Abstract
Rationale:
Pulmonary sarcomatoid carcinoma (PSC) represents <1% of all lung cancers and is characterized by a very poor prognosis. The optimal therapeutic regimen remains unclear. We describe a rare case of PSC with both anaplastic lymphoma kinase (ALK)-arranged and high levels of programmed death ligand 1 (PD-L1) expression.
Patient concerns:
A 46-year-old woman, nonsmoker, came to our attention due to uncontrolled pain in the lower left limb.
Diagnosis:
PSC with both ALK rearrangement and high levels of PD-L1 expression.
Interventions:
The patient started first-line systemic treatment with pembrolizumab reporting stable disease; at progression, she received second-line treatment with crizotinib. The treatment was not well-tolerated, and the patient then underwent 5 cycles of ceritinib treatment.
Outcomes:
The patient showed a partial response to targeted therapy. At progression, brigatinib was initiated, but the patients reported liver progression soon after the initiation of this therapy.
Lessons:
Molecular-driven investigation is necessary in PSC for treatment selection.
Keywords: ALK, ceritinib, crizotinib, PD-L1, pembrolizumab, pulmonary sarcomatoid carcinoma
1. Introduction
Pulmonary sarcomatoid carcinoma (PSC) accounts for <1% of all lung cancers and is associated with a worse prognosis than other non-small-cell lung cancers (NSCLCs).[1] PSCs show high levels of programmed death ligand 1 (PD-L1) expression and several genetic alterations, providing rationale for the potential use of immunotherapy or targeted therapy.[2–4] In more details, PD-L1 expression appears to be higher in PSC than in NSCLC, thus suggesting that targeting the PD-1/PD-L1 pathway could represent a suitable therapy in this setting.[2] Moreover, ALK amplification was shown to be a clonally related event in a subset of PSC.[4] These considerations pave the way for the use of anti-PD-1/PD-L1 agents or ALK-targeted therapies in PSC.
We describe a rare case of PSC with both ALK-arranged and high levels of PD-L1 expression.
2. Case report
In April 2017, a 46-year-old woman, nonsmoker, was admitted to our hospital because of uncontrolled pain in the lower left limb. She had no relevant comorbidities; Eastern Cooperative Oncology Group performance status (PS) was 1. A contrast-enhanced total body computed tomography (CT) scan revealed a stage IV lung neoplasm (The American Joint Committee on Cancer tumor-node-metastasis staging system, 8th edition) with bilateral hilar and mediastinal lymph nodes, a lateral subpleural mass on the left lung, multiple hepatic lesions, and diffuse infiltration of the D11 and L1 vertebrae (Fig. 1A). A coxo-femoral magnetic resonance imaging showed further bone involvement at this level. Positron emission tomography scan confirmed lung, liver, spinal column lesions and an abnormal hypermetabolic activity in soft tissues around the proximal tract of the left femur. Bone femur and liver biopsies revealed histological diagnosis of carcinosarcoma (Fig. 2). The tumor was ALK-rearranged (in 80% of tumor cells by Fluorescence in situ hybridization) (Fig. 3) and showed high levels of PD-L1 (>50% by immunohistochemistry) (Fig. 4). epidermal growth factor receptor (EGFR), KRAS, and ROS1 mutations were not present.
Figure 1.
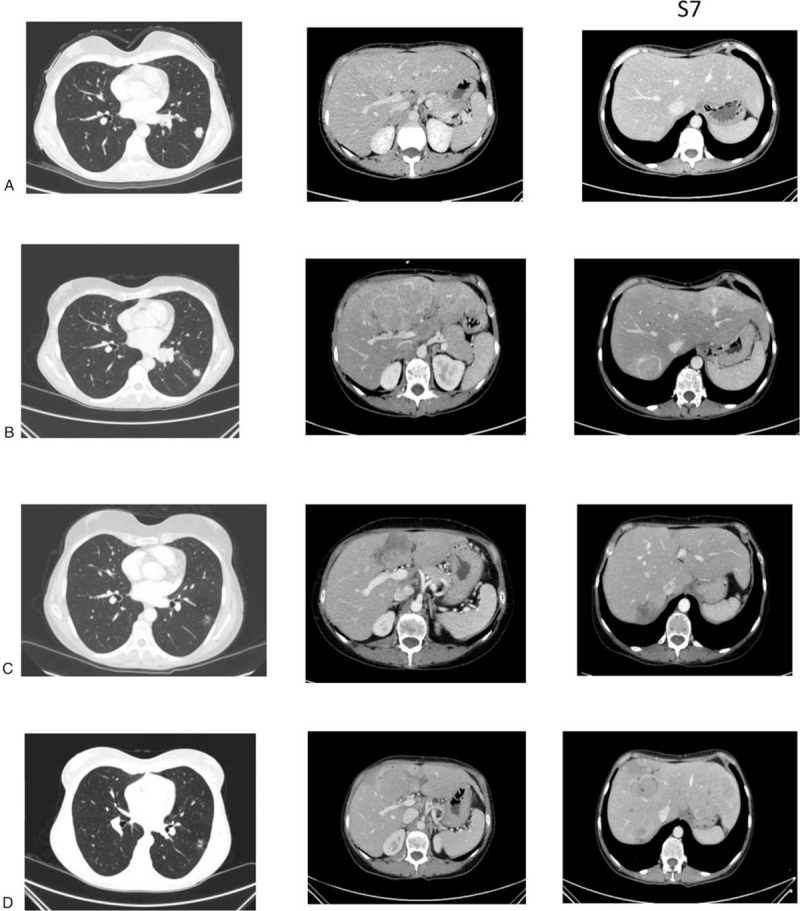
Computed tomography scans at baseline (A), after 5 cycles of pembrolizumab (B), at partial response (C), and progressive disease (D) during ceritinib. In detail, the first column represents lung lesions, while the second and the third ones represent liver lesions at the fourth and seventh segment, respectively.
Figure 2.
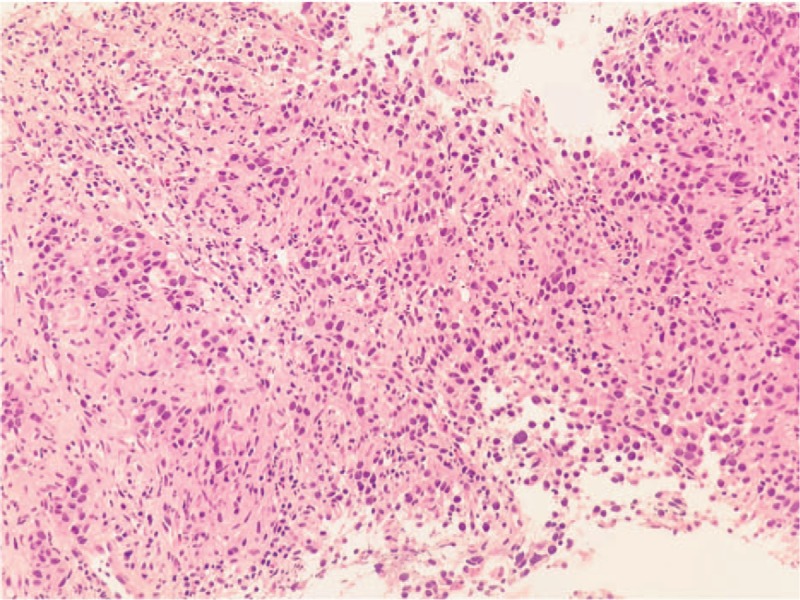
Hematoxylin and eosin staining.
Figure 3.
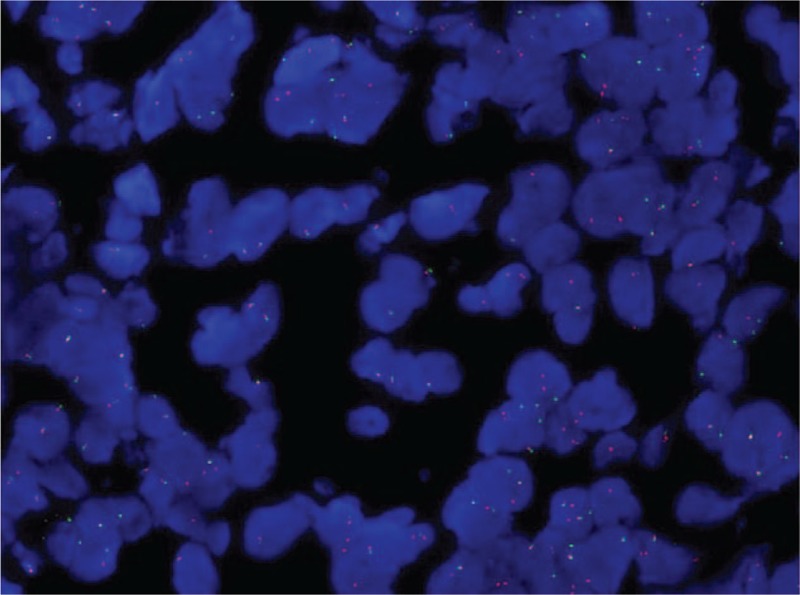
ALK FISH test (magnification: 40×; ZytoLight SPEC ALK Dual Color Break Apart Probe).
Figure 4.
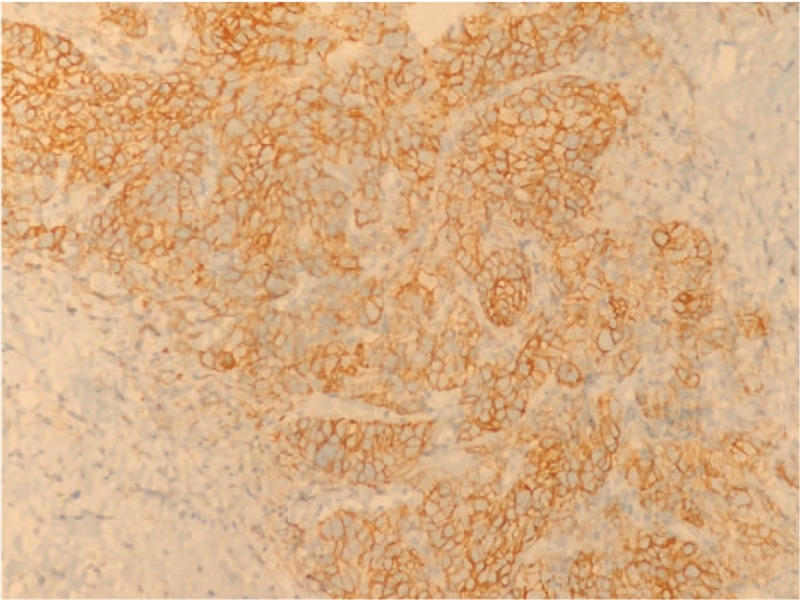
PD-L1 expression by immunohistochemistry.
In May 2017, she received palliative radiation therapy on the left hip, homolateral proximal femur and ischiatic tuberosity (25 Gy/5 fractions). In June 2017, based on the immunologic profile (PD-L1 >50%), she started a first-line systemic treatment with pembrolizumab 200 mg intravenous day 1 every 21 days. When immunotherapy was started, data on mutational status were not available. A CT scan after 2 cycles of pembrolizumab showed stable disease (according to both RECIST v1.1 criteria,[5] and modified RECIST v1.1 for immune-based therapeutics or iRECIST[6]). A re-evaluation CT was performed after 3 more cycles of pembrolizumab in October 2017, showing disease progression with liver and lymph node lesions (Fig. 1B; PD according to RECIST v1.1 and iUPD, unconfirmed progressive disease according to iRECIST), associated with clinical deterioration of PS and hemoptysis (clinical progression was considered when deciding to stop immunotherapy as per iRECIST). Considering the mutational profile (ALK-arranged), the patient received a second-line treatment with oral crizotinib 250 mg twice daily. After the first cycle she showed G1 diplopia and G2 peripheral edema (CTCAE v4.0), and therefore crizotinib was reduced to 250 mg once daily. After 3 cycles, in December 2017, CT scan showed stable disease (RECIST v1.1; these response evaluation criteria have been used in the following restaging imaging). Due to the persistent grade 2 liver toxicity (CTCAE v4.0) without disease shrinkage, we switched to the second-generation ALK inhibitor ceritinib 750 mg orally once daily. After 2 cycles she reported partial response at CT scan (Fig. 1C). While on ceritinib, her clinical conditions improved, with pain and fatigue reduction, and normalization of liver function tests. After 5 months of initiation of ceritinib, our patient experienced an abdominal lymph node oligoprogression treated with radiation therapy and ceritinib treatment was continued. In July 2018, CT scan revealed hepatic disease progression (Fig. 1D). Patient is starting third-generation therapy with brigatinib; however, she experienced immediate liver progression and started home-based palliative care.
3. Discussion
We reported the rare occurrence of a widespread metastatic PSC in a young woman, with ALK translocation and high levels of PD-L1. The peculiarity of our case, which is of course anecdotal in nature, is the rare coexistence of an ALK genetic alteration and of high levels of PD-L1. Indeed, in ALK-arranged NSCLC, high levels of PD-L1 are not frequent.[7,8] This unusual molecular profile allowed us to administer different and specific therapies.
According to PD-L1 positivity, being mutational status not yet available at the time of the first therapeutic decision, we started treatment with pembrolizumab. During immunotherapy, patient showed progressive disease with liver and lymph node lesions. Therefore, according to the genetic profile, we decided to administer a second-line treatment with an anti-ALK drug, since NSCLC patients with ALK rearrangements are very sensitive to ALK inhibitors. However, genetic alterations for targeting therapy are largely unexplored in PSC.[4] Our patient started second-line crizotinib treatment, but progression was reported after few months (in contrast to literature data on durable responses of NSCLC treated with ALK inhibitors), probably due to crizotinib dose reduction in our patient. Therefore, we switched to a second-generation ALK inhibitor (ceritinib). She reported higher hepatotoxicity during crizotinib rather than ceritinib, although her liver disease was stable, suggesting different mechanisms of liver toxicity.[9,10]
The patient experienced partial response during targeted therapy. Although pre-clinical evidence of the interaction between ALK and PD-L1 exists,[11,12] data in NSCLC and melanoma show that upfront targeted therapies could be the best therapeutic option compared with immunotherapy in patients with driver mutations.
However, molecular-driven clinical research is necessary in PSC to provide more information about the role of targeted therapy or immunotherapy in therapeutic algorithm of a cancer type without therapeutic alternatives.
Acknowledgments
Editorial assistance was provided by Luca Giacomelli, PhD, and Aashni Shah, of Polistudium srl, whose assistance was supported by internal funds.
Author contributions
Conceptualization: Federica D’Antonio, Rita De Sanctis, Armando Santoro.
Investigation: Federica D’Antonio, Rita De Sanctis, Isabella Bolengo, Annarita Destro, Daoud Rahal, Fabio De Vincenzo, Armando Santoro.
Supervision: Armando Santoro.
Writing – original draft: Federica D’Antonio, Rita De Sanctis, Armando Santoro.
Writing – review and editing: Federica D’Antonio, Rita De Sanctis, Isabella Bolengo, Annarita Destro, Daoud Rahal, Fabio De Vincenzo, Armando Santoro.
Footnotes
Abbreviations: NSCLC = non-small-cell lung cancer, PS = performance status, PSC = pulmonary sarcomatoid carcinoma.
Armando Santoro has received honoraria from BMS, AstraZeneca, MSD, Lilly, Bayer, Takeda, Roche, Mundipharma, Novartis, Servier, Amgen, ArQule, Celgene, Incyte, AbbVie, Gilead, Pfizer, Daiichi, Sandoz, and Sanofi.
The patient provided consent to the presentation of her data.
Informed written consent was obtained from the patient for publication of this case report and accompanying images.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent has been received from the patient.
The other authors have no funding and conflicts of interest to disclose.
References
- [1].Yendamuri S, Caty L, Pine M, et al. Outcomes of sarcomatoid carcinoma of the lung: a surveillance, epidemiology, and end results database analysis. Surgery 2012;152:397–402. [DOI] [PubMed] [Google Scholar]
- [2].Vieira T, Antoine M, Hamard C, et al. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1) and strong immune-cell infiltration by TCD3 cells and macrophages. Lung Cancer 2016;98:51–8. [DOI] [PubMed] [Google Scholar]
- [3].Kim S, Kim MY, Koh J, et al. Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: comparison of sarcomatous and carcinomatous areas. Eur J Cancer 2015;51:2698–707. [DOI] [PubMed] [Google Scholar]
- [4].Pelosi G, Gasparini P, Cavazza A, et al. Multiparametric molecular characterization of pulmonary sarcomatoid carcinoma reveals a nonrandom amplification of anaplastic lymphoma kinase (ALK) gene. Lung Cancer 2012;77:507–14. [DOI] [PubMed] [Google Scholar]
- [5].Ghobrial FE, Eldin MS, Razek AAKA, et al. Computed tomography assessment of hepatic metastases of breast cancer with revised response evaluation criteria in solid tumors (RECIST) criteria (Version 1.1): inter-observer agreement. Pol J Radiol 2017;82:593–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lin K, Cheng J, Yang T, et al. EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-κB. Biochem Biophys Res Commun 2015;463:95–101. [DOI] [PubMed] [Google Scholar]
- [8].Li D, Zhu X, Li N, et al. Correlation of PD-L1 expression with EGFR, KRAS, or ALK alterations and with survival of non-small cell lung cancer (NSCLC) treated with EGFR-TKIs: a meta-analysis of published trials. J Clin Oncol 2016;34: DOI: 10.1200/JCO.2016.34.15_suppl.e20576. [Google Scholar]
- [9].Ripault MP, Pinzani V, Fayolle V, et al. Crizotinib-induced acute hepatitis: first case with relapse after reintroduction with reduced dose. Clin Res Hepatol Gastroenterol 2013;37:e21–3. [DOI] [PubMed] [Google Scholar]
- [10].Sassier M, Mennecierc B, Gschwendc A, et al. Successful treatment with ceritinib after crizotinib induced hepatitis. Lung Cancer 2016;95:15–6. [DOI] [PubMed] [Google Scholar]
- [11].Ota K, Azuma K, Kawahara A, et al. Induction of PD-L1 expression by the EML4–ALK oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin Cancer Res 2015;21:4014–21. [DOI] [PubMed] [Google Scholar]
- [12].Hong S, Chen N, Fang W, et al. Upregulation of PD-L1 by EML4–ALK fusion protein mediates the immune escape in ALK positive NSCLC: implication for optional anti-PD-1/PD-L1 immune therapy for ALK-TKIs sensitive and resistant NSCLC patients. Oncoimmunology 2016;5:e1094598. [DOI] [PMC free article] [PubMed] [Google Scholar]


