Abstract
Interleukin-17A/F (IL-17A/F) might play a role in the pathophysiology of osteoarthritis (OA), but several studies exploring the association between IL-17A/F single nucleotide polymorphisms and OA in different populations present inconsistent results. Thus, this case–control study, involving 410 OA cases and 507 controls, was aimed to investigate such association in a Chinese population. Genotyping was performed using standard polymerase chain reaction and restriction fragment length polymorphism. It was found that AA genotype or A allele carriers of IL-17A gene rs2275913 polymorphism were associated with OA susceptibility. Stratified analyses showed IL-17A rs2275913 polymorphism was evidently associated with a significantly increased risk for OA among males, <60 years old patients, smokers, and drinkers. No significant association was observed between IL-17F gene rs763780 polymorphism and OA risk. In conclusion, IL-17A rs2275913 polymorphism is involved in the development of OA in Chinese Han population. This finding should be confirmed by a larger study from diverse ethnic populations.
Keywords: bioinformatics analysis, Chinese population, IL17, osteoarthritis, polymorphism
1. Introduction
Primary osteoarthritis (OA), a common late-onset arthritis, could cause progressive loss of joint function.[1] OA is responsible for activity limitations, particularly walking, and affects participation and quality of life.[2] However, the etiology of OA is not fully understood. There is an evidence that genetic risk factors play important roles in the development of OA.[3] Interleukin-17 (IL-17) family members (A, B, C, D, E, F) play active roles in autoimmune diseases and inflammatory diseases.[4] IL-17A is a pro-inflammatory cytokine related to rheumatoid arthritis,[5] systemic lupus erythematosus[6] and ankylosing spondylitis.[7] Increased serum level of IL-17A is associated with decreased vitamin D3 and increased pain in OA.[8] IL-17F and IL-17A are similar in many aspects[9] and are both involved in innate and adaptive immune responses.
Many studies have demonstrated the influence of genetic variability in the pathogenesis of OA.[10] The rs22759133 located at a transcription factor binding motif for tumor necrosis factor (TNF)-activated T cells regulates production of IL-17. Therefore, this polymorphism might alter the genetic expression profile and modulate the susceptibility of OA. The rs763780 polymorphism would cause a histidine to arginine substitution at amino acid 161 upon the change from T to C nucleotides, thereby affecting the expression or function of IL-17F. Two studies have investigated the association between IL-17A rs2275913 or IL-17F rs763780 polymorphism and OA in different populations, but yielded conflicting results.[11,12] Moreover, there is no gene association study of these 2 single nucleotide polymorphisms (SNPs) among Chinese Han populations. Thus, this case–control study was aimed to investigate whether IL-17A and IL-17F SNPs were associated with the risk and development of OA in a Chinese Han population.
2. Materials and methods
2.1. Patients
A total of 917 participants (410 OA cases and 507 controls) were consecutively recruited from the Second Affiliated Hospital, Zhejiang University School of Medicine and the Affiliated Changzhou No.2 People's Hospital of Nanjing Medical University between May 2012 and October 2017. All OA cases were diagnosed on basis of the American College of Rheumatology criteria, which included primary OA with any symptom or sign of OA, and radiographic signs of OA according to the Kellgren–Lawrence grade (K-L grade).[13] Other etiologies of OA were excluded, including inflammatory arthritis, post-traumatic or postseptic arthritis, skeletal dysplasia, or developmental dysplasia. Participants were chosen without restriction to age, sex, or occupation.
The healthy controls were free of OA and matched to the OA cases based on sex and age. No control ever had any sign or symptom of OA, other arthritis or joint diseases (pain, swelling, tenderness, or restriction of movement) at any site based on medical history, and a thorough examination conducted by an experienced physiatrist. No control had relation with the patients or family history of OA.
The demographic, lifestyle, and clinical characteristics of all participants, such as gender, age, body mass index (BMI), and K-L grading, were collected from medical records. Clinically, the standardized Lequesne–Algofunctional index (L-index) was used to evaluate severity for knee disease through the assessment of pain/discomfort, maximum distance walked, and activity of daily living.[14] The study used 10 as the threshold, which was reported in a previous article.[2] Smokers was defined as individuals who had smoked at least 1 cigarette per day for at least 2 years. Individuals were defined as alcohol consumers if they drank alcohol at least once a week for more than 1 year. Written informed consent was obtained from all participants. The study protocol was approved by the Ethics Committee of the 2 hospitals. The ethical approval was in line with the standards of the Declaration of Helsinki.
2.2. DNA extraction and genotyping
The OA patients did not take anti-inflammatory analgesics for a month before blood samples were collected. Peripheral blood samples were collected in the morning. DNA was extracted using a QIAamp DNA blood mini kit (Qiagen, Hilden, Germany).
Genotyping was carried out by standard polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP). PCRs were performed each in a 50 μL volume containing 1 μL of DNA template, 5 μL of 10 ∗ PCR buffer, 3 μL of 2.0 mM MgSO4, 1 μL of 1U/μL KOD-Plus-Neo, 5 μL of 0.2 mM dNTPs, 33 μL of sterilized distilled water, and 1 μL of forward/reverse primers. The conditions included denaturation for 10 minutes at 95°C; 30 cycles of denaturation for 30 seconds at 94°C, annealing for 30 seconds at 60°C, elongation for 30 seconds at 72°C; final extension for 10 minutes at 72°C. Amplification was performed using a PTC-100 thermal cycler (MJ Research Inc, Quebec, Canada). The digested products were subjected to gel electrophoresis and visualized by ethidium bromide staining. PCR-RFLP was performed without knowing the status of the participants, in order to control the genotyping quality.
2.3. IL-17 cytokine assay
We selected 60 patients and 60 controls from the test population (20 patients for each genotype) and measured their serum IL-17 expression levels using an enzyme-linked immunosorbent assay kit (ELISA) (eBioscience). The subjects were adequately matched for age and sex (P = .825 and .559, respectively). All analytical steps were performed in accordance with the manufacturer's recommendations.
2.4. Statistical analysis
Qualitive data were compared by the Chi-square test and 1-way analysis of variance (ANOVA) test was utilized to compare different levels of clinical parameters of 3 different genotypes. Differences in continuous variables between cases and controls were tested using Student test. The Hardy–Weinberg equilibrium (HWE) was assessed to test for deviation between observed and expected frequencies among controls using a goodness-of-fit chi-square. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated to estimate the association between IL-17A/F gene polymorphisms and risk of OA by logistic regression analyses. Power and sample size were calculated on the Power and Sample Size Program. The following parameters were used: type I error probability for a 2-sided test (α), 0.05; probability of exposure in controls (P0), 0.237 for rs2275913, 0.083 for rs763780; number of patients (N), 408; ratio of controls to patients (m), 506; odd ratio of exposure in cases relative to controls (Ψ), OR = 1.33 (rs2275913) and 0.83 (rs763780). All statistical analyses were performed using SAS 9.1.3 (SAS Institute, Cary, NC). P < .05 was the cutoff value of significance.
3. Results
3.1. Characteristics of the study population
The characteristics of OA cases and controls as well as K-L grading and L-index scores are summarized in Table 1. No significant differences between the case and control groups were found in average age (57.35 vs 57.88 years) and/or proportion of females (66.1% vs 68.6%), indicating the cases and controls were well matched in terms of gender and age. Significant between-group difference was observed in BMI, but not in the proportions of smokers or drinkers, indicating that BMI was a risk factor of OA.
Table 1.
Patient demographics and risk factors in osteoarthritis.
3.2. Association between IL-17A rs2275913 or IL-17F rs763780 and OA risk
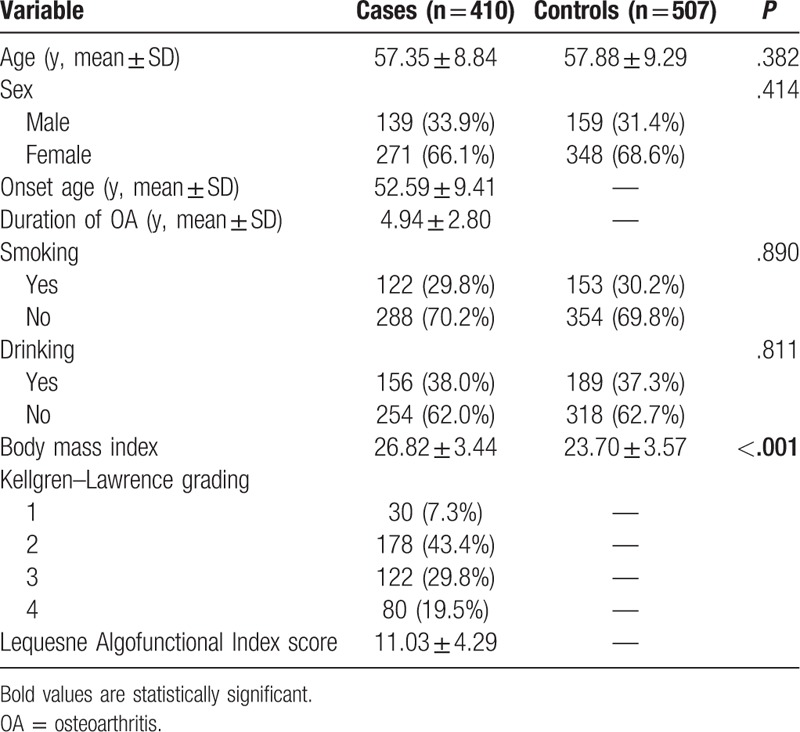
The genotype frequencies of rs2275913 and rs763780 polymorphisms in 2 groups are summarized in Table 2. The genotyping was successful in 408 cases and 506 controls for rs2275913; The genotyping was successful in 409 cases and 505 controls for rs763780. Genotype distributions of the 2 polymorphisms rs2275913 and rs763780 in the controls conformed to HWE (P = .180 and .383, respectively). The power analysis indicated that this meta-analysis had a power of 47.3% and 11.2% to detect the effect of rs2275913 or rs763780 polymorphism on OA susceptibility, assuming an OR of 1.33 and 0.83, respectively. Logistic regression analyses revealed that the risk of OA was significantly intensified by AA genotype [OR and 95% CI: 2.09 (1.20–3.66), P = .010 for AA vs GG; 1.33 (1.03–1.73), P = .032 for GA+AA vs GG] or A allele carriers [1.33 (1.07–1.63), P = .008 for A vs G] of IL-17A gene rs2275913 polymorphism. However, no such positive result was obtained in the CC genotype or C allele of IL-17F gene rs763780 polymorphism. Then, stratification analyses of rs2275913 polymorphism were conducted according to age, sex, smoking, and drinking. Stratified analysis by sex revealed an increased risk of OA among male patients. This finding also held true in the age < 60 individuals, smokers, and drinkers (Table 3).
Table 2.
Logistic regression analysis of associations between polymorphisms and risk of osteoarthritis.
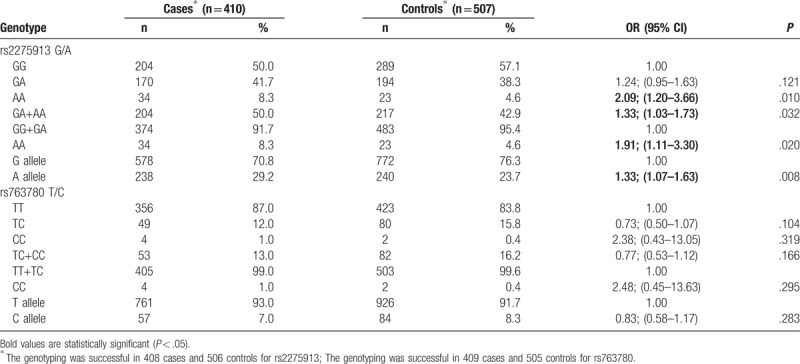
Table 3.
Stratified analyses between rs2275913 polymorphisms and the risk of osteoarthritis.
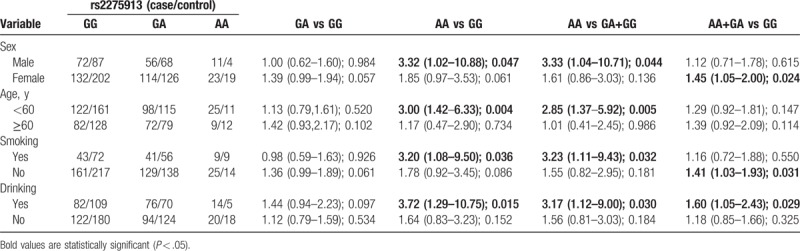
Furthermore, we also examined the association between rs2275913 polymorphism and clinical features of OA, including onset age, L-index, and K-L grading (Table 4). The results indicated that GA, AA, GA+AA genotype, and A allele of rs2275913 polymorphism were associated with the poor L-index.
Table 4.
The association between IL-17A rs2275913 polymorphism and clinical features of osteoarthritis.
3.3. Plasma levels of IL-17 in OA patients, controls, and different genotypes
The average plasma level of IL-17 was significantly higher in OA patients than in controls (Table 5). The mean plasma levels of IL-17 in OA patients with different genotypes were 119.03 ± 13.42 (GG genotype), 134.41 ± 18.69 (GA genotype), and 151.23 ± 13.88 (AA genotype) pg/mL, respectively. The AA genotype of rs2275913 polymorphism could increase significantly the production of IL-17 in OA patients compared with GG genotype (P < .001) (Fig. 1).
Table 5.
The demographics and risk factors in 120 OA patients measuring the expression levels of IL-17.
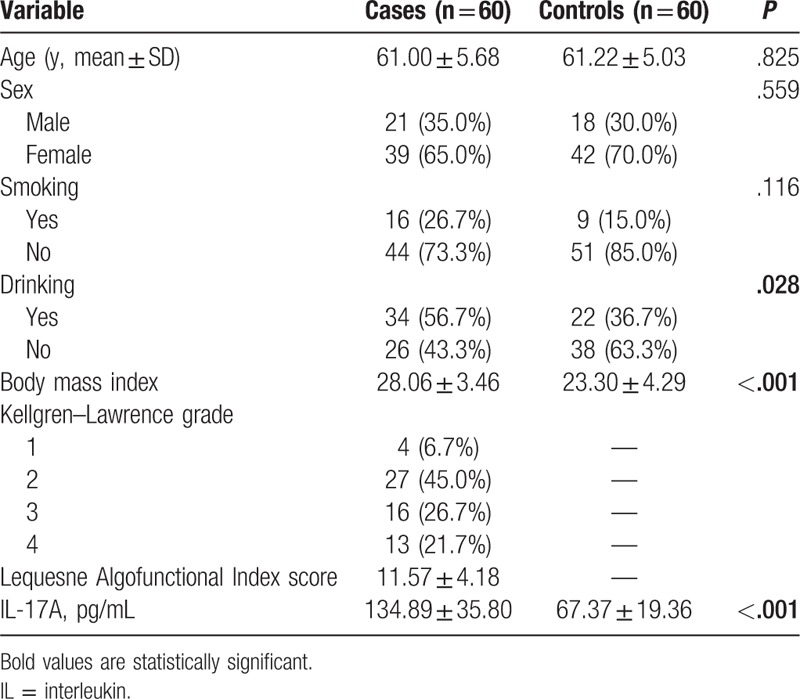
Figure 1.
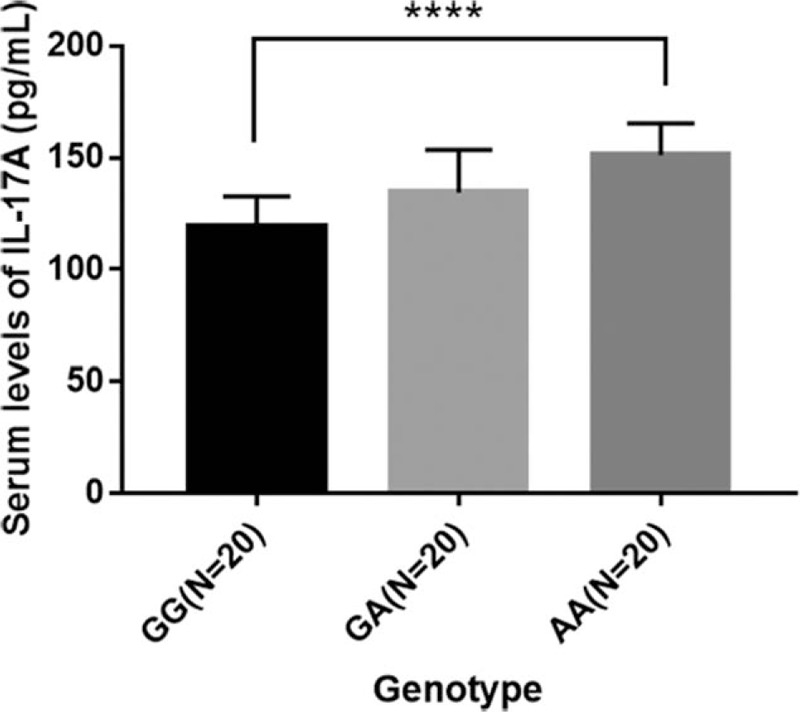
IL-17 plasma levels of different genotypes of rs2275913 polymorphism in OA patients. ∗P < .05 (t test).
4. Discussion
We found that IL-17A gene rs2275913 polymorphism conferred susceptibility to OA. Stratified analysis further indicated significant association with OA risks among male, age <60 individuals, smokers, and drinkers. AA genotype and A allele of rs2275913 polymorphism showed significantly correlation with poor L-index. However, no significant association between IL-17F rs763780 polymorphism and OA risk was found.
IL-17A, often referred to as IL-17, contributes to cartilage breakdown and synovial infiltration in OA by inducing the release of chemokines from chondrocytes and synovial fibroblasts and the synthesis of IL-1beta in chondrocytes.[15] IL-17A is associated with inflammation[16] and bears 50% homology to IL-17F.[17] IL-17A and F are overexpressed in OA patients.[18] IL-17A and IL-17F alone have similar regulatory effects in the presence of TNF-α.[17] Two polymorphisms (rs2275913 and rs763780) have been investigated in various diseases such as gastric cancer,[19] tuberculosis,[20] and inflammatory diseases.[21] However, little is known about their associations with OA susceptibility. Han et al[12] first examined the alleles and genotypes of IL-17A rs2275913 and IL-17F rs763780 in a Korean population, including 302 OA patients and 300 controls, and found a significant association between only rs2275913 and OA susceptibility. Later, Vrgoc et al[11] validated this significant association in a Croatian population (500 OA patients and 597 controls), but found no association of IL-17A rs2275913 with the risk of hip or knee OA. The C allele of the IL-17F gene rs763780 polymorphism increased the risk of hip OA, but not knee OA.[11] We found a significant association between the increased risk of OA and IL-17A rs2275913, but not IL-17F rs763780. Discrepancy in the above studies may be due to false-positive results, differences in genetic and/or environmental factors, small sample sizes, and differences in statistical methods.
The study conducted by Han et al[12] and this study found that IL-17A rs2275913 polymorphism conferred susceptibility to OA among Asians, but it was not confirmed among Caucasians.[11] The discrepancy could be attributed to differences in genetic backgrounds, as Asians had higher A allele frequency than Caucasians [0.292 (this study) vs 0.134[11]]. Three studies including this study investigated the association between IL-17F rs763780 polymorphism and risk of knee OA, but presented negative results.[11,12] A possible explanation was that small sample sizes increased the possibility of false-positive or false-negative associations caused by random errors. Notably, Vrgoc et al[11] found this SNP was associated with the increased risk of hip OA, indicating that the effect of rs763780 polymorphism may be tissue-specific. To our knowledge, this is the first case–control study to address the association between IL-A/F gene polymorphisms and OA risk in a Chinese population. The stratified analyses by smoking or drinking were not concerned in previous studies, but we found that AA genotype of rs2275913 polymorphism increased the risk of OA among smokers and drinkers. Furthermore, our results indicated that AA genotype of rs2275913 polymorphism has a higher level of IL-17 expression than GG genotype.
The potential limitations of this study merit careful consideration. First, most controls were admitted to the hospital for treatment of trauma, which may contribute to selection bias. Second, the polymorphisms selected on basis of functional considerations may not comprehensively uncover the genetic variability of IL-17A/F. Third, we cannot obtain details about OA severity, which restricted our analyses. Fourth, the degrees of genetic risk of IL-17A/F gene polymorphisms may differ under different environmental exposures because OA risk was influenced by gene–gene and gene–environment interactions. Fifth, we could not provide the follow-up data and update the survival analysis. Finally, this study may be underpowered by the relatively small sample size.
In summary, IL-17A rs2275913 polymorphism may contribute to the etiology of OA, especially among males, age <60 individuals, smokers, and drinkers. IL-17F rs763780 polymorphism is not associated with the risk of OA. Nevertheless, a larger-size prospective study from a more diverse ethnic population is needed to confirm these findings.
Author contributions
Conceptualization: Lifeng Jiang.
Data curation: Lifeng Jiang.
Formal analysis: Lifeng Jiang, Xindie Zhou.
Funding acquisition: Lifeng Jiang, Xindie Zhou.
Investigation: Xindie Zhou.
Methodology: Lifeng Jiang, Xindie Zhou.
Project administration: Xindie Zhou, Yan Xiong.
Resources: Yan Xiong, Jiapeng Bao.
Software: Yan Xiong, Jiapeng Bao.
Supervision: Jiapeng Bao.
Validation: Kai Xu.
Visualization: Lidong Wu.
Writing – original draft: Kai Xu, Lidong Wu.
Writing – review & editing: Lidong Wu.
Footnotes
Abbreviations: BMI = body mass index, CIs = confidence intervals, HWE = Hardy–Weinberg equilibrium, IL-17A/F = Interleukin-17A/F, K-L grade = Kellgren–Lawrence grade, L-index = Lequesne-Algofunctional index, OA = osteoarthritis, ORs = odds ratios, PCR-RFLP = polymerase chain reaction and restriction fragment length polymorphism, SNPs = single nucleotide polymorphisms, TNF = tumor necrosis factor.
This study was supported in part by National Natural Science Foundations of China (81801371).
No potential conflict of interest was reported by the authors.
References
- [1].Valdes AM, Spector TD. Genetic epidemiology of hip and knee osteoarthritis. Nat Rev Rheumatol 2011;7:23–32. [DOI] [PubMed] [Google Scholar]
- [2].Diab SM, Kamal HM, Mansour AI, et al. Clinical significance of Matrilin-3 gene polymorphism in Egyptian patients with primary knee osteoarthritis. Eur J Rheumatol 2017;4:200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang T, Liang Y, Li H, et al. Single nucleotide polymorphisms and osteoarthritis: an overview and a meta-analysis. Medicine (Baltimore) 2016;95:e2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity 2004;21:467–76. [DOI] [PubMed] [Google Scholar]
- [5].Paradowska A, Masliniski W, Grzybowska-Kowalczyk A, et al. The function of interleukin 17 in the pathogenesis of rheumatoid arthritis. Arch Immunol Ther Exp (Warsz) 2007;55:329–34. [DOI] [PubMed] [Google Scholar]
- [6].Rana A, Minz RW, Aggarwal R, et al. Gene expression of cytokines (TNF-alpha, IFN-gamma), serum profiles of IL-17 and IL-23 in paediatric systemic lupus erythematosus. Lupus 2012;21:1105–12. [DOI] [PubMed] [Google Scholar]
- [7].Chen WS, Chang YS, Lin KC, et al. Association of serum interleukin-17 and interleukin-23 levels with disease activity in Chinese patients with ankylosing spondylitis. J Chin Med Assoc 2012;75:303–8. [DOI] [PubMed] [Google Scholar]
- [8].Askari A, Naghizadeh MM, Homayounfar R, et al. Increased serum levels of IL-17A and IL-23 are associated with decreased vitamin D3 and increased pain in osteoarthritis. PLoS One 2016;11:e0164757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chang SH, Dong C. IL-17F: regulation, signaling and function in inflammation. Cytokine 2009;46:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yucesoy B, Charles LE, Baker B, et al. Occupational and genetic risk factors for osteoarthritis: a review. Work 2015;50:261–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vrgoc G, Vrbanec J, Eftedal RK, et al. Interleukin-17 and Toll-like receptor 10 genetic polymorphisms and susceptibility to large joint osteoarthritis. J Orthop Res 2018;36:1684–93. [DOI] [PubMed] [Google Scholar]
- [12].Han L, Lee HS, Yoon JH, et al. Association of IL-17A and IL-17F single nucleotide polymorphisms with susceptibility to osteoarthritis in a Korean population. Gene 2014;533:119–22. [DOI] [PubMed] [Google Scholar]
- [13].Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res 2016;474:1886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lequesne MG, Mery C, Samson M, et al. Indexes of severity for osteoarthritis of the hip and knee. Validation--value in comparison with other assessment tests. Scand J Rheumatol Suppl 1987;65:85–9. [DOI] [PubMed] [Google Scholar]
- [15].Honorati MC, Bovara M, Cattini L, et al. Contribution of interleukin 17 to human cartilage degradation and synovial inflammation in osteoarthritis. Osteoarthritis Cartilage 2002;10:799–807. [DOI] [PubMed] [Google Scholar]
- [16].Rosu A, Margaritescu C, Stepan A, et al. IL-17 patterns in synovium, serum and synovial fluid from treatment-naive, early rheumatoid arthritis patients. Rom J Morphol Embryol 2012;53:73–80. [PubMed] [Google Scholar]
- [17].Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol 2009;182:3112–20. [DOI] [PubMed] [Google Scholar]
- [18].Sarkar S, Justa S, Brucks M, et al. Interleukin (IL)-17A, F and AF in inflammation: a study in collagen-induced arthritis and rheumatoid arthritis. Clin Exp Immunol 2014;177:652–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Elshazli RM, Salman DO, Kamel MM, et al. Genetic polymorphisms of IL-17A rs2275913, rs3748067 and IL-17F rs763780 in gastric cancer risk: evidence from 8124 cases and 9873 controls. Mol Biol Rep 2018;45:1421–44. [DOI] [PubMed] [Google Scholar]
- [20].Yu ZG, Wang BZ, Li J, et al. Association between interleukin-17 genetic polymorphisms and tuberculosis susceptibility: an updated meta-analysis. Int J Tuberc Lung Dis 2017;21:1307–13. [DOI] [PubMed] [Google Scholar]
- [21].Eskandari-Nasab E, Moghadampour M, Tahmasebi A. Meta-analysis of risk association between interleukin-17A and F gene polymorphisms and inflammatory diseases. J Interferon Cytokine Res 2017;37:165–74. [DOI] [PubMed] [Google Scholar]


