Abstract
Ulcerative colitis (UC) is a chronic inflammatory process that is occasionally associated with complications that cause significant morbidity and mortality. Studies in experimental animal models have demonstrated a beneficial effect of cannabis on intestinal inflammation. It is however unknown if this corresponds to fewer complications for patients with Ulcerative Colitis.
We aimed to compare the prevalence of UC related complications and certain key clinical endpoints among cannabis users and nonusers hospitalized with a primary diagnosis of UC, or primary diagnosis of a UC-related complication with a secondary diagnosis of UC.
Using data from the Healthcare Cost and Utilization Project-National Inpatient Sample (NIS) during 2010–2014, a total of 298 cannabis users with UC were compared to a propensity score matched group of nonusers with UC. We evaluated several UC-related complications and clinical endpoints.
Within our matched cohort, prevalence of partial or total colectomy was lower in cannabis users compared to nonusers (4.4% vs 9.7%, P = .010) and there was a trend toward a lower prevalence of bowel obstruction (6.4% vs 10.7%, P = .057). Cannabis users had shorter hospital length-of-stay (4.5 vs 5.7 days P < .007) compared to their nonuser counterparts.
Cannabis use may mitigate some of the well described complications of UC among hospitalized patients. Our findings need further evaluation, ideally through more rigorous clinical trials.
Keywords: cannabis, complications, inflammatory bowel disease, ulcerative colitis
1. Introduction
Ulcerative colitis (UC) is a chronic, relapsing, immune-mediated disorder that affects mainly the colon, causing abdominal pain, bloody diarrhea, and weight loss.[1] The estimated prevalence of UC is about 263 cases per 100,000 persons among adults and 34 cases per 100,000 persons among children and adolescents (<20 years).[2] The cost of inpatient care is a significant contributor to the overall health care costs for UC patients. Between 1998 and 2004 total inpatient charges attributable to UC within the United States rose from $592 to $945 million per year.[3]
While the definite cause of UC remains unknown, literature converges on the idea that the disease process is as a result of a combination of genetic and environmental factors, as well as a dysregulation of the intestinal immune system.[4,5] Conventional therapy for UC involves the use of 5-aminosalicylic acid, corticosteroids, azathioprine, 6-mercaptopurine, antitumor necrosis factor agents, cyclosporine, and other more recently approved agents (vedolizumab and tofacitinib).[6,7] While conventional treatments may be effective in maintaining remission, these treatments have side effects and a significant proportion of patients with UC are resistant to biologic therapy or high doses of standard therapies and patients often look for other alternative treatment.[8]
As of November 2016, more than 50% of states in the United States allowed physicians to prescribe cannabis to patients.[9] Also, population-based studies show that the likelihood of cannabis use is higher among individuals with IBD compared to their counterparts without IBD.[10] Together, broad interest in cannabis as an alternative treatment option for IBD and availability of cannabis legally in the United States highlight the need to better understand the effects of cannabis use on UC outcomes. Thus, we analyzed data from the National Inpatient Sample database to evaluate the association between cannabis use and complications related to UC.
2. Methods
2.1. Cohort and variables
The present study is a population-based cohort study based on the healthcare cost and utilization project's (HCUP) National Inpatient Sample (NIS) dataset. We extracted five years of data, calendar years 2010 through 2014. The NIS is a yearly survey of 20% of total admissions from more than 4000 hospitals across over 30 states in the United States and the District of Columbia. The NIS has been validated in several studies to provide reliable estimates of disease and comorbidity prevalence among inpatient admissions in the United States.[11]
In the present study, we analyzed the inpatient data for a cohort of patients with UC by either primary diagnosis code: 556.x; or well-documented complications of UC (i.e., intestinal perforation, active fistulizing disease and intraabdominal abscess, bowel obstruction, anemia, malnutrition, gastrointestinal hemorrhage, and dehydration), and a secondary diagnosis of UC (ICD9 codes as in Supplementary Table S1). We also compared the average duration of hospital stay among cannabis users and nonusers. The same criteria for Ulcerative Colitis disease cohort have been used in prior studies, albeit with different clinical outcomes.[12] Cannabis use was defined by ICD-9-CM codes 304.3, 304.3x, and 305.2x: as either mild (nondependent use) or moderate/severe (dependent use), which has also been used in previous studies.[13] To minimize potential confounders, we excluded patients with a diagnosis of abuse of other substances including opioid, amphetamine, psychostimulants, cocaine, sedative, antidepressant, and hallucinogens while retaining patients that use tobacco and alcohol. We retained tobacco and alcohol use to assess the effect between these common substances and cannabis use in modulating UC related complications. All missing variables were excluded.
For each dataset, we extracted demographic factors (gender, age, race), hospital-level characteristics (hospital size, teaching status [teaching vs nonteaching] and geographic location [region of the United States and rural vs urban]), and health insurance and income status. Comorbidity burden was collected and quantified using the Elixhauser comorbidity index.[14] Our 1:1 propensity-score match used the nearest neighbor nonreplacement matching method.[15] We assessed match success by performing chi-square test for categorical variables and a paired t test for normally distributed continuous variables (Table 1). We assessed the association between cannabis use and the clinical outcomes related to ulcerative colitis disease (Table 2).
Table 1.
Descriptive statistics of patients admitted with ulcerative colitis disease and propensity score match.
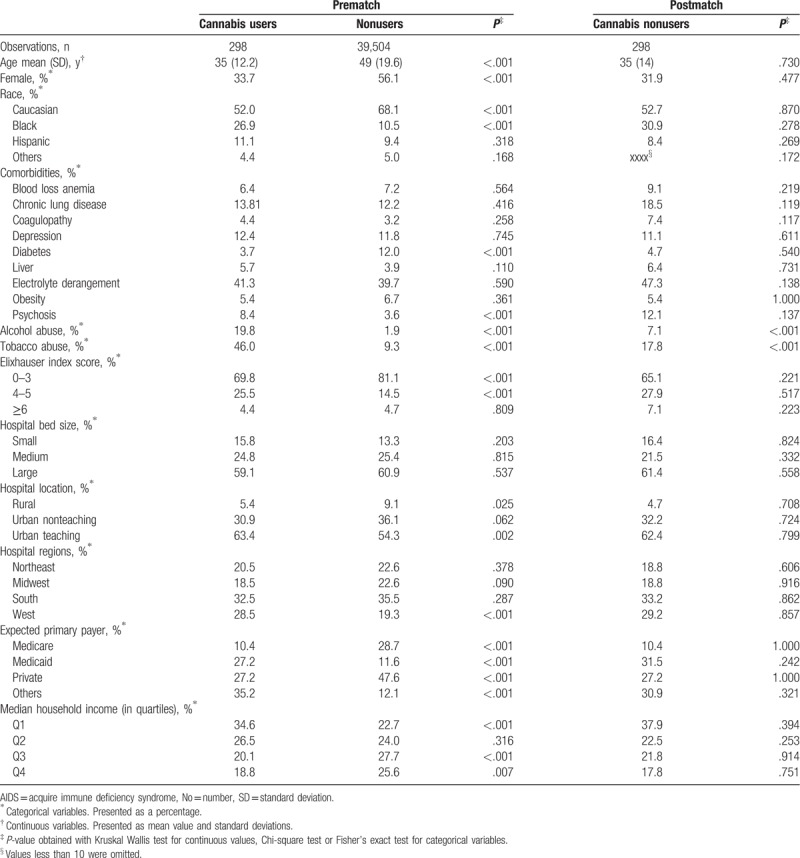
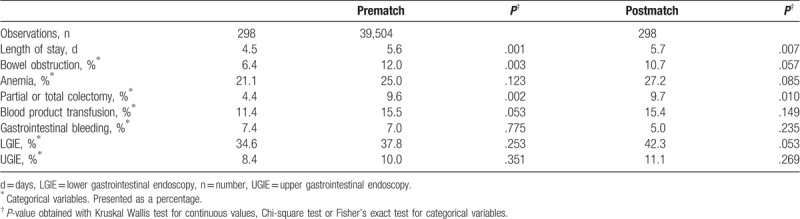
Table 2.
Clinical outcomes of patients admitted with ulcerative colitis disease and propensity score match.
To evaluate the statistical significance of differences in the above clinical end-points after adjusting for additional variables, we built forward stepwise multivariable logistic regression models to establish adjusted odds ratios for cannabis use on the rate of anemia, stricturing bowel disease, bowel obstruction, anemia, transfusion of blood products, parenteral nutrition requirement, small bowel resection, small bowel anastomosis, partial or total colectomy, lower GI endoscopy, upper GI endoscopy, and abdominal CT. The propensity to use cannabis was estimated by building models that included patient and group-level variables, (e.g., age, sex, gender, comorbidities; and hospital size and location). All statistical analyses were performed using STATA (version 14.0, College Station, TX). We considered P-values of < .05 to be statistically significant.
The data was extracted and reviewed for accuracy by two separate authors (MC and YW), also, as the NIS is a publicly available deidentified dataset, IRB approval was not required.
3. Results
3.1. Cohort characteristic and direct comparison
The sample population consisted of a total of 39,802 adult patients among which 298 (0.7%) were coded as cannabis users.
Compared to patients without recognized cannabis use, cannabis users were significantly younger (mean age 35 years vs 49 years, P < .001), more likely male (66.3% vs 43.9%, P < .001), African American (26.9% vs 10.5%, P < .001), in the lowest quartile of median household income (34.6% vs 22.7%, P < .001), and more likely to use alcohol (19.8% vs 1.9%, P < .001), and tobacco (46.0% vs 9.3%, P < .001) (Table 1). Comparison of hospital characteristics revealed significant differences between users and nonusers. Cannabis users were more common at urban-teaching hospitals (63.4% vs 54.3%, P = .002), more likely to have had Medicaid as their expected primary payer (27.2% vs 11.6%, P < .001) and less likely to list private insurance (27.2% vs 47.6% P < .001) and Medicare (10.4% vs 28.7% P < .001) as their expected primary payer. A direct comparison of co-morbidity profiles between users and nonusers showed a significantly lower prevalence of selected disease among cannabis users including congestive heart failure, diabetes, hypothyroidism, neurologic disease, peripheral vascular disease, pulmonary circulation disorder, renal failure, tumors, and valvular heart disease but a significantly higher rate of concurrent psychiatric diseases.
When we evaluated clinical end-points among unmatched sample, we found that among cannabis users there were lower rates of bowel obstruction (6.4% vs 12.0%, P = .003), and partial or total colectomy (4.4% vs 9.6%, P = .002). Cannabis users also had shorter hospital lengths of stay (4.5 days vs 5.6 days, P < .001).
3.2. Propensity score match and postmatch
The propensity score was calculated using multivariable logistic regression models that included 37 hospitals; clinical and demographic covariates, and Elixhauser comorbidity index were entered into the model to generate a probability for cannabis use among the study population. After matching by propensity score, the populations were similar (i.e., no statistically significant differences) for the matched cohort for most variables (Table 1). For the propensity-score analyses, we found that, similar to our analysis on the full cohort, cannabis use was associated with a significantly lower prevalence of partial or total colectomy (4.41% vs 9.7%, P = .010) and on average shorter hospital lengths of stay (4.5 days vs 5.7 days, P < .007). There was a trend towards lower prevalence of bowel obstruction and lower GI endoscopy requirement (Table 2).
In the postmatch univariate and multivariable logistic regression analysis, cannabis use remained associated with a reduced prevalence of colectomy (Table 3 and Fig. 1).
Table 3.
Univariate logistic regression of clinical outcomes.
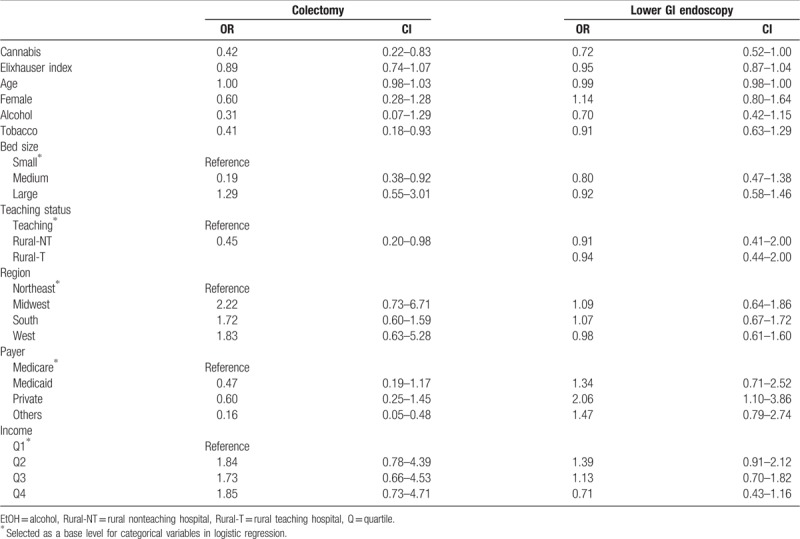
Figure 1.
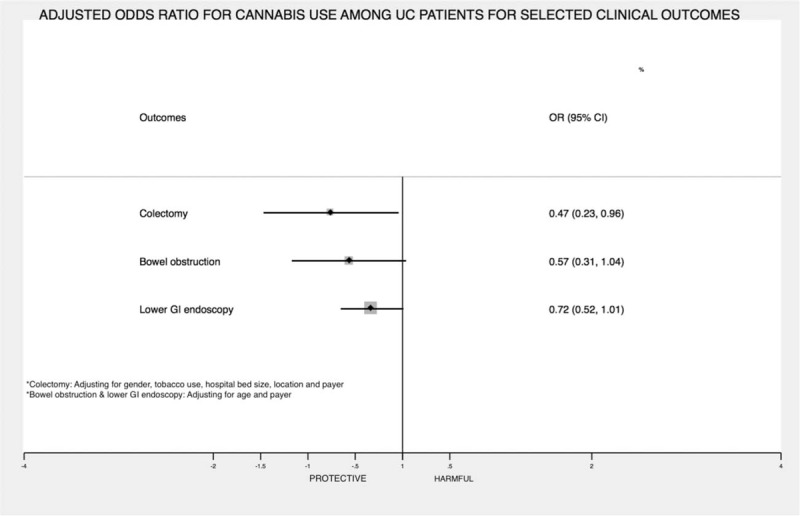
XXX.
4. Discussion
The present study is the first, large-scale nationwide cohort study to evaluate the association between cannabis use and clinical outcomes in hospitalized patients with UC. We found that amongst matched cohorts, cannabis users had a lower prevalence of partial or total colectomies and there was a trend towards lower prevalence of bowel obstruction. Cannabis users also had significantly shorter hospital length of stay compared to noncannabis users.
The efficacy of cannabis for the symptomatic relief of UC is still controversial. A study by Lahat et al reported statistically significant improvement in general health perception, social functioning, ability to work, physical pain, depression, and average Harvey-Bradshaw index for patients with IBD following three months treatment with inhaled cannabis.[16] However, it is unclear whether these benefits are a result of attenuation of the disease process or due to the psychotropic effect of cannabis.
Other studies have shown that activation of enteric cannabinoid (CBD) receptors may reduce colonic motility and propulsion,[17] promote wound healing,[18] and be protective against excessive inflammation in the colon.[19,20] These findings are supported by a recent randomized control trial which demonstrated, clinical and endoscopic improvements among UC patients on cannabis compared to placebo.[21] Conversely, another recent randomized control trial suggested that while there were significant improvements in subject global impression of change scores among trial participants on cannabinoid (CBD)-rich botanical extracts compared to patients on the placebo, remission rates were similar for patients on CBD-rich botanical extracts and placebo.[22]
In clinical practice, colectomies are occasionally indicated for toxic megacolon, perforation, and severe colorectal bleeding. Also, colectomies may be indicated when there is failure of medical management with intractable symptoms, and less commonly with intestinal strictures.[23,24] It is therefore plausible that the lower prevalence of partial or total colectomy seen amongst cannabis users could possibly be due to fewer complications such as bowel obstruction, while shorter length of stay could possibly reflect a less severe exacerbation amongst cannabis users.
While Cannabis use may be associated with potential benefit in patients with UC, several side effects have also been associated with the use of cannabis ranging from mild side effects like nausea and vomiting[25–28] to more serious side effects like postural hypotension, delirium, psychosis, and seizure.[29,30] Cannabis use is also associated with the use of other illicit drugs and this raises potential public health concerns. In order to reduce the risk of most of the adverse effects of cannabis, CB2 receptors could be a potential target as most side effects of cannabis are due to activation of CB1 receptors in the brain.[31] This creates an opportunity for directed therapy targeting CB2 receptors while maintaining its potential anti-inflammatory effect in colitis.
While previous research has been limited in terms of sample size, this study used a large database of hospitalized patients in the US. Also, cannabis users are likely abusers of other substances which could have influenced the outcomes of prior studies. However, in this study, we excluded patients with other drug abuse to avoid interference in our result. Nevertheless, our study has limitations. First, we couldn’t control for the time of diagnosis of ulcerative colitis, the severity of the disease, extent of the disease, historical and ongoing immunomodulator, or biologic therapy. Second, the NIS data is only generalizable to hospitalized populations of the United States and lack postdischarge follow up. Third, some data could have inaccurate coding as demonstrated in other administrative databases. Particularly, there could be under coding of cannabis use, due to which the prevalence of cannabis use is much lower in our population as compared to other studies.[32,33] Despite the mentioned limitations, we believe the large sample, our rigorous methodology, and the scientific rationale adds to the current literature on cannabis use in patients with UC. It should be emphasized that our study lacks data regarding cannabis consumption route, amount, frequency and side-effects related to cannabis use. Therefore, we recommend cautious interpretation of our results until they can be validated through prospective randomized studies.
In summary, our study suggests that cannabis use may mitigate some complications of UC among hospital inpatients and this could be due to an antiinflammatory effect of cannabis and potential improvement in gastrointestinal mucosal healing. Our study has important clinical findings and warrants further investigations.
Author contributions
Conceptualization: Bashar Attar, Wang Yuchen, Isaac Paintsil, Mathew Madhu, Oyintayo Ajiboye, Simons-Linares C. Roberto, William E. Trick, Vikram Kotwal.
Data curation: Wang Yuchen.
Formal analysis: Chimezie Mbachi, Wang Yuchen.
Methodology: Wang Yuchen, William E. Trick, Vikram Kotwal.
Software: Wang Yuchen.
Supervision: Bashar Attar, Wang Yuchen, Isaac Paintsil, Mathew Madhu, Simons-Linares C. Roberto, William E. Trick, Vikram Kotwal.
Visualization: Simons-Linares C. Roberto.
Writing – original draft: Chimezie Mbachi, Bashar Attar, Olamide Oyenubi, Wang Yuchen, Isaac Paintsil, Mathew Madhu, Oyintayo Ajiboye, Simons-Linares C. Roberto, Vikram Kotwal.
Writing – review & editing: Chimezie Mbachi, Olamide Oyenubi, Wang Yuchen, Aisien Efesomwan, Isaac Paintsil, Oyintayo Ajiboye, William E. Trick, Vikram Kotwal.
Chimezie Mbachi orcid: 0000-0002-4046-282X.
Footnotes
Abbreviations: CBD = cannabinoid, HCUP = health care utilization project, ICD = international classification of disease, NIS = national inpatient sample, THC = tetrahydro cannabinoid, UC = ulcerative colitis, USA = United States of America.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Rembacken B, Snelling A, Hawkey P, et al. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 1999;354:635–9. [DOI] [PubMed] [Google Scholar]
- [2].Kappelman MD, Moore KR, Allen JK, et al. Recent trends in the prevalence of Crohn's disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci 2013;58:519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nguyen GC, Tuskey A, Dassopoulos T, et al. Rising hospitalization rates for inflammatory bowel disease in the United States between 1998 and 2004. Inflamm Bowel Dis 2007;13:1529–35. [DOI] [PubMed] [Google Scholar]
- [4].Rutgeerts P, Geboes K. Understanding inflammatory bowel disease—the clinician's perspective. Eur J Surg Suppl 2001;66–72. [DOI] [PubMed] [Google Scholar]
- [5].Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011;474:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American college of gastroenterology, practice parameters committee. Am J Gastroenterol 2010;105:501. [DOI] [PubMed] [Google Scholar]
- [7].Novak G, Hindryckx P, Khanna R, et al. The safety of vedolizumab for the treatment of ulcerative colitis. Expert opin Drug Saf 2017;16:501–7. [DOI] [PubMed] [Google Scholar]
- [8].Ng SC, Kamm MA. Therapeutic strategies for the management of ulcerative colitis. Inflamm Bowel Dis 2008;15:935–50. [DOI] [PubMed] [Google Scholar]
- [9].Kilmer B. Recreational cannabis—minimizing the health risks from legalization. N Engl J Med 2017;376:705–7. [DOI] [PubMed] [Google Scholar]
- [10].Weiss A, Friedenberg F. Patterns of cannabis use in patients with inflammatory bowel disease: a population based analysis. Drug Alcohol Depend 2015;156:84–9. [DOI] [PubMed] [Google Scholar]
- [11].Adegbala O, Martin KD, Otuada D, et al. Diabetes mellitus with chronic complications in relation to carotid endarterectomy and carotid artery stenting outcomes. J Stroke Cerebrovasc Dis 2017;26:217–24. [DOI] [PubMed] [Google Scholar]
- [12].Micic D, Gaetano JN, Rubin JN, et al. Factors associated with readmission to the hospital within 30 days in patients with inflammatory bowel disease. PLoS One 2017;12:e0182900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rumalla K, Reddy AY, Mittal MK. Association of recreational marijuana use with aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 2016;25:452–60. [DOI] [PubMed] [Google Scholar]
- [14].Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- [15].Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lahat A, Lang A, Ben-Horin S. Impact of cannabis treatment on the quality of life, weight and clinical disease activity in inflammatory bowel disease patients: a pilot prospective study. Digestion 2012;85:1–8. [DOI] [PubMed] [Google Scholar]
- [17].Pinto L, Izzo AA, Mascolo N, et al. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology 2002;123:227–34. [DOI] [PubMed] [Google Scholar]
- [18].Wright K, Rooney N, Feeney M, et al. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology 2005;129:437–53. [DOI] [PubMed] [Google Scholar]
- [19].Massa F, Marsicano G, Hermann H, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest 2004;113:1202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Maccarrone M, Bab I, Bíró T, et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci 2015;36:277–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Naftali T, Bar Lev Schlieder L, Sklerovsky Benjaminov F, et al. P398 Cannabis induces clinical and endoscopic improvement in moderately active ulcerative colitis (UC). J Crohn's Colitis 2018;12Suppl 1:S306–1306. [Google Scholar]
- [22].Irving PM, Iqbal T, Nwokolo C, et al. A randomized, double-blind, placebo-controlled, parallel-group, pilot study of cannabidiol-rich botanical extract in the symptomatic treatment of ulcerative colitis. Inflamm Bowel Dis 2018;24:714–24. [DOI] [PubMed] [Google Scholar]
- [23].Andersson P, Söderholm JD. Surgery in ulcerative colitis: indication and timing. Dige Dis 2009;27:335–40. [DOI] [PubMed] [Google Scholar]
- [24].Hwang JM, Varma MG. Surgery for inflammatory bowel disease. World J Gastroenterol 2008;14:2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers 2007;4:1770–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry 2001;178:101–6. [DOI] [PubMed] [Google Scholar]
- [27].Adams IB, Martin BR. Cannabis: pharmacology and toxicology in animals and humans. Addiction 1996;91:1585–614. [PubMed] [Google Scholar]
- [28].Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 2003;42:327–60. [DOI] [PubMed] [Google Scholar]
- [29].Dow GJ, Meyers FH, Stanton W, et al. Serious reactions to oral delta-9-tetrahydrocannabinol in cancer chemotherapy patients. Clin Pharm 1984;3:14. [PubMed] [Google Scholar]
- [30].Devine ML, Dow GJ, Greenberg BR, et al. Adverse reactions to delta-9-tetrahydrocannabinol given as an antiemetic in a multicenter study. Clin Pharm 1987;6:319–22. [PubMed] [Google Scholar]
- [31].Izzo AA, Borrelli F, Capasso R, et al. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci 2009;30:515–27. [DOI] [PubMed] [Google Scholar]
- [32].Merikangas KR, McClair VL. Epidemiology of substance use disorders. Hum Genet 2012;131:779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ravikoff Allegretti J, Courtwright A, Lucci M, et al. Marijuana use patterns among patients with inflammatory bowel disease. Inflamm Bowel Dis 2013;19:2809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


