Abstract
Rationale:
Glioblastoma (GBM) is the most aggressive malignant brain tumor in adults. The first choice for GBM is surgery, and followed by a combination of radiotherapy and chemotherapy. There are limited treatments for patients with recurrent GBM. Relapsed patients usually have a worse prognosis, and with a median survival time of <6 months. Anlotinib is a novel small molecule multi-target tyrosine kinase inhibitor that can inhibit tumor angiogenesis and inhibit tumor cell growth. This drug has been used to treat advanced lung cancer.
Patient concerns:
We present a case of recurrent GBM was treated with anlotinib in this report. The patient was diagnosed with GBM in August 2016 and treated with surgery and temozolomide (TMZ) chemotherapy. She was diagnosed with recurrence in February 2017 following which she was treated with gamma knife and TMZ chemotherapy. In November 2017, the patient presented with decreased vision in left eye. She was given radiation and her left eye vision returned to normal after radiation. On May23, 2018, the patient reported a decrease in left visual acuity again.
Diagnoses:
Brain magnetic resonance imaging (MRI) showed progression of the disease, and the tumor invaded the left optic nerve.
Interventions:
This patient was administer anlotinib 12 mg po qd (d1–14, 21days as a cycle). Three cycles anlotinib were given to this patient.
Outcomes:
The patient reported her left visual acuity increased over 10 days after first cycle of anlotinib treatment. MRI scan revealed tumor volume shrinks, especially the part that invades the left optic nerve shrinks significantly at 26 days after anlotinib treatment on August 11, 2018. However, the tumor progressed in 2 months after using of anlotinib. From the beginning of the application of anlotinib to death, her survival time was 110 days.
Lessons:
Anlotinib treatment with mild side effects may be a new option for the patients with recurrent glioblastoma.
Keywords: anlotinib, case report, glioblastoma, targeted therapy
1. Introduction
Glioblastoma (GBM) is the most aggressive malignant brain tumor in adults and is characterized by poor prognosis. The median survival time (OS) for GBM patients is only 13 to 16 months and the 2-year survival rate is only 26.9%.[1] Surgery is still the first choice for GBM patients. Radiotherapy combined with temozolomide (TMZ) is recommended by the National Comprehensive Cancer Network (NCCN) guidelines as standard treatment for postoperative GBM patients.[1] The prognosis for patients with recurrent GBM remains poor, showing a lack of improvement in the therapeutic options. These patients only have a median OS of <6 months.[2] Recently, some targeted drugs have been used for treatment of patients with GBM. The common targeted drugs are bevacizumab, thalidomide, cetuximab, etc. However, these drugs have limited effectiveness for patients with recurrent GBM.[3] Anlotinib is a novel multitarget tyrosine kinase inhibitor that targets angiogenesis-related kinases such as vascular endothelial growth factor receptor (VEGFR)1/2/3, fibroblast growth factor receptors (FGFR)1/2/3, and other tumor-associated kinases such as c-Kit, Ret.[4] Anlotinib has been reported for the treatment of non-small cell lung cancer, metastatic renal cell carcinoma and sarcoma, with good effect and mild side effect.[5] However, anlotinib has not been reported for the treatment of patients with GBM. The efficacy and security of a case with recurrent GBM after taking anlotinib was reported in our article.
2. Case presentation
A 61-year-old woman was first admitted to our hospital complaining from headache and vomiting. Magnetic resonance imaging (MRI) revealed a large abnormal mass in the left temporal lobe. The patient was underwent total resection in August 2016 and was diagnosed of GBM. She received concomitant TMZ chemotherapy after surgery. MRI scan showed recurrence of the left temporal lobe tumor in February 2017. And then gamma knife was given to this patient with a single dose of 28 Gy. After radiation, 7 adjuvant TMZ cycles (150 mg/m2/d, qd, d1–5, every 28 days as 1 cycle) were given to this patient. This patient developed 1° of myelosuppression and mild gastrointestinal reactions during chemotherapy. The patient successfully finished the last cycles of TMZ in September, 2017. However, the patient reported a decrease of left visual acuity in November 2017. MRI revealed the relapse of the tumor invading the skull base, meninges, left tibia, and left optic nerve. Neurosurgeons suggested that it is difficult to resection, and recommended palliative radiation. In January 2018, the patient was given cerebral palliative radiation with a dose of 3000 Gy/10 fractions. After radiation, the patient's left eye vision returned to normal, and TMZ was given for 2 cycles (the same dose as before). MRI scan revealed a decrease in tumor volume in April 2018 compared with that before radiation. Then 2 cycles of TMZ was given to this patient. On May23, 2018, the patient reported a decrease in left visual acuity. Brain MRI (Fig. 1A1 and B1) showed progression of the disease, and the tumor invaded the left optic nerve. At the same time, grade IV myelosuppression occurred in blood analysis, the lowest neutrophils count is 1.58 × 109/L, and the lowest platelets count is 13 × 109/L. TMZ chemotherapy was stopped. Platelet intravenous infusion and interleukin-11 were given to this patient. Neutrophils and platelets count returned to normal after a month. Given the above-mentioned results, we decided to stop TMZ and give anlotinib 12 mg po qd from July16, 2018 (d1–14, 21 days as a cycle). The patient reported an increase in her left visual acuity on July 26, 2018. The patient did not develop nausea and any grade myelosuppression. MRI scan revealed tumor volume shrinks, especially the tumor that invades the left optic nerve shrinks significantly at 26 days after anlotinib treatment on August 11, 2018 (Fig. 1A2 and B2). Then anlotinib 12 mg po qd was given to the patient for the second cycle. Follow up MRI scans on September 13 (Fig. 1A3 and B3) revealed tumor volume increased than that in August 11 (Fig. 1A2 and B2). But the tumor was not invade the left optic nerve again and remained stable. And anlotinib stopped to use anymore on October 9 due to decreased platelets and she was given the best support treatment. The patient had not any gastrointestinal reaction and her left eye vision did not decreased anymore. She had myelosuppression in the second and the third cycle of anlotinib treatment. The lowest neutrophil is 1.65 × 109/L, and the lowest platelet is 31 × 109/L. These 2 blood indicators return to normal after symptomatic treatment. Her highest blood pressure was 160/110 during the anlotinib treatment, and she was given nifedipine release tablets (30 mg po bid), her blood pressure remained in normal level. The patient developed coma on October 21, 2018 and died on November 2, 2018.
Figure 1.
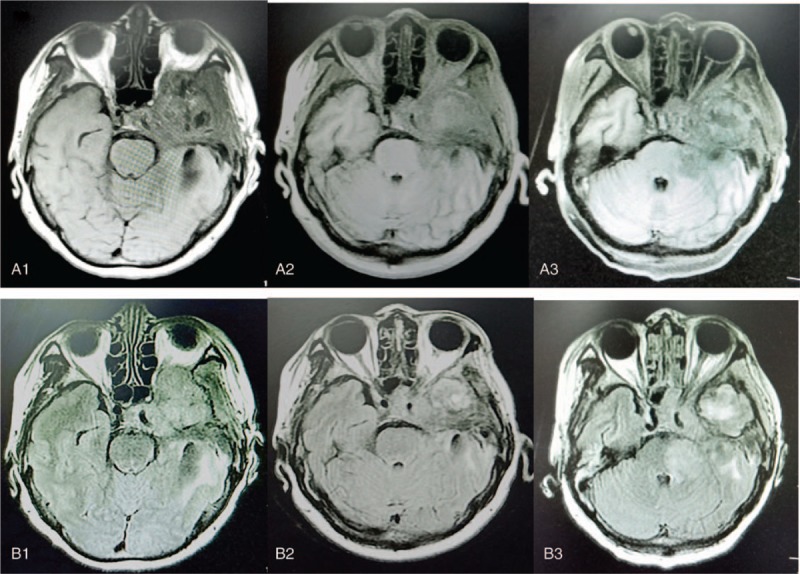
MRI findings in patient presented with glioblastoma. A and B represent MRI T1 weighting and T2 weighting, respectively. 1, 2, and 3 represent before, 1 month after treatment, and 2 months after treatment of anlotinib, respectively. MRI = magnetic resonance imaging.
3. Discussion
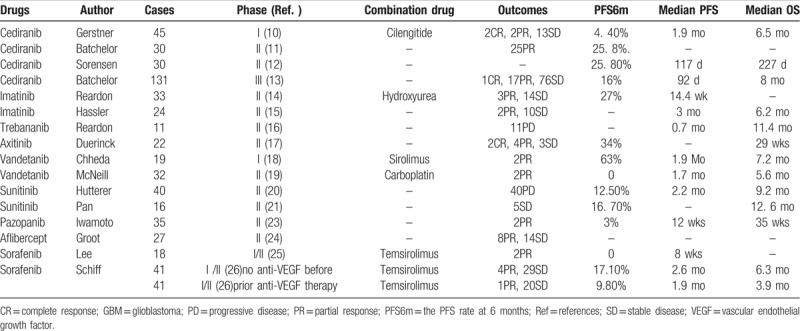
The prognosis of patients with recurrent GBM was poor. Conventional cytotoxic chemotherapies have little efficacy may be due to the presence of the blood–brain barrier. At present, anti-VEGF drugs have been widely used in the treatment of various malignant tumors, including GBM.[6] GBM is one of the most vascularized tumors and GBM cells can produce VEGF. The elevated levels of VEGF usually induce worse prognosis in patients with cancer.[7] Bevacizumab (bev), a monoclonal antibody directed against VEGF, has become the standard treatment for patients with recurrent GBM in NCCN guideline. A meta-analysis showed bev combined with chemotherapy versus single bev in recurrent GBM was significantly improve progression-free survival (PFS) and objective response rate (ORR), but did not prolong OS.[8] In addition, the other meta-analysis involving 548 patients with recurrent GBM showed the median OS was 9.3 months and the median time to tumor progression (TTP) was 6.1 months. However, this analysis was not compared the differences of efficiency between single bev and bev combined with chemotherapy.[9] In addition to bev, several other anti-angiogenic drugs were reported for the treatment of patients with recurrent GBM (Table 1, reference 10–26). A phase I study of cediranib combination with cilengitide in patients with recurrent GBM reported the median OS was 6.5 months, median PFS was 1.9 months, and the PFS rate at 6 months (PFS6m) was 4.4%.[10] A phase II study showed cediranib, an oral anti-VEGF receptor tyrosine kinase inhibitor, was administered to 31 patients with recurrent GBM. The proportion of patients alive and progression free at 6 months was 25.8%. Radiographic partial responses (PR) were observed by MRI in 17 (56. 7%) of 30 evaluable patients.[11] The other phase II revealed the median PFS of the 30 patients who received cediranib was 117 days and the median OS was 227 days.[12] A phase III trial comparing the progression-free survival (PFS) of single cediranib, in combination with lomustine, versus single lomustine in patients with recurrent GBM. The PFS among these 3 groups was not significantly different.[13] Thirty-three patients were given imatinib mesylate and hydroxyurea enrolled in a phase II study. The median PFS of these patients was 14.4 weeks. There were no grade 4 or 5 side effects.[14] Hassler et al[15] reported a trial of imatinib was given 24 recurrent GBM patients. Six patients survived over 1 year and the median PFS was 3 months and median OS was 6.2 months. Imatinib was well tolerated. A trial of trebananib with and without bev for patients with recurrent GBM showed that trebananib was ineffective as monotherapy and did not enhance the efficacy combined with bev to improve the OS of patients.[16] A phase II clinical trial was investigated the efficiency of axitinib in patients with recurrent GBM. This trial showed that the PFS6m rate was 34% for patients treated with axitinib and 28% for patients with alternative treatment; and the median OS was 29 and 17 weeks of these 2 different groups, respectively. And objective responses were got in 28% of patients treated with axitinib and 23% of patients treated with alternative therapy.[17] Vandetanib combined with sirolimus were given to patients in a phase I trial. And there were 2 cases got PR and PFS6m was 15.8%.[18] Another trial showed that the combination of vandetanib and carboplatin did not have better efficacy than single carboplatin treatment in patients with recurrent GBM.[19] Forty patients were given continuous daily sunitinib enrolled in a study, and the median PFS was 2.2 months, and median OS was 9.2 months.[20] In the other study, sunitinib was given to 16 patients with recurrent GB and the most common side effects were fatigue, neutropenia, and thrombocytopenia. Five patients had stable disease (SD) and median OS was 12.6 months.[21] A review indicates that sunitinib was not superior to bev or conventional chemotherapy for recurrent GBM.[22] Iwamoto et al[23] conducted a phase II trial of pazopanib in 35 patients with recurrent GBM. The study reported a median progression survival of 12 weeks and with PFS6m was 3%. The median OS from pazopanib application was 35 weeks. Groot et al[24] reported the results of a phase II trial of single aflibercept for patients with recurrent GBM. The response rate was 30% (14SD, 8PR of 27 evaluable patients). There were 2 trials showed sorafenib combined with temsirolimus had minimal anti-tumor activity.[25,26] Interestingly, Schiff et al[26] showed the subgroup of patients without using anti-VEGF treatment before had higher efficacy than those who have previously used anti-VEGF treatment.
Table 1.
Clinical trials of anti-angiogenic drugs were reported for recurrent GBM.
In conclusion, these anti-VEGF drugs have mild toxicities, but their efficacy of anti-tumor was not satisfactory. At present, some anti-angiogenic drugs are still controversial for the application for patients with recurrent GBM. The anti-tumor mechanism of these drugs is may be that they can normalize brain tumor vasculature by decreasing vessel diameter and permeability, and thinning the abnormally thick basement membrane. The change in microvessel volume was correlated with duration of OS and PFS.[12] Moreover, anti-VEGF drugs can inhibit the binding of VEGF to receptors which located on endothelial cells. These drugs can inhibit biological activity of VEGF to inhibit tumor angiogenesis, and result in a hypoxic environment that inhibits the growth of tumor cells.[9]
Anlotinib is a drug that can inhibit both tumor angiogenesis and tumor cell proliferation. It can inhibit more targets than other target drugs can, including sorafenib, sunitinib, and pazopanib.[5] The anti-angiogenic activity of anlotinib is stronger than that of 3 other anti-angiogenesis drugs, including sunitinib, sorafenib, and nintedanib.[27] Bevacizumab was recommended to this patient after tumor progression, but her son refused and required oral medication. This drug was given to this patient because it has an anti-angiogenic effect similar to bevacizumab. We selected the same dosage and cycle of anlotinib as lung cancer treatment, and this dosage has been confirmed to be safe by clinical trial.[5] Our article firstly reported that anlotinib was used for patients with recurrent GBM. This PFS of this patient was about 2 months and toxicities were well tolerated. And her visual acuity was significantly increased than before using anlotinib. Although the tumor progressed again after 2 months of application, this patient still had OS of 110 days. It may provide a new option for patients with recurrent GBM after TMZ or bev treatment. However, the efficacy and side effects of this drug for GBM patients still require large-scale clinical trials to confirm. In addition, whether anlotinib combined with chemotherapy or radiotherapy is superior to single anlotinib for patients with recurrent GBM still needs to be studied in the future.
Author contributions
Data curation: Meijuan Song, Yong Hou, Ning Liang.
Resources: Yajuan Lv, Fengjun Liu, Yong Hou, Meijuan Song.
Writing - Original Draft: Yajuan Lv.
Writing - Review & Editing: Jiandong Zhang.
Jiandong Zhang orcid: 0000-0003-0169-7092.
Footnotes
Abbreviations: bev = Bevacizumab, FGFR = fibroblast growth factor receptors, GBM = glioblastoma, MRI = magnetic resonance imaging = Diffusion weighted imaging, OS = survival time, ORR = objective response rate, PFS = progression-free survival, PFS6m = the progression-free survival rate at 6 months, TMZ = temozolomide, TTP = time to tumor progression, VEGFR = vascular endothelial growth factor receptor.
Ethical policy: The ethical was approved by ethics committee of Qianfoshan Hospital.
Consent for publication: We have obtained the informed written consent of the patient's son for publication of this case report and accompanying images.
The authors declare that they have no competing interests.
References
- [1].Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- [2].Clarke J, Butowski N, Chang S. Recent advances in therapy for glioblastoma. Arch Neurol 2010;67:279–83. [DOI] [PubMed] [Google Scholar]
- [3].Olson JJ, Nayak L, Ormond DR, et al. The role of targeted therapies in the management of progressive glioblastoma:a systematic review and evidence-based clinical practice guideline. J Neurooncol 2014;118:557–99. [DOI] [PubMed] [Google Scholar]
- [4].Xie C, Wan X, Quan H, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci 2018;109:1207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol 2018;11:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Reardon DA, Turner S, Peters KB, et al. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J Natl Compr Canc Netw 2011;9:414–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen Z, Xu N, Zhao C, et al. Bevacizumab combined with chemotherapy vs single-agent therapy in recurrentglioblastoma: evidence from randomized controlled trials. Cancer Manag Res 2018;10:2193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wong ET, Gautam S, Malchow C, et al. Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. J Natl Compr Canc Netw 2011;9:403–7. [DOI] [PubMed] [Google Scholar]
- [10].Gerstner ER, Ye X, Duda DG, et al. A phase I study of cediranib incombination with cilengitide inpatients with recurrent glioblastoma. Neuro Oncol 2015;17:1386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Batchelor TT, Duda DG, di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol 2010;28:2817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sorensen AG, Batchelor TT, Zhang WT, et al. A “vascular normalization index” as potential mechanistic biomarker to predictsurvival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res 2009;69:5296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tracy T, Batchelor TT, Mulholland P, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol 2013;31:3212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Reardon DA, Egorin MJ, Quinn JA, et al. Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J Clin Oncol 2005;23:9359–68. [DOI] [PubMed] [Google Scholar]
- [15].Hassler MR, Vedadinejad M, Flechl B, et al. Response to imatinib as a function of target kinase expression in recurrentglioblastoma. Springerplus 2014;3:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Reardon DA, Lassman AB, Schiff D, et al. Phase 2 and biomarker study of trebananib, an angiopoietin-blocking peptibody, with and without bevacizumab for patients with recurrent glioblastoma. Cancer 2018;124:1438–48. [DOI] [PubMed] [Google Scholar]
- [17].Duerinck J, Du Four S, Vandervorst F, et al. Randomized phase II study of axitinib versus physicians best alternative choiceof therapy in patients with recurrent glioblastoma. J Neurooncol 2016;128:147–55. [DOI] [PubMed] [Google Scholar]
- [18].Chheda MG, Wen PY, Hochberg FH, et al. Vandetanib plus sirolimus in adults with recurrent glioblastoma: results of a phase I and dose expansion cohort study. J Neurooncol 2015;121:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McNeill K, Iwamoto F, Kreisl T, et al. A randomized phase ii trial of vandetanib (zd6474) in combination with carboplatin versus carboplatin alone in adults with recurrent glioblastoma. Neurooncology 2014;16:v8–22. [Google Scholar]
- [20].Hutterer M, Nowosielski M, Haybaeck F, et al. A single-arm phase II Austrian/German multicenter trial on continuous daily sunitinib in primary glioblastoma at first recurrence (SURGE 01-07). Neuro Oncol 2014;16:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pan E, Yu D, Yue B, et al. A prospective phase II single-institution trial of sunitinib for recurrent malignant glioma. J Neurooncol 2012;110:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Grisanti S, Ferrari VD, Buglione M, et al. Second line treatment of recurrent glioblastoma with sunitinib: results of a phase II study and systematic review of literature. J Neurosurg Sci 2016;Se28. [DOI] [PubMed] [Google Scholar]
- [23].Iwamoto FM, Lamborn KR, Robins HI, et al. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02). Neuro Oncol 2010;12:855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].De Groot JF, Wen PY, Lamborn K, et al. Phase II single arm trial of aflibercept in patients with recurrent temozolomide-resistant glioblastoma: NABTC 0601. J Clin Oncol 2008;26:2020.18421055 [Google Scholar]
- [25].Lee EQ, Kuhn J, Lamborn KR, et al. Phase I/II study of sorafenib in combination with temsirolimus for recurrentglioblastoma or gliosarcoma: North American Brain Tumor Consortium study 05-02. Neuro Oncol 2012;14:1511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schiff D, Kurt A, Jaeckle SK, et al. Phase 1/2 trial of temsirolimus and sorafenib in the treatment of patients with recurrent glioblastoma: North Central Cancer Treatment Group Study/Alliance N0572. Cancer 2018;124:1455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lin B, Song X, Yang D, et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2 PDGFR and FGFR1 Gene. Gene 2018;654:77–86. [DOI] [PubMed] [Google Scholar]


