Abstract
Freeze-drying of spermatozoa is a convenient and safe method to preserve mammalian genetic material without the use of liquid nitrogen or a deep freezer. However, freeze-dried spermatozoa (FD sperm) are not frequently used because of the low success rate of offspring after intracytoplasmic spermatozoa injection (ICSI). In this study, we determined the optimal concentration and a point of action of trehalose as a protectant for the preservation of FD sperm from different mouse strains at room temperature (RT). Although trehalose demonstrated no potential to protect the FD sperm of ICR mice against the freeze-drying procedure itself, the blastocyst rate was significantly improved when FD sperm was preserved for more than 1 month at RT (56–63% vs. 29% without trehalose). The optimal concentration of trehalose was 0.5 M. Importantly, remarkable results were obtained when spermatozoa of inbred mouse strains (C57BL/6N, C3H/He, and 129/Sv) were used, and many offspring were obtained when FD sperm that was preserved for 3 months at RT (26–28% vs. 6–11% of without trehalose) was used. However, when DNA damage in FD sperm was examined by gamma-H2Ax assays, it was found that trehalose failed to protect the FD sperm from DNA damage. These results suggest that trehalose has the potential to protect other sperm factors rather than sperm DNA during preservation at RT for longer periods and trehalose is more effective for inbred mouse strains.
Keywords: Freeze-dry, Intracytoplasmic spermatozoa injection (ICSI), Spermatozoa, Trehalose
The preservation of mammalian gametes is important for the treatment of infertility, the preservation of genetically modified mouse strains, and the transportation of the genetic material of mouse strains instead of live animals. Currently, liquid nitrogen (LN2) is used for the long-term cryopreservation of spermatozoa [1]. Those sperm that are still alive after thawing can result in healthy offspring after in vitro fertilization or ICSI [2]. However, the constant supply of LN2 and electricity lead to high costs, and in these conditions, sample handling becomes difficult because of the very low temperature and the risk of oxygen deficiency. Moreover, the samples may not be preserved continuously at a cold enough temperature when accidents or natural disasters occur.
On the other hand, freeze-drying is also a reliable and commonly used technique in many countries. Freeze-drying is a dehydration process that is typically used to preserve live, perishable materials such as food or microorganisms for long-term storage at room temperature (RT) without the use of any preservatives. Previously, we demonstrated that, although mouse spermatozoa cannot survive after freeze-drying, their DNA is still viable and can generate offspring when injected into oocytes [3]. Since then, this method has been used not only for mouse spermatozoa but also for spermatozoa of other animals such as rats and rabbits [4,5,6]. If spermatozoa can be preserved at RT, the maintenance cost will decrease and the samples will be easier to handle compared with those in a deep freezer or in LN2 storage. However, at that time, the rate of full-term development of embryos derived from freeze-dried spermatozoa (FD sperm) was very low compared with fresh spermatozoa [3, 7], and the preservation period at RT was only a few months [8,9,10,11]. Consequently, the preservation of FD sperm at RT has not been reliable and is not routinely practiced.
Recently, we demonstrated that the vacuum condition in the ampoule was one of the most important factors in the maintenance of the integrity of FD sperm [12]. By improving the freeze-drying method and applying a leak detector to the ampoules, we succeeded in producing healthy offspring from FD sperm preserved for more than 1 year at RT. This study suggests that FD sperm can maintain a full-term developmental capacity after fertilization, even when it has been preserved at RT for longer periods.
However, the lower birth rate of embryos derived from FD sperm is an unresolved issue. Recently, Men et al. reported that 15 mM trehalose can improve the DNA integrity of boar FD sperm [13]. Liu et al. reported that 0.3 M trehalose had the potential to protect evaporatively dried spermatozoa, as live offspring were obtained from spermatozoa preserved at RT for 2 years [14]. We also reported that trehalose can increase tolerance of FD sperm to extremely high and low temperatures [15]. Those reports suggest that when the optimal concentration and appropriate method of trehalose are discovered, the success rate of offspring from FD sperm will improve, even after preservation at RT for many years.
In this study, we determined the optimal concentration and a point of action of trehalose as a protectant for the preservation of FD sperm at RT. The protective effect of trehalose was remarkable when the storage period was extended and inbred mouse strains were used.
Methods
Animals
ICR female mice, ICR, BDF1 (C57BL/6N × DBA2), C57BL/6N, C3H/He, and 129/Sv male mice (8–10 weeks of age) were obtained from SLC Inc (Hamamatsu, Japan). The surrogate pseudopregnant ICR females, which were used as recipients of the embryos, were mated with vasectomized ICR males, whose sterility had been previously demonstrated. On the day of the experiment or after all experiments were completed, the mice were euthanized by CO2 inhalation or cervical dislocation and were used in the experiments described below. All animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Committee of Laboratory Animal Experimentation of the University of Yamanashi.
Media
HTF medium was used for capacitation of spermatozoa [16]. Tris–EGTA medium was used for the freeze-drying of spermatozoa [17, 18]. HEPES-CZB medium [7] and CZB medium [19] were used for oocyte/embryo manipulation and for incubation in 5% CO2 at 37°C, respectively.
Preparation of FD spermatozoa
Both epididymides were collected from male mice (ICR, BDF1(C57BL/6N × DBA2), C57BL/6N, C3H/He, and 129/Sv), and the ducts were severed with sharp scissors. A few drops of the dense spermatozoa mass were then placed into a centrifuge tube containing 750 µl HTF medium, which was then incubated for 30 min at 37°C in 5% CO2. The concentration and motility of the spermatozoa were determined, and 500 µl supernatant was collected and centrifuged at 5000 rpm for 10 min. The supernatant was removed, and the sperm pellet was suspended in 200 µl Tris–EGTA, which did not contain trehalose. Next, 50 µl aliquots of the spermatozoa suspension were dispensed into four other centrifuge tubes containing 450 µl Tris–EGTA with different concentrations of trehalose (0, 0.1, 0.5, or 2.0 M). The 50 µl aliquots of the spermatozoa suspension were then dispensed into glass ampoules. The ampoules were frozen in LN2 and freeze-dried using FDU-2200 freeze dryer (EYELA, Tokyo, Japan). The cork of the freeze-dryer was opened for at least 6 h until all samples were completely dry. After drying, the ampoules were sealed by melting the ampoule necks using a gas burner under vacuum, as previously described (Fig. 1a) [20].
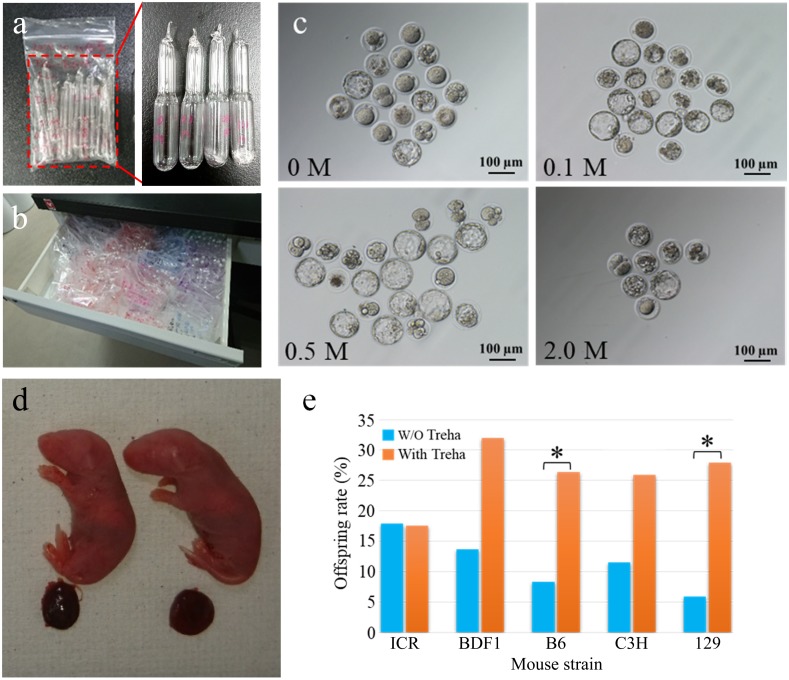
Fig. 1.
Preservation of freeze-dried (FD) sperm for up to 3 months at RT and production of offspring. (a, b) Ampoules of FD sperm in plastic bags were preserved in a desk drawer. (c) Blastocysts derived from oocytes fertilized with FD sperm treated with 0.1 to 2.0 M trehalose or without trehalose and preserved at RT for 1 month. (d) Healthy offspring derived from FD sperm preserved at RT for 1 month with 0.5 M trehalose. (e) Birth rates of embryos derived from the FD sperm of inbred strains. Those FD sperm were stored with or without 0.5 M trehalose and were preserved for 3 months at RT. Asterisk indicates the significant difference between samples treated and non-treated with trehalose in each strain (P < 0.01).
Preservation of FD sperm
All ampoules were placed in small plastic bags and stored in a desk drawer at RT (15–25°C) (Fig. 1b) until further use.
Detection of trapped air in ampoules using the Tesla coil leak detector
Ampoules containing air were identified using a Tesla coil leak detector (Sanko Electronic Laboratory, Kanagawa, Japan) according to the manufacturer's instructions. When the tip of the Tesla coil is brought near the ampoule, the tip will spark around the glass. If much air is trapped inside the ampoule, the air cannot be ionized. However, if the ampoule contains only a small amount of residual air, its ionization produces a spark inside the ampoule. Only Tesla-positive ampoules, which suggest highly vacuumed ampoules, were used for all experiments [12].
Oocyte preparation
Female mice were superovulated by the injection of 5 IU of equine chorionic gonadotropin, followed by 5 IU of human chorionic gonadotropin after 48 h. Cumulus-oocyte complexes (COCs) were collected from the oviducts of females 14–16 h later and moved to a Falcon dish containing HEPES-CZB media. To disperse the cumulus, COCs were transferred into a 50 µl droplet of HEPES-CZB medium containing 0.1% bovine testicular hyaluronidase for 3 min. Cumulus-free oocytes were washed twice and moved to a 20 µl droplet of CZB for culture.
ICSI and embryo transfer
ICSI was performed as previously described [7]. Just prior to starting the ICSI, the neck of an ampoule was punctured and 50 µl of sterile distilled water was immediately added and mixed with a pipette. For microinjection of spermatozoa, 1–2 µl of the spermatozoa suspension was moved directly to the injection chamber. The spermatozoa suspension was replaced every 30 min during the ICSI procedure. Application of several piezo pulses separated the spermatozoa head from the tail, and the head was then injected into the oocyte. The oocytes that survived ICSI were incubated in CZB medium at 37°C with 5% CO2. Pronucleus formation was verified 6 h after ICSI. Embryos at the 2-cell stage were transferred to a day 0.5 pseudopregnant mouse that had been mated with a vasectomized male the night before transfer. Five to twelve embryos were transferred into each oviduct. At day 18.5 of gestation, offspring were delivered by cesarean section and allowed to mature. The remaining unused embryos were cultured for up to 4 days to evaluate their potential for developing into blastocysts.
Gamma-H2Ax assay
Histone H2Ax is one of the H2A variants. The serine at position 139 of H2Ax is rapidly phosphorylated within seconds of DNA damage. The phosphorylated form of H2Ax, designated as gamma-H2Ax, forms foci at sites of DNA damage, which leads to the recruitment of various repair and cell-cycle checkpoint proteins [21]. Therefore, gamma-H2Ax foci formation was used as a marker of DNA double-strand breaks in male and female pronuclei, and histone H3K9 me2 signals were used to distinguish female and male pronuclei. All specimens were fixed 10 h after ICSI with 4% paraformaldehyde (PFA; Wako Pure Chemical, Osaka, Japan) containing 0.2% Triton X at RT for 20 min and stored in a refrigerator until staining. Primary antibodies used for the immunostaining of zygotes included the anti-phospho-H2Ax (Ser139) rabbit polyclonal antibody (1:500; Millipore-Merck, Darmstadt, Germany) and anti-histone H3 (dimethyl K9) mouse monoclonal antibody (1:500; Abcam, Cambridge, UK). The secondary antibodies used were Alexa Fluor 488-labeled goat anti-mouse IgG (1:500; Molecular Probes, Eugene, OR, USA) and Alexa Fluor 568-labeled goat anti-rabbit IgG (1:500; Molecular Probes). DNA was stained with 4′6-diamidino-2-phenylindole (2 µg/ml; Molecular Probes). The brightness of each male pronucleus was measured using ImageJ software and was subtracted from the brightness of the zygote cytoplasm.
Statistical analysis
Results of the gamma-H2Ax assay were analyzed using the Wilcoxon-Mann-Whitney nonparametric test. The blastocyst formation and birth rates were evaluated using chi-squared tests. Statistically significant differences between variables were determined at P < 0.01.
Results
Optimal concentration of trehalose for FD sperm and its point of action as a protectant
To determine the optimal concentration of trehalose for the preservation of FD sperm at RT, ICR mouse spermatozoa were freeze-dried in 0, 0.1, 0.5, or 2.0 M trehalose and stored at −30°C for 1 day or at RT for 1 week or 1 month. Preservation for 1 day was used to detect whether trehalose could protect spermatozoa against the freeze-drying treatment itself, whereas preservation for 1 week or 1 month was used to detect the effect of trehalose during the preservation of FD sperm at RT for longer periods. After preservation, those spermatozoa were injected into fresh oocytes and cultured for 4 days in vitro or transferred into recipient females at the 2-cell stage.
When FD sperm were stored at −30°C for 1 day, the rate of blastocyst development and full-term slightly but not significantly increased when trehalose was added to the medium (47–69% vs. 46% without trehalose and 24–43% vs. 25% without trehalose, respectively) (Table 1). Next, FD sperm cells were preserved for 1 week at RT (Table 2). Although the blastocyst development rate and the full-term development rate were not significantly different among all the groups, when 0.5 M trehalose was used, the both rates were higher than that of other groups (53% vs. 36–43% and 35% vs. 15–17%, respectively). Then, FD sperm were preserved for 1 month at RT (Table 3). The blastocyst development rate increased significantly when spermatozoa were freeze-dried in 0.1 or 0.5 M trehalose compared with spermatozoa freeze-dried without trehalose (63% or 56% vs. 29%, respectively) (Fig. 1c). Similarly, when 2-cell embryos were transferred to recipients, the highest birth rate was obtained when 0.5 M trehalose was used (47% vs. 17–27%, respectively) (Fig. 1d). However, when 2.0 M trehalose was used, the rate of blastocyst development and full-term were decreased.
Table 1. The rate of blastocyst development and full-term embryonic development after fertilization with freeze-dried sperm preserved for 1 day.
| Conc. of trehalose | No. of oocytes surviving after ICSI |
No. (%) of fertilized zygotes |
No. (%) of embryos developed to |
No. of transferred embryos (no. of recipients) |
No. (%) [min–max] of offspring | Mean body weight (g) | |||
|---|---|---|---|---|---|---|---|---|---|
| 2 cell (%) | 4–8 cell (%) | Morula (%) | Blastocyst (%) | ||||||
| 0 M | 44 | 41 (93) | 35 (85) | 31 (76) | 22 (54) | 19 (46) | – | – | – |
| 79 | 73 (92) | 56 (77) | – | – | – | 56 (4) | 14 (25) [8–37] | 1.71 ± 0.17 | |
| 0.1 M | 43 | 42 (98) | 37 (88) | 34 (81) | 30 (71) | 24 (57) | – | – | – |
| 70 | 64 (91) | 56 (88) | – | – | – | 56 (4) | 24 (43) [14–71] | 1.73 ± 0.16 | |
| 0.5 M | 48 | 43 (90) | 36 (84) | 30 (70) | 26 (60) | 20 (47) | – | – | – |
| 64 | 61 (95) | 51 (83) | – | – | – | 51 (4) | 17 (33) [14–53] | 1.82 ± 0.12 | |
| 2.0 M | 36 | 36 (100) | 36 (100) | 28 (78) | 25 (69) | 25 (69) | – | – | – |
| 65 | 63 (97) | 58 (92) | – | – | – | 58 (4) | 14 (24) [0–53] | 1.73 ± 0.10 | |
Table 2. The rate of blastocyst development and full-term embryonic development after fertilization with freeze-dried sperm preserved for 1 week.
| Conc. of trehalose | No. of oocytes surviving after ICSI |
No. (%) of fertilized zygotes |
No. (%) of embryos developed to |
No. of transferred embryos (no. of recipients) |
No. (%) [min-max] of offspring | Mean body weight (g) | |||
|---|---|---|---|---|---|---|---|---|---|
| 2 cell (%) | 4–8 cell (%) | Morula (%) | Blastocyst (%) | ||||||
| 0 M | 47 | 46 (98) | 43 (93) | 33 (72) | 28 (61) | 19 (41) | – | – | – |
| 35 | 35 (100) | 27 (77) | – | – | – | 27 (3) | 4 (15) [9–20] | 2.06 ± 0.21 | |
| 0.1 M | 36 | 35 (97) | 32 (91) | 23 (66) | 23 (66) | 15 (43) | – | – | – |
| 35 | 35 (100) | 35 (100) | – | – | – | 35 (3) | 6 (17) [0–33] | 2.08 ± 0.07 | |
| 0.5 M | 52 | 49 (94) | 43 (88) | 39 (80) | 36 (73) | 26 (53) | – | – | – |
| 46 | 46 (100) | 40 (87) | – | – | – | 40 (3) | 14 (35) [30–7] | 1.85 ± 0.17 | |
| 2.0 M | 41 | 36 (88) | 29 (81) | 22 (61) | 20 (56) | 13 (36) | – | – | – |
| 63 | 61 (97) | 50 (82) | – | – | – | 34 (4) | 5 (15) [0–40] | 2.09 ± 0.14 | |
Table 3. The rate of blastocyst development and full-term embryonic development after fertilization with freeze-dried sperm preserved for 1 month.
| Conc. of trehalose | No. of oocytes surviving after ICSI |
No. (%) of fertilized zygotes |
No. (%) of embryos developed to |
No. of transferred embryos (no. of recipients) |
No. (%) [min–max]* of offspring | Mean body weight (g) | |||
|---|---|---|---|---|---|---|---|---|---|
| 2 cell (%) | 4–8 cell (%) | Morula (%) | Blastocyst (%)* | ||||||
| 0 M | 57 | 55 (96) | 45 (82) | 30 (55) | 23 (42) | 16 (29) a | – | – | – |
| 91 | 88 (97) | 69 (78) | – | – | – | 60 (5) | 13 (22) [0–75] | 1.76 ± 0.32 | |
| 0.1 M | 46 | 40 (87) | 35 (88) | 30 (75) | 26 (65) | 25 (63) b | – | – | – |
| 56 | 51 (91) | 45 (88) | – | – | – | 44 (4) | 12 (27) [20–38] | 1.83 ± 0.12 | |
| 0.5 M | 96 | 90 (94) | 76 (84) | 61 (68) | 61 (68) | 50 (56) b | – | – | – |
| 41 | 38 (93) | 33 (87) | – | – | – | 32 (3) | 15 (47) [25–56] a | 1.93 ± 0.14 | |
| 2.0 M | 40 | 37 (93) | 29 (78) | 25 (68) | 20 (54) | 14 (38) | – | – | – |
| 64 | 58 (91) | 52 (90) | – | – | – | 46 (5) | 8 (17) [0–50] b | 1.94 ± 0.14 | |
* Different letters mean significant differences (P < 0.01).
From these reasons, we decided that 0.5 M trehalose is the optimal concentration to protect FD sperm for longer preservation at RT and used this concentration for the following experiments.
Effect of trehalose on the preservation of inbred FD sperm for 3 months at RT
We examined the effect of trehalose on not only the FD sperm of outbred (ICR) spermatozoa but also that of hybrid (BDF1) and inbred (C57BL/6N, C3H/He, and 129/Sv) spermatozoa after 3 months preservation at RT. In the ICR strain, the birth rate was not different between the spermatozoa treated with or without trehalose (18% vs. 18%) (Table 4). However, when BDF1 and C3H spermatozoa were examined, although no significant differences were observed, the birth rates were increased (32% vs. 14% and 26% vs. 11%, respectively) by 0.5 M trehalose. This tendency was demonstrated remarkably well when spermatozoa from other inbred strains were examined. The success rates of full-term development in C57BL/6N and 129/Sv using trehalose were 26% and 28%, respectively, which were significantly higher than the rates for them not preserved in trehalose (8% and 6%, respectively) (Fig. 1e).
Table 4. The rate of full-term embryonic development after fertilization with freeze-dried sperm derived from outbred, hybrid and inbred mouse strain, preserved for 3 month.
| Strain | Trehalose | No. of oocytes surviving after ICSI |
No. (%) of fertilized zygotes |
No. (%) of 2 cell embryos | No. of transferred embryos (no. of recipients) |
No. (%) [min–max]* of offspring |
Mean body weight (g) |
|---|---|---|---|---|---|---|---|
| ICR | – | 87 | 81 (93) | 67 (83) | 67 (6) | 12 (18) [0–35] | 1.81 ± 0.20 |
| + | 79 | 70 (89) | 57 (81) | 57 (6) | 10 (18) [0–33] | 1.85 ± 0.25 | |
| BDF1 | – | 52 | 37 (71) | 22 (59) | 22 (3) | 3 (14) [0–38] | 1.75 ± 0.06 |
| + | 42 | 34 (81) | 25 (74) | 25 (3) | 8 (32) [18–57] | 1.81 ± 0.16 | |
| B6 | – | 122 | 108 (89) | 72 (67) | 72 (7) | 6 (8) [0–21] a | 1.76 ± 0.16 |
| + | 125 | 119 (95) | 91 (76) | 91 (7) | 24 (26) [12–43] b | 1.76 ± 0.13 | |
| C3H | – | 117 | 108 (92) | 88 (81) | 87 (6) | 10 (11) [0–31] | 1.82 ± 0.09 |
| + | 113 | 100 (88) | 81 (81) | 81 (6) | 21 (26) [18–40] | 1.64 ± 0.16 | |
| 129 | – | 79 | 67 (85) | 51 (70) | 51 (4) | 3 (6) [0–13] a | 2.10 ± 0.16 |
| + | 93 | 87 (94) | 68 (78) | 68 (4) | 19 (28) [0–40] b | 1.64 ± 0.25 | |
* Different letters mean significant difference (P < 0.01).
Detection of DNA damage in FD sperm by gamma-H2Ax assay
To detect DNA damage in FD sperm, C57BL/6N mouse FD sperm treated with or without 0.5 M trehalose were used for a gamma-H2Ax assay after ICSI. The protective effect against freeze-drying treatment was detected in FD sperm preserved for 1 day, whereas protective effect against long-term preservation at RT was detected in FD sperm preserved for more than 3 months.
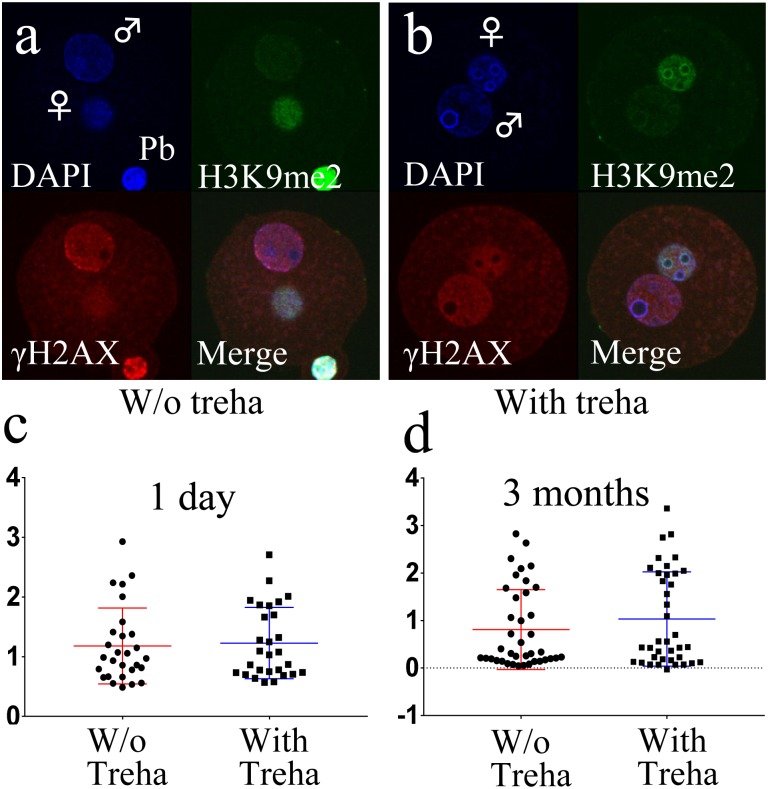
After ICSI, zygotes were immunostained with an anti-gamma-H2Ax antibody. Several foci were detected in the male pronuclei derived from one day preserved FD sperm (Fig. 2a, b). Since it was difficult to count the number of foci within the pronuclei, we measured the brightness of the whole male pronucleus and subtracted the brightness of the zygote cytoplasm. As a results, no significant differences were detected between the trehalose-treated and non-treated spermatozoa, irrespective of the storage period (Fig. 2c, d).
Fig. 2.
DNA damage analysis using gamma-H2Ax assay. (a, b) Gamma-H2Ax assay using a zygote fertilized with freeze-dried sperm preserved for one day with or without trehalose. Images show male and female pronuclei stained with 4′6-diamidino-2-phenylindole in blue (upper left), female pronuclei labeled with anti-H3K9 me2 antibody in green (upper right), gamma-H2Ax signals indicating double-stranded DNA breaks in red (lower left), and merged images (lower right). (c, d) The brightness of each male pronucleus derived from FD sperm preserved for 1 day (c) and 3 months (d) was plotted. Circle dots indicate no trehalose treatment, whereas square dots indicate trehalose treatment.
Discussion
It is known that trehalose, which is a non-reducing disaccharide, exerts some important effects such as water-holding capacity [22], protein denaturation prevention [23, 24], and oxidation inhibition of unsaturated fatty acids [25]. In case of lower species, including tardigrades and the sleeping chironomid, some of them can be revived after exposure to extreme environments by accumulation of trehalose [26]. On the other hand, regarding “higher species”, they cannot produce and accumulate trehalose in their body, and therefore, cannot survive when they are exposed to extreme environments. However, if trehalose is added to the culture medium, it can suppress the damage of mammalian cells or spermatozoa caused by freezing [27, 28]. Trehalose is likely to function in keeping the cell membrane intact regardless of the species. On the contrary, when spermatozoa were freeze-dried, all spermatozoa were killed by that treatment [3]. Thus far, no mammalian spermatozoa have survived after freeze-drying even if trehalose was used, which suggests that trehalose cannot protect the cell membrane against freeze-drying treatment.
Importantly, even though spermatozoa were killed by freeze-dried treatment, healthy offspring could be obtained when FD sperm was injected into oocytes [3], which suggests that, live or dead, and whether the membrane is intact or not, are not important to generate offspring. However, the birth rate of embryos fertilized with FD sperm was largely decreased compared with that of embryos fertilized with fresh spermatozoa due to DNA damage [29]. Kusakabe suggested that DNA damage was induced by mechanical or oxidative stress not only during freeze-drying but also during long-term preservation [30]. Kaneko et al. also reported that storage of FD sperm at RT for 3 months resulted in chromosomal abnormalities [9]. Therefore, until recently, the preservation period of FD sperm at RT was limited to only a few months.
Recently, we succeeded in generating offspring from FD sperm preserved for more than 1 year at RT [12]. That study demonstrated that the oxidation and denaturation caused by air and moisture in the ampoules led to the decreased quality of FD sperm after preservation at RT, and therefore, maintaining a high vacuum in ampoules is critical to ensure the viability of FD sperm. However, even in the ampoules judged to have a high vacuum according to nondestructive inspection, all ampoules still contain a small amount of air [12]. With the current technology, it is difficult to completely remove all air and moisture from inside the ampoules, and oxidation and denaturation will occur even from such slightest remaining air, which will disrupt the long-term preservation of FD sperm at RT. Because of its ability to prevent protein denaturation and oxidation, if trehalose is added to the ampoules, it may be effective in the protection of FD sperm from the slightest air and moisture remaining in the ampoule [13, 31, 32].
In this study, even though spermatozoa were freeze-dried with trehalose, we discovered that the trehalose could not protect live spermatozoa at the time of “freeze-drying treatment” against its damage but could protect FD sperm during “preservation period at RT” (Fig. 1c, d; Tables 1–3). This was an expected result because trehalose is a large molecule, and therefore, it cannot enter cells via the cell membrane before freeze-drying treatment [14]. Since trehalose could not protect the cell membrane, all spermatozoa died after freeze-dried treatment. However, when FD sperm was preserved for longer periods at RT, because the spermatozoa membrane was already compromised and trehalose could enter the cells, trehalose could protect FD sperm during preservation against air and moisture that remained in the ampoules.
Interestingly, although the birth rate of offspring from trehalose-treated FD sperm was improved significantly, the DNA damage was not different between trehalose-treated and untreated FD sperm (Fig. 1e, 2c, d; Table 4). Very recently, we reported that trehalose could increase the tolerance of FD sperm against extremely high temperatures by protecting FD sperm from burning [15]. Those results suggest that trehalose has the potential to protect several factors (such as the integrity of acrosome or cytoplasm) of FD sperm but not DNA, and therefore, even though the birth rate was increased, DNA damage was not reduced by trehalose treatment.
A finding of this study is that a remarkable effect was obtained when inbred mouse strains were used (Fig. 1e; Table 4). It is known that many researchers use inbred mouse strains such as C57BL/6, C3H/He, and 129/Sv to produce genetically modified mice or for whole-genome analysis using next-generation sequencing. Therefore, cryopreservation of spermatozoa, oocytes, and embryos of those inbred strains is important to maintain those strains. However, the gametes of inbred mouse strains are sensitive to assisted reproductive technology, and the production rate of offspring from inbred strains was reported to be lower than that of hybrid strains [9, 33]. Because DNA was common in all organisms, those differences were likely caused by the tolerance of the cell membrane or some organelle rather than the DNA [15, 34]. Therefore, trehalose could work more effectively in the “weaker” spermatozoa of inbred strains compared with the “stronger” spermatozoa of outbred or hybrid strains.
The optimal concentration of trehalose for the preservation of FD sperm at RT was 0.5 M, and higher concentrations of trehalose (2.0 M) resulted in lower blastocyst and birth rates compared with FD sperm not freeze-dried in trehalose (Fig. 1c; Table 2, 3). The reason for this is not clear, but after freeze-drying treatment, crystallized trehalose may cause physical damage to the spermatozoa. At low concentrations of trehalose, there is less damage from crystallization, but the protective effects are less potent. At high concentrations, physical damage caused by crystallization may be greater than the protective effect. For this reason, 0.5 M trehalose results in the best balance to obtain the highest protective effect.
In conclusion, we demonstrated that, although trehalose exerted no protective effects on spermatozoa against freeze-drying treatment, it could improve the birth rate of embryos fertilized with FD sperm preserved at RT for longer periods. Specifically, the protective effect was remarkable when inbred strains of mice were used. However, the average success rate of offspring was still lower than that of fresh spermatozoa. To obtain reliability so that this method may be used practically, it is necessary to increase the success rate of offspring, such as the optimization of the method of freeze-drying treatment or the discovery of a better cryoprotectant.
Acknowledgments
We thank Drs S Kishigami, and Y Fujimoto, and Miss C Yamaguchi for assistance in preparing this manuscript. This work was partially funded by the Japan Society for the Promotion of Science to TW (16H02593); the Naito Foundation to SW; Asada Science Foundation to TW; and the Takeda Science Foundation to TW; and International Scientific Partnership Program (ISPP) at King Saud University.
References
- 1.Benson JD, Woods EJ, Walters EM, Critser JK. The cryobiology of spermatozoa. Theriogenology 2012; 78: 1682–1699. [DOI] [PubMed] [Google Scholar]
- 2.Sztein JM, Takeo T, Nakagata N. History of cryobiology, with special emphasis in evolution of mouse sperm cryopreservation. Cryobiology 2018; 82: 57–63. [DOI] [PubMed] [Google Scholar]
- 3.Wakayama T, Yanagimachi R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat Biotechnol 1998; 16: 639–641. [DOI] [PubMed] [Google Scholar]
- 4.Gil L, Olaciregui M, Luño V, Malo C, González N, Martínez F. Current status of freeze-drying technology to preserve domestic animals sperm. Reprod Domest Anim 2014; 49 (Suppl 4): 72–81. [DOI] [PubMed] [Google Scholar]
- 5.Hirabayashi M, Kato M, Ito J, Hochi S. Viable rat offspring derived from oocytes intracytoplasmically injected with freeze-dried sperm heads. Zygote 2005; 13: 79–85. [DOI] [PubMed] [Google Scholar]
- 6.Liu JL, Kusakabe H, Chang CC, Suzuki H, Schmidt DW, Julian M, Pfeffer R, Bormann CL, Tian XC, Yanagimachi R, Yang X. Freeze-dried sperm fertilization leads to full-term development in rabbits. Biol Reprod 2004; 70: 1776–1781. [DOI] [PubMed] [Google Scholar]
- 7.Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod 1995; 52: 709–720. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko T, Nakagata N. Relation between storage temperature and fertilizing ability of freeze-dried mouse spermatozoa. Comp Med 2005; 55: 140–144. [PubMed] [Google Scholar]
- 9.Kaneko T, Serikawa T. Long-term preservation of freeze-dried mouse spermatozoa. Cryobiology 2012; 64: 211–214. [DOI] [PubMed] [Google Scholar]
- 10.Kawase Y, Suzuki H. A study on freeze-drying as a method of preserving mouse sperm. J Reprod Dev 2011; 57: 176–182. [DOI] [PubMed] [Google Scholar]
- 11.Kawase Y, Araya H, Kamada N, Jishage K, Suzuki H. Possibility of long-term preservation of freeze-dried mouse spermatozoa. Biol Reprod 2005; 72: 568–573. [DOI] [PubMed] [Google Scholar]
- 12.Kamada Y, Wakayama S, Shibasaki I, Ito D, Kamimura S, Ooga M, Wakayama T. Assessing the tolerance to room temperature and viability of freeze-dried mice spermatozoa over long-term storage at room temperature under vacuum. Sci Rep 2018; 8: 10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Men NT, Kikuchi K, Nakai M, Fukuda A, Tanihara F, Noguchi J, Kaneko H, Linh NV, Nguyen BX, Nagai T, Tajima A. Effect of trehalose on DNA integrity of freeze-dried boar sperm, fertilization, and embryo development after intracytoplasmic sperm injection. Theriogenology 2013; 80: 1033–1044. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Lee GY, Lawitts JA, Toner M, Biggers JD. Live pups from evaporatively dried mouse sperm stored at ambient temperature for up to 2 years. PLoS One 2014; 9: e99809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wakayama S, Ito D, Kamada Y, Yonemura S, Ooga M, Kishigami S, Wakayama T. Tolerance of the freeze-dried mouse sperm nucleus to temperatures ranging from -196 °C to 150 °C. Sci Rep 2019; 9: 5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinn P, Moinipanah R, Steinberg JM, Weathersbee PS. Successful human in vitro fertilization using a modified human tubal fluid medium lacking glucose and phosphate ions. Fertil Steril 1995; 63: 922–924. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko T, Nakagata N. Improvement in the long-term stability of freeze-dried mouse spermatozoa by adding of a chelating agent. Cryobiology 2006; 53: 279–282. [DOI] [PubMed] [Google Scholar]
- 18.Kusakabe H, Kamiguchi Y. Ability to activate oocytes and chromosome integrity of mouse spermatozoa preserved in EGTA Tris-HCl buffered solution supplemented with antioxidants. Theriogenology 2004; 62: 897–905. [DOI] [PubMed] [Google Scholar]
- 19.Chatot CL, Lewis JL, Torres I, Ziomek CA. Development of 1-cell embryos from different strains of mice in CZB medium. Biol Reprod 1990; 42: 432–440. [DOI] [PubMed] [Google Scholar]
- 20.Wakayama S, Kamada Y, Yamanaka K, Kohda T, Suzuki H, Shimazu T, Tada MN, Osada I, Nagamatsu A, Kamimura S, Nagatomo H, Mizutani E, Ishino F, Yano S, Wakayama T. Healthy offspring from freeze-dried mouse spermatozoa held on the International Space Station for 9 months. Proc Natl Acad Sci USA 2017; 114: 5988–5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair (Amst) 2004; 3: 959–967. [DOI] [PubMed] [Google Scholar]
- 22.Cordone L, Cottone G, Giuffrida S, Palazzo G, Venturoli G, Viappiani C. Internal dynamics and protein-matrix coupling in trehalose-coated proteins. Biochim Biophys Acta 2005; 1749: 252–281. [DOI] [PubMed] [Google Scholar]
- 23.Olsson C, Jansson H, Swenson J. The role of trehalose for the stabilization of proteins. J Phys Chem B 2016; 120: 4723–4731. [DOI] [PubMed] [Google Scholar]
- 24.Jain NK, Roy I. Effect of trehalose on protein structure. Protein Sci 2009; 18: 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oku K, Watanabe H, Kubota M, Fukuda S, Kurimoto M, Tsujisaka Y, Komori M, Inoue Y, Sakurai M. NMR and quantum chemical study on the OH...pi and CH...O interactions between trehalose and unsaturated fatty acids: implication for the mechanism of antioxidant function of trehalose. J Am Chem Soc 2003; 125: 12739–12748. [DOI] [PubMed] [Google Scholar]
- 26.Rothschild LJ, Mancinelli RL. Life in extreme environments. Nature 2001; 409: 1092–1101. [DOI] [PubMed] [Google Scholar]
- 27.Woelders H, Matthijs A, Engel B. Effects of trehalose and sucrose, osmolality of the freezing medium, and cooling rate on viability and intactness of bull sperm after freezing and thawing. Cryobiology 1997; 35: 93–105. [DOI] [PubMed] [Google Scholar]
- 28.Barbas JP, Mascarenhas RD. Cryopreservation of domestic animal sperm cells. Cell Tissue Bank 2009; 10: 49–62. [DOI] [PubMed] [Google Scholar]
- 29.Ward MA, Kaneko T, Kusakabe H, Biggers JD, Whittingham DG, Yanagimachi R. Long-term preservation of mouse spermatozoa after freeze-drying and freezing without cryoprotection. Biol Reprod 2003; 69: 2100–2108. [DOI] [PubMed] [Google Scholar]
- 30.Kusakabe H. Chromosomal integrity and DNA damage in freeze-dried spermatozoa. Reprod Med Biol 2011; 10: 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyers SA. Dry storage of sperm: applications in primates and domestic animals. Reprod Fertil Dev 2006; 18: 1–5. [DOI] [PubMed] [Google Scholar]
- 32.Sitaula R, Elmoazzen H, Toner M, Bhowmick S. Desiccation tolerance in bovine sperm: a study of the effect of intracellular sugars and the supplemental roles of an antioxidant and a chelator. Cryobiology 2009; 58: 322–330. [DOI] [PubMed] [Google Scholar]
- 33.Kawase Y, Iwata T, Toyoda Y, Wakayama T, Yanagimachi R, Suzuki H. Comparison of intracytoplasmic sperm injection for inbred and hybrid mice. Mol Reprod Dev 2001; 60: 74–78. [DOI] [PubMed] [Google Scholar]
- 34.Kaneko T, Whittingham DG, Overstreet JW, Yanagimachi R. Tolerance of the mouse sperm nuclei to freeze-drying depends on their disulfide status. Biol Reprod 2003; 69: 1859–1862. [DOI] [PubMed] [Google Scholar]