- 36.Abe H, Yanagawa Y, Kanbara K, Maemura K, Hayasaki H, Azuma H, Obata K, Katsuoka Y, Yabumoto M, Watanabe M. Epithelial localization of green fluorescent protein-positive cells in epididymis of the GAD67-GFP knock-in mouse. J Androl 2005; 26: 568–577. [DOI] [PubMed] [Google Scholar]