Abstract
Failure in evaluation of smoke inhalation injury (SII) is related to increased morbidity and mortality. Prognostic biomarkers that reflect the injury are undoubtedly needed. Cell-free DNA (CFD) concentrations are associated to the extent of tissue damage and inflammation in various pathologies. We have developed a simple assay for CFD quantification and previously found it prognostic in various pathologies including burns, lung disease, and sepsis. The aim of this study was to evaluate admission CFD as an injury severity marker in patients with SII.
In a prospective study, we measured admission CFD levels in 18 SII patients and matched control subjects. Daily CFD levels were also performed in 4 hospitalized patients. Serum CFD levels were measured by our direct rapid fluorometric assay.
Admission CFD levels of SII patients were significantly higher than those of healthy controls, 879 (236–3220) ng/mL vs. 339 (150–570) ng/mL, [median (range)], P < .0001. Admission CFD levels of hospitalized patients were significantly higher than those of nonhospitalized patients, 1517 (655–3220) ng/mL vs. 675 (236–1581) ng/mL, P < .05. Admission CFD positively correlated with hospitalization time (Rho = 0.578, P < .05) and was in linear correlation with CO poisoning (carboxyhemoglobin (COHb) levels, R2 = 0.621, P < .0001). Additionally, along with the recovery of hospitalized patients, we observed a matched reduction of CFD levels.
CFD appears to be a potentially valuable marker for severity and follow-up of SII. We believe this rapid assay can help introduce the routine use of CFD measurement into daily practice.
Keywords: biomarker, carboxyhemoglobin, circulating cell free DNA, smoke inhalation injury
1. Introduction
Smoke inhalation is the leading cause of death in the event of a fire.[1,2] Injury is usually a result of several mechanisms including thermal injury to the upper airway, irritation or chemical damage to the airways from soot, choking, and toxicity from carbon monoxide and other gases. Injury from smoke inhalation is mostly expressed as direct damage to exposed epithelial surfaces, and may result in inflammation of the conjunctiva, corneal edema, rhinorrhea, pharyngitis, tracheitis, bronchitis, inflammation of the alveoli, and pneumonia. Systemic absorption of toxins may also occur. Respiratory insufficiency is likely the result of direct damage to lung tissue combined with the inflammatory response that accompanies injury.[1]
Several morbidity and mortality indicators have been identified for burns, the strongest of which is the proportion of total body surface area (TBSA) burned.[2,3] An additional prognostic marker is the presence of smoke inhalation injury (SII), which increases mortality by nearly 24 times.[1] In most cases, the initial diagnosis of the extent of SII and subsequent treatment relies on limited clinical tools. Fiber-optic bronchoscopy is commonly used to identify inhalation injury in the supraglottic and infra-glottic airway.[4] Higher plasma carboxyhemoglobin (COHb) levels in combination with greater airway neutrophilia and cytokine release have been suggested for grading the severity of SII.[5] The difficulty in reliably grading the severity of SII is at least partly related to increased morbidity and mortality in burn patients.[1] Therefore, useful biomarkers that directly reflect the primary injury and secondary damage from inflammation are needed.
Several studies have shown elevated concentrations of circulating cell free DNA (CFD) in burn patients and correlated to the affected TBSA and duration of hospitalization.[6–8] Fox et al. suggested using CFD as a direct marker of cellular injury, which can provide a single, objective evaluation of the severity of the burn injury as part of a global pathological process.[9] Chiu et al. found that CFD levels were elevated in burn patients and related to hospitalization length and to the TBSA burned.[6] In our previously published work, we found a strong correlation between TBSA and CFD levels and an even stronger correlation between Total Burn Volume (TBSA × burn degree) and CFD levels.[8] In various pathologies, including lung diseases, elevated CFD concentrations are associated to necrosis and apoptosis.[10–13] In addition, it is now clear that degradation of DNA from neutrophil extracellular traps (NETs) significantly contributes to elevation of CFD.[12] “NETosis” denotes neutrophil cell death in inflamed tissue and formation of NETs, an active trap for bacteria composed of DNA filaments and antibacterial protein.[14] NETs production is associated to acute and chronic lung diseases[15,16] and it has been shown that host DNA released by NETosis itself promotes allergic asthma exacerbation.[12] Increasing evidence suggests that NETosis also occurs in noninfectious, sterile inflammation and plays an important role in autoimmunity, coagulation, acute injuries, and cancer.[14]
We have developed a simple, fast, and reliable fluorometric assay to measure serum CFD. This method does not require PCR and prior processing of the samples, and was validated for serum.[17] Using this method, we have already found that elevated CFD levels are strongly associated with poor prognosis in COPD, sepsis, traumatic brain injury, and burn patients.[8,11,18,19] We hypothesized that circulating CFD released from smoke inhalation injured lungs will effectively reflect lung injury and inflammation. The aim of this study was to evaluate admission CFD levels as an injury severity marker in isolated SII patients.
2. Patients and methods
We conducted a prospective study in trauma centers of two large Israeli hospitals (∼1000 beds), in the Soroka University Medical Center Beer-Sheva, and Rabin Medical Center, Petah Tikva. The study was approved by the institutional review boards of both centers and informed consent was obtained from all individual participants included in the study.
Study cohort: Eighteen patients diagnosed in the emergency department as suffering from isolated SII, aged 18 years or over, who arrived at the hospital within 2 h from the time of injury. All patients were found to have evidence of SII by flexible fiber-optic endoscopy. All patients received oxygen on their way to the emergency department. Patients were either hospitalized or followed up in the emergency department for up to 24 h, according to the clinical judgement of the treating emergency physician. This decision was influenced mainly by the history of the injury (exposure time to smoke in closed quarters), the patient's respiratory condition, COHb levels, and the recommendation of the ENT performing the fiber-optic endoscopy.
To exclude the effect of comorbidities, we chose to investigate healthy, nonsmoking patients, without concomitant trauma including cutaneous burns, pregnant women, and patients suffering from pre-existing severe chronic illnesses (cancer, heart disease, lung disease, kidney disease, autoimmune diseases).
Data recorded: Time and cause of injury, demographics, medical history, complete blood count, blood chemistry panel, arterial/venous blood gases, and COHb admission levels.
Controls: Eighteen healthy nonsmoking volunteers, age and gender matched to the patients.
CFD analysis: Blood samples were obtained at arrival to the emergency department, within two hours of injury, and during routine daily blood tests for hospitalized patients. Blood samples were collected using BD Vacutainer gel tubes (Becton-Dickinson, Plymouth, UK) and stored at 4°C for up to 24 h before centrifugation. Serum was separated from the cellular fraction and frozen at −20°C until assayed. CFD was detected directly in sera, according to our previously published novel method.[17] Briefly, 20 μL of sera or DNA standard solutions were applied in duplicate to black 96-well plates (Greiner Bio-One; Frickenhausen, Germany), and 80 μL of diluted SYBR Gold were added to each well (final dilution 1:10,000). Fluorescence was measured with a 96-well fluorometer at an emission wavelength of 535 nm and an excitation wavelength of 485 nm. Concentrations of unknown samples were calculated from a standard curve by extrapolation in a linear regression model. As described previously, our assay strongly correlates with the conventional quantitative PCR assay of β-globin (R2 = 0.9987, P < .001).[17]
Statistical analysis: Statistical analysis was performed using GraphPad Prism (Version 6.01, La Jolla, CA). Wilcoxon matched test was used to compare SII patients and matched controls, and Mann Whitney test to compare hospitalized and nonhospitalized patients. Spearman's rank correlation coefficient was used to correlate between CFD levels of the study group upon admission with other patient parameters (time, cause, severity level, and complete blood count and blood chemistry panels). Results are presented in median and range.
3. Results
Eighteen patients and 18 age and gender matched healthy control subjects were recruited between January 2014 and June 2015 (Table 1). Three of the patients were female (16.7%) and 15 (83.3%) male. The patients’ median age (Table 2) was 35 (range 27–65) years, not different from matched control group, 35 (23–70) years.
Table 1.
Patient group characteristics.
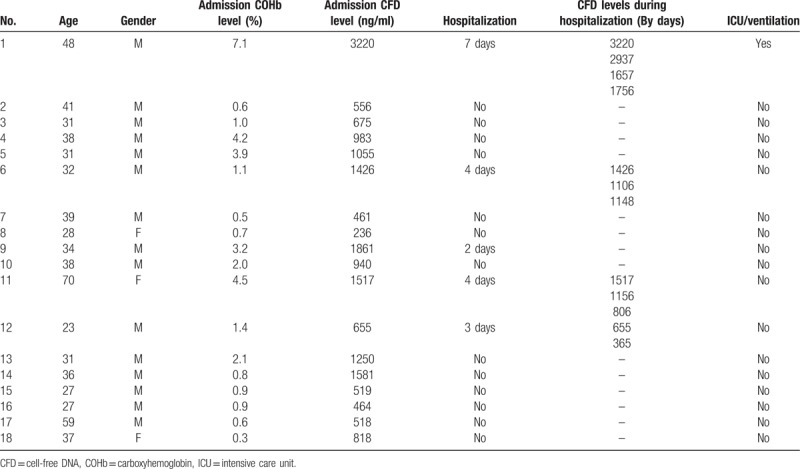
Table 2.
Study groups.

As shown in Table 1, five (27.7%) of the patients were hospitalized and thirteen (72.3%) were treated in the emergency department and discharged within 24 hours. One patient (5.5%) was ventilated and admitted to the intensive care unit (ICU) for 2 days.
As shown in Table 2 and Figure 1A, median admission CFD level of smoke inhalation patients was significantly higher than that of the age and gender matched healthy control group 879 (236–3220) ng/mL vs. 339 (150–570) ng/mL (P < .0001). Additionally, admission CFD levels of the smoke inhalation patients who were hospitalized were significantly higher than of those who were discharged from the emergency department within 24 h 1517 (655–3220) ng/mL vs. 675 (236–1581) ng/mL (Figure 1B, P < .05). Admission CFD was in correlation with hospitalization time (Rho = 0.578, P < .05) similar to the correlation of COHb to hospitalization time (Rho = 0.557, P < .05). We found a positive linear correlation between the patients’ admission COHb levels and CFD levels (R2 = 0.621, P < .0001, Figure 2).
Figure 1.
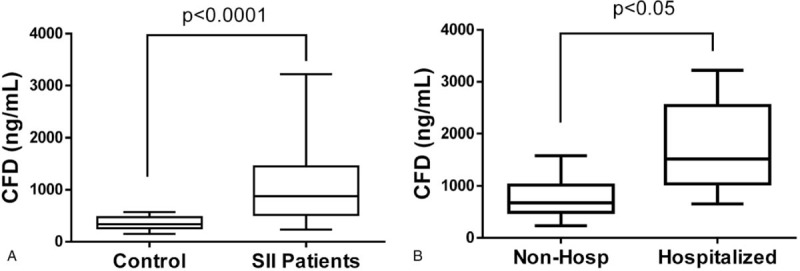
(A) Median admission cell-free DNA (CFD) levels of smoke inhalation patients (SII) versus controls. Circulating serum concentrations were measured using our fluorescent DNA assay in 18 SII patients and matched healthy controls. (B) Median admission CFD levels of hospitalized vs. nonhospitalized smoke inhalation patients.
Figure 2.
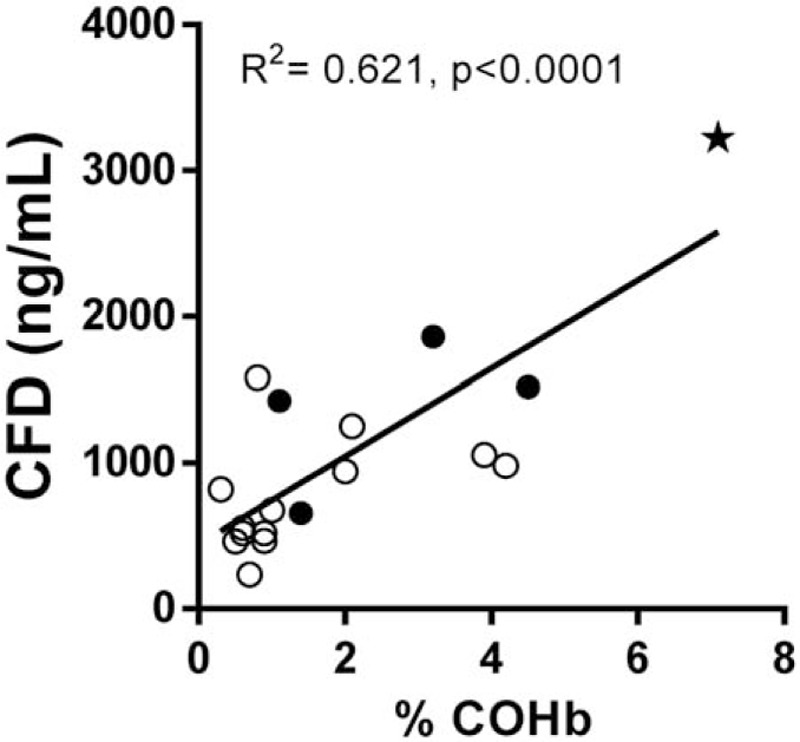
Correlation between admission CFD concentrations and carboxyhemoglobin (COHb) levels. Full circles indicate hospitalized patients and hollow circles indicate discharged patients. Star indicates patient ventilated in ICU.
As shown in Figure 3, we found that smoke inhalation patients who were hospitalized showed a gradual decrease in CFD levels during the days after admission (patients #1, #6, #11 and #12).
Figure 3.
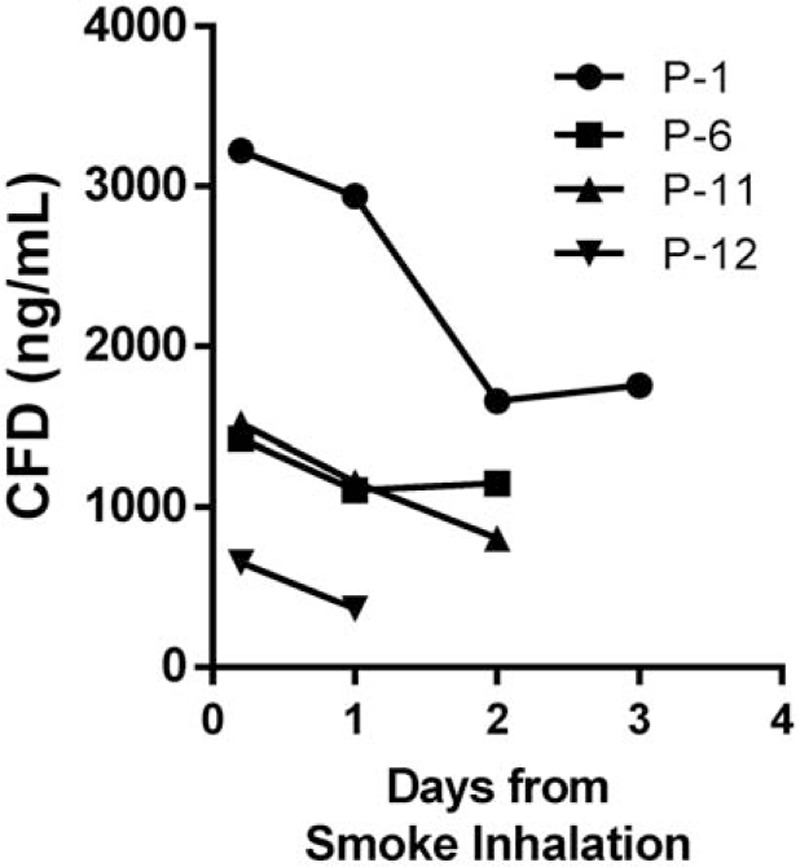
Daily CFD levels of four hospitalized patients (patient numbers 1, 6, 11, and 12).
We did not find significant correlations between admission CFD levels and complete blood count or blood chemistry panel variables.
4. Discussion
Early diagnosis of inhalation injury is crucial for recognition of a possibly compromised airway and to manage fluid resuscitation. There is no current consensus on the diagnostic criteria for inhalation injury.[20] In most cases, evaluation of inhalation injury is based on assessment of airway, breathing, and circulation, history of injury in an enclosed space, facial burns, and a few other physical criteria. These criteria are limited in prediction of the inflammatory response to the initial injury, which may be delayed for a day or two.[20] We hypothesized that admission CFD levels would reflect the severity of thermal destruction and toxicity of inhaled gases. Therefore, the aim of this study was to evaluate admission CFD levels as an injury severity marker in SII patients. In order to assess the CFD levels originating solely from SII injury, we chose to enroll only patients who had isolated SII, without any concomitant trauma or known pathological process capable of releasing additional CFD into the bloodstream.
In recent years, several reports have discussed the use of CFD levels as a prognostic factor for burn victims.[6–8] To the best of our knowledge, the current study is the first to address CFD in SII. We found that SII patients had significantly higher admission CFD levels than healthy control subjects. Furthermore, the correlation of CFD levels with the hospitalization period suggests that circulating CFD levels reflect the severity of SII. Furthermore, the strong correlation of CFD with admission COHb levels is indicative of the power of this biomarker to quantitate the damage from the toxic effect of CO and possible other noxious substances released during combustion.[20] In addition to necrosis from heat and toxic smoke, it is possible that part of the elevation in CFD in SII patients is contributed by degradation of neutrophils NETs, in which DNA is the main constituent.[12] It is possible that the prognostic power of CFD comes from its integrative reflection of primary injury and secondary damage from inflammation and hypoxia.
Additional support for the validity of this marker is the reduction of CFD along with the recovery of our hospitalized patients, suggesting the possible use of this marker for follow-up.
Our work demonstrates the routine clinical applicability of measuring admission CFD levels as a prognostic indicator for SII. Compared to the complex standard PCR-based assay,[7] the “mix and measure” assay that we have developed gives almost immediate results, which is essential for evaluation of trauma patients. We believe this rapid assay can help introduce the routine use of CFD measurement into daily practice.
Study limitations: Our study was conducted in 2 medical centers in a country in which fires leading to isolated SII are not common, thus limiting our cohort. This limited patient cohort may also explain why we did not find correlations between CFD levels and blood count or chemistry panel variables. Additionally, we find it important to point out that when measuring CFD in SII patients, severe background illness must be considered. CFD is not a specific biomarker of SII and may be affected by severe injuries or by rigorous chronic illness of the patients.
To conclude, CFD levels, measured by our simple “mix and measure” assay, appear to be an integrative promising marker for SII severity. We were able to measure admission CFD concentrations in 18 SII patients and matched control subjects. Patients had elevated CFD levels and their levels correlated with hospitalization time. CFD correlated CO intoxication levels and daily CFD measurements reflected the recovery of hospitalized patients. Our data from this preliminary study suggest that admission circulating CFD may serve as an effective triage tool for early diagnosis of SII. Additional studies are needed to further validate our results in isolated SII, and investigate the effect of concomitant trauma and comorbidities on CFD levels.
Author contributions
Conceptualization: Yaron Shoham, Amos Douvdevani.
Data curation: Yehiel Hayun, Yaron Shoham, Yuval Krieger, Eldad Silberstein, Dean Ad-El.
Funding acquisition: Amos Douvdevani.
Investigation: Yehiel Hayun.
Methodology: Yaron Shoham.
Project administration: Amos Douvdevani.
Supervision: Yaron Shoham, Amos Douvdevani, Dean Ad-El.
Validation: Amos Douvdevani.
Writing – original draft: Yehiel Hayun.
Writing – review & editing: Yaron Shoham, Amos Douvdevani.
Footnotes
Abbreviations: CFD = cell-free DNA, COHb = carboxyhemoglobin, ICU = intensive care unit, NETs = neutrophil extracellular traps, ROC = receiver operating characteristic, SII = smoke inhalation injury, TBSA = total body surface area.
YH and YS indicates first two authors with equal contribution.
This work was supported by the “Dr. Montague Robin Fleisher Kidney Transplant Unit Fund.”
All authors declare that they have no conflicts of interest.
References
- [1].Enkhbaatar P, Pruitt BA, Jr, Suman O, et al. Pathophysiology, research challenges, and clinical management of smoke inhalation injury. Lancet 2016;388:1437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fujioka M, Yakabe A. Does inhalation injury increase the mortality rate in burn patients? Investigation of relationship between inhalation injury and severity of burn surface. Signa Vitae 2009;4:20–2. [Google Scholar]
- [3].Neuwalder JM, Sampson C, Breuing KH, et al. A review of computer-aided body surface area determination: SAGE II and EPRI's 3D Burn Vision. J Burn Care Rehabil 2002;23:55–9. discussion 54. [DOI] [PubMed] [Google Scholar]
- [4].Kwon HP, Zanders TB, Regn DD, et al. Comparison of virtual bronchoscopy to fiber-optic bronchoscopy for assessment of inhalation injury severity. Burns 2014;40:1308–15. [DOI] [PubMed] [Google Scholar]
- [5].Albright JM, Davis CS, Bird MD, et al. The acute pulmonary inflammatory response to the graded severity of smoke inhalation injury. Crit Care Med 2012;40:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chiu TW, Young R, Chan LY, et al. Plasma cell-free DNA as an indicator of severity of injury in burn patients. Clin Chem Lab Med 2006;44:13–7. [DOI] [PubMed] [Google Scholar]
- [7].Fox A, Gal S, Fisher N, et al. Quantification of circulating cell-free plasma DNA and endothelial gene RNA in patients with burns and relation to acute thermal injury. Burns 2008;34:809–16. [DOI] [PubMed] [Google Scholar]
- [8].Shoham Y, Krieger Y, Perry ZH, et al. Admission cell free DNA as a prognostic factor in burns: quantification by use of a direct rapid fluorometric technique. Biomed Res Int 2014;2014:306580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Saukkonen K, Lakkisto P, Varpula M, et al. Association of cell-free plasma DNA with hospital mortality and organ dysfunction in intensive care unit patients. Intensive Care Med 2007;33:1624–7. [DOI] [PubMed] [Google Scholar]
- [10].Uzuelli JA, Dias-Junior CA, Izidoro-Toledo TC, et al. Circulating cell-free DNA levels in plasma increase with severity in experimental acute pulmonary thromboembolism. Clin Chim Acta 2009;409:112–6. [DOI] [PubMed] [Google Scholar]
- [11].Avriel A, Paryente Wiessman M, Almog Y, et al. Admission cell free DNA levels predict 28-day mortality in patients with severe sepsis in intensive care. PLoS One 2014;9:e100514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Toussaint M, Jackson DJ, Swieboda D, et al. Host DNA released by NETosis promotes rhinovirus-induced type-2 allergic asthma exacerbation. Nat Med 2017;23:681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tsang JC, Lo YM. Circulating nucleic acids in plasma/serum. Pathology 2007;39:197–207. [DOI] [PubMed] [Google Scholar]
- [14].Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med 2017;23:279–87. [DOI] [PubMed] [Google Scholar]
- [15].Wright TK, Gibson PG, Simpson JL, et al. Neutrophil extracellular traps are associated with inflammation in chronic airway disease. Respirology 2016;21:467–75. [DOI] [PubMed] [Google Scholar]
- [16].Cheng OZ, Palaniyar N. NET balancing: a problem in inflammatory lung diseases. Front Immunol 2013;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Goldshtein H, Hausmann MJ, Douvdevani A. A rapid direct fluorescent assay for cell-free DNA quantification in biological fluids. Ann Clin Biochem 2009;46(Pt 6):488–94. [DOI] [PubMed] [Google Scholar]
- [18].Avriel A, Rozenberg D, Raviv Y, et al. Prognostic utility of admission cell-free DNA levels in patients with chronic obstructive pulmonary disease exacerbations. Int J Chron Obstruct Pulmon Dis 2016;11:3153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shaked G, Douvdevani A, Yair S, et al. The role of cell-free DNA measured by a fluorescent test in the management of isolated traumatic head injuries. Scand J Trauma Resusc Emerg Med 2014;22:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tanizaki S. Assessing inhalation injury in the emergency room. Open Access Emerg Med 2015;7:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


