Abstract
The objectives of this study were to analyze the clinical features of patients with bone involved lymphoma and identify the prognostic factors and to explore the optimized treatment strategy for bone involved lymphoma.
A total of 1948 patients with lymphoma in our cancer center from September 2006 to October 2017 were retrospectively evaluated. Among these, 109 patients with skeletal involvement in lymphoma were enrolled. According to the pathologic subtypes, the patients were divided into 3 subgroups: classic Hodgkin lymphoma (cHL), B-cell non-Hodgkin lymphoma (B-NHL), and T-cell non-Hodgkin lymphoma (T-NHL). The clinical characteristics and overall survival (OS) of 3 groups of patients were reviewed, and the prognostic factors were analyzed.
There were 9 (3 unifocal, 6 multifocal) patients with primary bone lymphoma. The 5-year OS of cHL, B-NHL, and T-NHL patients was 88.24%, 54.09%, and 61.58%, respectively. Advanced stage, elevated lactate dehydrogenase (LDH), age above 60, high International Prognostic Index score, and treatment without radiotherapy for the bone involved were significant poor prognostic factors for OS of all patients in univariate analysis. There was a trend toward better OS not only in limited-stage but also in advanced-stage patients with radiotherapy for the bone involved compared with the patients without radiotherapy. Elevated LDH level and age above 60 were the independent unfavorable prognostic factor in multivariate analysis.
Elevated LDH level and age above 60 predict the poor prognosis of patients with bone involvement. The potential for long-term survival suggests that additional consolidative radiotherapy for the site of skeleton involvement may have a better chance of long-term success.
Keywords: bone lymphoma, Hodgkin lymphoma, lactate dehydrogenase, non-Hodgkin lymphoma, radiotherapy
1. Introduction
Bone involvement in lymphoma is an uncommon form of extra-nodal lymphoma. Although a primary lesion can originate from bone as a primary bone lymphoma, this is extremely rare compared to secondary bone involvement by systemic lymphoma. Primary bone lymphomas account for <1% of all malignant lymphomas and 7% of malignant bone tumors. Compared with systemic lymphomas, most primary lymphomas are treated with combined chemotherapy and radiotherapy and have a better prognosis. Malignant lymphoma of bone accounts for 5% of extranodal lymphoma.[1,2] Every lymphoma category can involve the skeleton, as an exclusive lesion or as a part of a disseminated disease. Because bone involvement is observed in advanced stages of lymphoma with high tumor burdens, patients with lymphoma with bone involvement may have worse outcomes than patients without bone involvement. Chemotherapy combined with involved bone radiotherapy is the consistent strategy for primary bone lymphoma. However, there is no standard treatment for the involved bone in systemic lymphomas, and several questions remain open: the role of radiotherapy and surgery, the best doses and volumes of radiotherapy, the most optimal combination of radiotherapy and chemotherapy.
We describe a relatively large group of 109 lymphoma patients with bone involvement with the clinical features, management, and prognosis, collected from a single center database from September 2006 to October 2017.
2. Patients and methods
2.1. Patients
We retrospectively reviewed a total of 1948 patients with lymphoma in Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology from September 2006 to October 2017. About 109 patients with skeletal involvement in lymphoma were finally enrolled, and clinical data of these patients were collected from medical records. Data collection of the study conformed to the provisions of the Declaration of Helsinki. All investigations had been approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. Due to the retrospective nature of the study, informed consent was not required.
Pathologic diagnosis was based on the World Health Organization classification (2001 or 2008 edition). All patients were staged according to the Ann Arbor staging system, including serum lactate dehydrogenase (LDH) level determination, enhanced total-body computed tomography (CT) scan and bone marrow assessment. Positron emission tomography (PET)/CT scan has been routinely performed since 2012. Bone involvement was defined either by radiologic procedures or histopathologic examination of surgical biopsies. Positive lesions in PET/CT scans that were due to diffuse involvement of bone marrow were not defined as bone involvement.
Treatment response was assessed according to the International Working Group Guidelines of 2007.[3] The decision to offer consolidative radiotherapy was generally made based on the referring physicians’ decision. Overall survival (OS) was calculated from the date of diagnosis to the date of last follow-up or death from any cause.
2.2. Survival analysis
Survival curves were calculated according to the Kaplan–Meier method and compared using the log-rank test. Differences were considered significant if P-values were ≤.05 (2-tailed). Univariate and multivariate analysis was performed using a Cox model using a forward variable selection procedure. Only the variables with significant values (P ≤ .05) in univariate analysis were included in the multivariate analysis. All data analyses were performed by SPSS software for Windows, version 20 (SPSS Inc, Chicago, IL).
3. Results
3.1. Patient characteristics
A total of 109 lymphoma patients with bone involvement were included in our study. Among them, 9 patients had primary bone lymphoma, and the others had secondary bone involvement of systemic lymphoma. Seventeen patients were diagnosed with classic Hodgkin lymphoma (cHL), 73 patients were diagnosed with B-cell non-Hodgkin lymphoma (B-NHL), and 19 patients had T-cell non-Hodgkin lymphoma (T-NHL). The major clinical characteristics of the patients are summarized in Table 1. As shown in Figure 1, diffuse large B-cell lymphoma (DLBCL, n = 48, 44.04%) was the most common histologic subtype, followed by cHL (n = 16, 14.68%) and follicular lymphoma (n = 8, 7.04%). The male/female ratio was 1.2:1, and the median age was 48 years. Most patients in our study had ECOG performance status of 0-1, and more than 80% of patients had advanced-stage disease at presentation.
Table 1.
Clinical characteristics of lymphoma patients with bone involvement.

Figure 1.
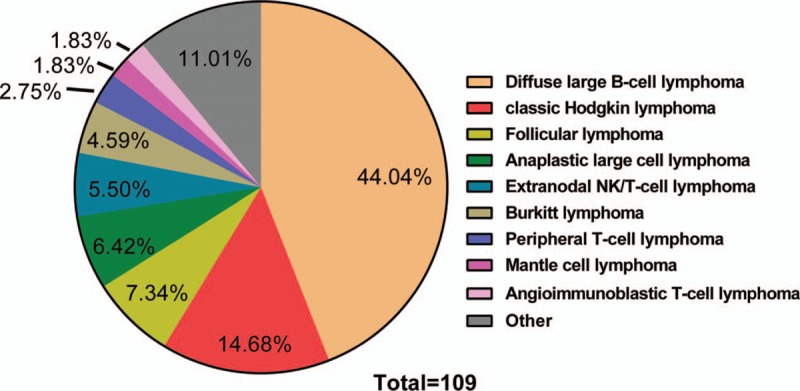
Distribution of 109 cases by histologic type. NK = natural killer.
As shown in Table 2, 17 patients had unifocal bone lesions at presentation, with the femur (23.53%) as the most commonly involved bone, followed by the spine (17.65%). However, in patients with multifocal bone disease, spine (51.09%) was the most commonly involved site, followed by pelvis (44.57%). There were 9 primary bone lymphoma patients. Three patients had unifocal bone lesions, and 6 patients had multifocal bone lesions.
Table 2.
Sites of bone involvement of lymphoma patients.

3.2. Treatments
Various treatment strategies were employed according to histologic type, physician discretion, and economic capability. Among 17 cHL patients, 14 (82.35%) received the ABVD (doxorubicin, bleomycin, vincristine, dacarbazine) chemotherapy regimen and 3 proceeded to radiotherapy for the bone involved; the radiation dose was 30, 30, and 46 Gy. CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) or CHOP-like were the most commonly used regimens for NHL patients. Thirty-six B-NHL patients received 1 to 8 cycles of rituximab plus chemotherapy. Sixteen B-NHL (21.92%) patients and 5 T-NHL (26.32%) patients were treated with radiotherapy for the bone involved, and the median radiation doses were 38 and 40 Gy, respectively.
3.3. Survival and prognostic factor analyses
The median follow-up for OS of cHL, B-NHL, and T-NHL patients was 18.5 months (range, 2–86 months), 10 months (range, 1–120 months), and 9.5 months (range, 0.7–144 months), respectively. The 5-year OS was 88.24%, 54.09%, and 61.58%, respectively. The OS at 10 years for B-NHL and T-NHL patients was 36.06% and 61.58%, respectively (Fig. 2A). The OS at 3 years for primary and secondary bone lymphoma patients was 74.07% and 59.30%, respectively (Fig. 2B).
Figure 2.
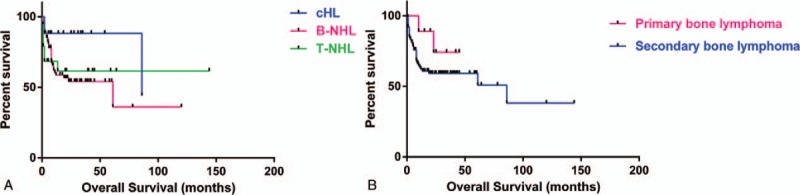
Overall survival (OS) for patients with skeletal involvement in different subgroups. (A) OS for classic Hodgkin lymphoma (cHL), B-cell non-Hodgkin lymphoma (B-NHL), and T-cell non-Hodgkin lymphoma (T-NHL) patients. (B) OS for primary and secondary bone lymphoma patients.
We analyzed the effects of the following individual factors on survival: stage, International Prognostic Index (IPI) risk group, serum LDH level, age, and radiotherapy to sites of skeletal involvement (Table 3). In univariate analysis, advanced stage, high IPI score, elevated LDH, age above 60 (Fig. 3), and treatment without radiotherapy for the bone involved (Fig. 4A) were significant poor prognostic factors for OS of all the patients in this study. Among limited-stage patients, only one who had not received radiotherapy for the bone involved died and the rest survived (Fig. 4B). There was a trend toward better OS in advanced-stage patients with radiotherapy for the bone involved compared with patients without radiotherapy (P = .09), but the difference did not reach statistical significance (Fig. 4C). Using Cox regression for multivariate analysis, elevated LDH level and age above 60 were independent unfavorable prognostic factors (Table 4).
Table 3.
Univariate analysis of prognostic factors for patients with bone involvement.

Figure 3.

Overall survival according to (A) stage, (B) International Prognostic Index, (C) serum lactate dehydrogenase (LDH), and (D) age for all patients with skeletal involvement.
Figure 4.

Overall survival according to radiotherapy (RT) to the bone involved for all patients (A), limited-stage (B), and advanced-stage patients (C).
Table 4.
Multivariate analysis of prognostic factors for patients with bone involvement.

4. Discussion
This is a retrospective analysis of lymphoma patients with bone involvement, including primary and secondary bone lymphoma. Secondary bone involvement by systemic lymphoma is more common than primary bone lymphomas. The exact incidence of primary bone lymphomas is difficult to define, but it appears to account for approximately 5% of extranodal lymphomas, <1% of all NHL, and approximately 1% of Hodgkin lymphoma.[4] Our data also confirm that primary bone lymphoma is extremely rare, in contrast to secondary bone lymphoma. There are only 9 primary bone lymphoma patients, accounting for 8.26% of the patients in this study. Although DLBCL was the most common histologic subtype in our study (44%), the percentage was lower than what was previously reported (70–80%).[5] Anaplastic large-cell lymphoma (ALCL) was the most common in our T-NHL subgroup. The international incidence of ALCL is similar to that in China.[6,7] Extranodal natural killer (NK)/T-cell lymphoma is more prevalent in Asians than in Western populations,[8–10] and it is the 2nd most common lymphoma in China,[7] accounting for more than 10% of all cases of NHL. In our data, extranodal NK/T-cell lymphoma, accounting for 5%, was the 2nd most common in the T-NHL subgroup.
In agreement with previous studies,[5] a male predominance was found, especially in the T-NHL subgroup, with a male/female ratio of 1.7. The median age was 53 years in the B-NHL group, compared with 32 to 33 years in the other groups.
In many primary bone lymphoma studies,[11–13] the most frequent site of bone involved is the femur, a feature that was observed in a small number of primary solitary bone cases in our study. However, spine was the most common site of bone infiltration with multifocal lesions.
By analyzing the 5-year OS of these 3 subgroups, we found that the 5-year OS rates for the cHL and T-NHL groups were both superior to that for the B-NHL group. One reason is that the B-NHL subgroup was a composite population, including patients of different degrees of aggression and pathologic type. The other reason is that most patients in this group had advanced-stage disease. The 5-year OS for T-NHL is better than that for many types of T-cell lymphoma in the literature,[6] which may be due to the fact that the largest proportion in our study was anaplastic cell lymphoma, and ALK-positive anaplastic cell lymphoma has the best prognosis of T-cell lymphoma. Given the small number of primary bone lymphoma cases and the shorter median follow-up times, there were only 3 years of OS data, which were slightly shorter than in previous literature, but the OS was also better than that of secondary bone lymphoma.[14–17]
Stage of disease in our data is defined according to the Ann Arbor staging system. In our univariate analyses of prognostic factors, advanced stage and high IPI score predicted a poorer outcome, consistent with many other investigations. In this study, many fewer patients had limited stage and lower IPI scores than patients with high tumor burdens and disseminated disease. For DLBCL of the bone, the International Extranodal Lymphoma Study Group (IELSG) has proposed the IELSG staging system[18]: stage IE = single bony lesion, IIE = single bony lesion plus regional lymphadenopathy, IVE = polyostotic lymphoma, and IV = conventional stage IV lymphoma with skeleton involvement. This staging system is based on the different prognoses of the primary bone lymphomas. Distinct prognostic indexes were applied in different pathologic types of lymphoma. However, it appears that there is no prognostic index consensus for primary or secondary bone lymphomas.
Elevated LDH level was one of independent unfavorable prognostic factors in multivariate analysis. In our univariate analysis, it is considered to be an unfavorable prognostic factor in the B-NHL and T-NHL groups, but not in the cHL group. Although the number of cHL patients was relatively small, with only 5 patients presenting elevated LDH levels, the result is also consistent with the international prognostic factor of classic HL.[19] Elevated LDH level is included neither in the risk factors for early stage HL nor in the International Prognostic Score for advanced-stage HL. However, an analysis of 137 patients with HL suggests that LDH is the most important prognostic factor for HL.[20] A previous investigation suggested that normal values of LDH were associated with a better response to therapy and longer survival, independent of histologic type and clinical stage in NHL.[21] LDH levels also correlate with survival in patients with bone malignancies[22] or bone metastases[23] from solid cancers, such as breast cancer.
Age over 60 years was another independent unfavorable prognostic factors in our multivariate analysis. Age impacts on prognosis of patients in at least 2 ways: on one hand, it is intrinsically associated with patients’ genetic changes, and on the other hand, older age often is associated with co-morbidity and reduced tolerability of chemotherapeutic regimens used in younger patients.
For early stage primary bone lymphoma, the therapeutic strategy of chemotherapy followed by radiotherapy was suggested to be the standard approach,[11,24–26] and it has demonstrated its superiority compared with single therapeutic approaches. Our study was consistent with many more patients having secondary bone involvement by systemic lymphoma. Another finding of our univariate analyses is the better survival of patients with skeletal disease who received additive radiotherapy to the sites involved, especially in the B-NHL group. There was a trend toward better OS in advanced-stage patients. In the rituximab era, the role of radiotherapy appears to be diminishing. For patients with diffuse large B-cell lymphoma, the combined data of the MabThera International Trial (MInT) for young good-prognosis patients and the Rituximab With CHOP Over 60 Years (RICOVER-60) suggest a beneficial effect of radiotherapy to sites of skeletal involvement, although rituximab failed to improve the outcome of DLBCL patients with skeletal involvement.[27] The clinical benefit of irradiation of involved bone in patients with disseminated lymphoma has been questioned. The major reason for avoiding radiotherapy is likely the risk of late effects from this treatment modality, especially delayed bone growth in children and development of a 2nd cancer within a previously irradiated field.[28,29] Given the limitation of our single-center experience, the clinical and prognostic significance of radiotherapy for the bone involved warrants further clarification in large, multicenter, prospective studies.
In summary, this retrospective study describes our single-center experience with 109 lymphoma patients with bone involvement. We found that DLBCL was the most common histologic subtype and confirmed that primary bone lymphoma was extremely rare. Elevated LDH level was the only one independent unfavorable prognostic factor according to multivariate analysis. Additive radiotherapy to the sites of bone involved may improve survival for advanced-stage patients. Given the limitation of the single-center experience, the effect of additive radiotherapy needs further multicenter, prospective studies.
Author contributions
Conceptualization: Gang Wu, Liling Zhang.
Data curation: Yin Xiao, Fang Zhu, Tao Liu, Qiuhui Li, Juan Li.
Formal analysis: Xiaoqian Li.
Funding acquisition: Yin Xiao, Liling Zhang.
Investigation: Yin Xiao, Fang Zhu, Tao Liu, Qiuhui Li, Juan Li.
Methodology: Yin Xiao, Xiaoqian Li.
Project administration: Juan Li, Liling Zhang.
Resources: Fang Zhu, Tao Liu, Qiuhui Li.
Supervision: Gang Wu, Liling Zhang.
Validation: Gang Wu, Liling Zhang.
Writing – original draft: Yin Xiao.
Writing – review & editing: Liling Zhang.
Footnotes
Abbreviations: ABVD = doxorubicin, bleomycin, vincristine, dacarbazine, ALCL = anaplastic large-cell lymphoma, B-NHL = B-cell non-Hodgkin lymphoma, cHL = classic Hodgkin lymphoma, CHOP = cyclophosphamide, doxorubicin, vincristine, prednisone, CT = computed tomography, DLBCL = diffuse large B-cell lymphoma, IELSG = International Extranodal Lymphoma Study Group, IPI = International Prognostic Index, LDH = lactate dehydrogenase, NHL = non-Hodgkin lymphoma, OS = overall survival, PET/CT = positron emission tomography/computed tomography, T-NHL = T-cell non-Hodgkin lymphoma.
This research was supported by grants from the National Natural Science Foundation of China (Nos. 81300412, 81672940) and the Clinical Research Physician Program of Tongji Medical College, Huazhong University of Science and Technology (No. 5001530053).
The authors have no conflicts of interest to disclose.
References
- [1].Ostrowski ML, Unni KK, Banks PM, et al. Malignant lymphoma of bone. Cancer 1986;58:2646–55. [DOI] [PubMed] [Google Scholar]
- [2].Lim CY, Ong KO. Imaging of musculoskeletal lymphoma. Cancer Imaging 2013;13:448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–86. [DOI] [PubMed] [Google Scholar]
- [4].Jain A, Alam K, Maheshwari V, et al. Primary bone lymphomas-Clinical cases and review of literature. J Bone Oncol 2013;2:132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Messina C, Christie D, Zucca E, et al. Primary and secondary bone lymphomas. Cancer Treat Rev 2015;41:235–46. [DOI] [PubMed] [Google Scholar]
- [6].Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008;26:4124–30. [DOI] [PubMed] [Google Scholar]
- [7].Sun J, Yang Q, Lu Z, et al. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol 2012;138:429–34. [DOI] [PubMed] [Google Scholar]
- [8].Macon WR. Peripheral T-cell lymphomas. Hematol Oncol Clin North Am 2009;23:829–42. [DOI] [PubMed] [Google Scholar]
- [9].Barrionuevo C, Zaharia M, Martinez MT, et al. Extranodal NK/T-cell lymphoma, nasal type: study of clinicopathologic and prognosis factors in a series of 78 cases from Peru. Appl Immunohistochem Mol Morphol 2007;15:38–44. [DOI] [PubMed] [Google Scholar]
- [10].Gualco G, Domeny-Duarte P, Chioato L, et al. Clinicopathologic and molecular features of 122 Brazilian cases of nodal and extranodal NK/T-cell lymphoma, nasal type, with EBV subtyping analysis. Am J Surg Pathol 2011;35:1195–203. [DOI] [PubMed] [Google Scholar]
- [11].Beal K, Allen L, Yahalom J. Primary bone lymphoma: treatment results and prognostic factors with long-term follow-up of 82 patients. Cancer 2006;106:2652–6. [DOI] [PubMed] [Google Scholar]
- [12].Heyning FH, Hogendoorn PC, Kramer MH, et al. Primary lymphoma of bone: extranodal lymphoma with favourable survival independent of germinal centre, post-germinal centre or indeterminate phenotype. J Clin Pathol 2009;62:820–4. [DOI] [PubMed] [Google Scholar]
- [13].Marshall DT, Amdur RJ, Scarborough MT, et al. Stage IE primary non-Hodgkin's lymphoma of bone. Clin Orthop Relat Res 2002;216–22. [PubMed] [Google Scholar]
- [14].Demircay E, Hornicek FJ, Mankin HJ, et al. Malignant lymphoma of bone: a review of 119 patients. Clin Orthop Relat Res 2013;471:2684–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Messina C, Ferreri AJ, Govi S, et al. Clinical features, management and prognosis of multifocal primary bone lymphoma: a retrospective study of the international extranodal lymphoma study group (the IELSG 14 study). Br J Haematol 2014;164:834–40. [DOI] [PubMed] [Google Scholar]
- [16].Govi S, Christie D, Mappa S, et al. The clinical features, management and prognosis of primary and secondary indolent lymphoma of the bone: a retrospective study of the International Extranodal Lymphoma Study Group (IELSG #14 study). Leuk Lymphoma 2014;55:1796–9. [DOI] [PubMed] [Google Scholar]
- [17].Bruno VM, Ferreri AJ, Gospodarowicz M, et al. Clinical features, management, and prognosis of an international series of 161 patients with limited-stage diffuse large B-cell lymphoma of the bone (the IELSG-14 study). Oncologist 2014;19:291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vitolo U, Seymour JF, Martelli M, et al. Extranodal diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27Suppl 5:v91–102. [DOI] [PubMed] [Google Scholar]
- [19].Cuccaro A, Bartolomei F, Cupelli E, et al. Prognostic factors in Hodgkin lymphoma. Mediterr J Hematol Infect Dis 2014;6:e2014053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Garcia R, Hernandez JM, Caballero MD, et al. Serum lactate dehydrogenase level as a prognostic factor in Hodgkin's disease. Br J Cancer 1993;68:1227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Endrizzi L, Fiorentino MV, Salvagno L, et al. Serum lactate dehydrogenase (LDH) as a prognostic index for non-Hodgkin's lymphoma. Eur J Cancer Clin Oncol 1982;18:945–9. [DOI] [PubMed] [Google Scholar]
- [22].Li S, Yang Q, Wang H, et al. Prognostic significance of serum lactate dehydrogenase levels in Ewing's sarcoma: a meta-analysis. Mol Clin Oncol 2016;5:832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brown JE, Cook RJ, Lipton A, et al. Serum lactate dehydrogenase is prognostic for survival in patients with bone metastases from breast cancer: a retrospective analysis in bisphosphonate-treated patients. Clin Cancer Res 2012;18:6348–55. [DOI] [PubMed] [Google Scholar]
- [24].Zinzani PL, Carrillo G, Ascani S, et al. Primary bone lymphoma: experience with 52 patients. Haematologica 2003;88:280–5. [PubMed] [Google Scholar]
- [25].Catlett JP, Williams SA, O’Connor SC, et al. Primary lymphoma of bone: an institutional experience. Leuk Lymphoma 2008;49:2125–32. [DOI] [PubMed] [Google Scholar]
- [26].Mendenhall NP, Jones JJ, Kramer BS, et al. The management of primary lymphoma of bone. Radiother Oncol 1987;9:137–45. [DOI] [PubMed] [Google Scholar]
- [27].Held G, Zeynalova S, Murawski N, et al. Impact of rituximab and radiotherapy on outcome of patients with aggressive B-cell lymphoma and skeletal involvement. J Clin Oncol 2013;31:4115–22. [DOI] [PubMed] [Google Scholar]
- [28].Probert JC, Parker BR. The effects of radiation therapy on bone growth. Radiology 1975;114:155–62. [DOI] [PubMed] [Google Scholar]
- [29].Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin's lymphoma. N Engl J Med 2015;373:2499–511. [DOI] [PubMed] [Google Scholar]


