Abstract
The purpose of this study was to investigate the influences of varied anesthetic methods and depths on inflammatory cytokines and stress hormone levels in radical operation among colon cancer patients during perioperative period.
A total of 120 patients were collected in the study and randomly divided into 4 groups, A: general anesthesia + Narcotrend D1, B: general anesthesia + Narcotrend D2, C: general anesthesia + epidural anesthesia + Narcotrend D1, D: general anesthesia + epidural anesthesia + Narcotrend D2. The levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-10, cortisol (Cor), adrenocorticotropic hormone (ACTH), and endothelin-1 (ET-1) were measured adopting commercial kits before anesthesia (T0), 4 hours after surgery (T1), 24 hours after surgery (T2), and 72 hours after surgery (T3).
There was no significant difference in basic clinical characteristics among the groups. In comparison with group A, B and C, group D showed significantly lower levels of TNF-α, IL-6, IL-10, Cor, ACTH, and ET-1 at T1 and T2 (all, P < .05). Significantly higher levels of TNF-α, IL-6, IL-10, Cor, and ACTH were detected at T1 and T2 than those at T0 (all, P < .05), whereas, at T3, the levels of inflammatory cytokines and stress hormones were all decreased near to preoperation ones.
General anesthesia combined with epidural anesthesia at Narcotrend D2 depth plays an important role in reducing immune and stress response in patients with colon cancer from surgery to 24 hours after surgery.
Keywords: anesthesia, colon cancer, cytokines, Narcotrend, stress hormone
1. Introduction
Colon cancer is one of the most common cancers in the adults worldwide,[1] with increasing incidence, and seriously threatens human health.[2] At present, surgical removal is the primary strategy in treating this malignancy. However, postoperative metastasis and recurrence, possibly mediated by tumor propensity and immunosurveillance of host,[3] could cause dismal survival and prognosis among the patients. Moreover, operation can lead to stress response depending on the severity and duration of tissue injury. The stress response may have positive effects, but excessive stimulation of cytokine production is associated with the risk of postoperative organ dysfunction, and postoperative metastasis as well.[4,5] The production of cytokines benefits tissue healing, but excessive secretion of cytokines may help catch circulating tumor cells and enhance cell adhesion, and thus leading to local and distant tumor growth and to novel formation of metastasis loci.[6] Therefore, protecting immune function and stress response during perioperative period might be crucial for improving the outcome of patients with colon cancer.
Anesthesia is essential for postoperative clinical outcomes among patients with cancer. Anesthesia may modulate cancer growth and metastasis through directly influencing biological behaviors of cancer cells, or improving tumor microenvironment.[7] Compared to general anesthesia, regional anesthesia has been postulated to have positive effect on cancer outcome.[8] Thoracic epidural anesthesia and analgesia have commonly been used for the management of intra- and postoperative pains during colon cancer surgery. General anesthesia and epidural anesthesia may suppress immunity via directly affecting the immune system or sympathetic nervous system.[9,10] Furthermore, numerous studies have demonstrated that intraoperative monitoring anesthesia depth is important to ensure successful surgery and rapid recovery.[11] Immunocompetent cells secrete different cytokines and effector molecules, such as tumor necrosis factor (TNF)-α, interleukin (IL)-4, IL-6, and IL-10, as well as stress hormones, which mediate anti-tumor immunity and body stress reaction to the operation.[12–14] Although previous studies have reported the effects of general anesthesia and anesthesia depth on human immunity and stress response, few reports have compared the effects between different anesthesia methods and anesthesia depths on immune and stress responses in patients with colon cancer.
In this study, we aimed to detect the influence of general + epidural anesthesia combined with Narcotrend D1+D2 depth on inflammatory reactions and stress response in patients receiving surgery.
2. Materials and methods
2.1. Patients’ collection
A total of 120 patients undergoing colon cancer surgery were continually recruited from the inpatient service of the Hebei University of Engineering Affiliated Hospital. These patients were randomly divided into 4 groups according to their anesthesia treatment. Group A (n=30): receiving general anesthesia with anesthesia depth at Narcotrend D1; group B (n = 30): receiving general anesthesia with anesthesia depth at Narcotrend D2; group C (n = 30): receiving epidural anesthesia combined with general anesthesia and Narcotrend D1 level; group D (n = 30): receiving epidural anesthesia combined with general anesthesia and Narcotrend D2. Among the patients in groups C and D, epidural anesthesia was firstly administrated, followed by general anesthesia. Narcotrend index based on brainwave analysis detected the depth of clinical anesthesia and hypnosis using Narcotrend anesthesia depth monitor.[15] Narcotrend algorithm classifies EEG epochs into different stages from A (awake) to F (increasing burst suppression down to electrical silence), and a total of 15 different stages (A, B0–2, C0–2, D0–2, E00–2, F0–1).[16] Exclusion criteria included the history of abdominal surgery, endocrine, or immune system dysfunction (such as diabetes, thyroid disease, multiple sclerosis, and rheumatoid arthritis), preoperative treatment with hormone or immunomodulatory substances and contraindication to epidural anesthesia. The study was approved by the Ethic Committee of Hebei University of Engineering Affiliated Hospital and all patients provided written informed consents in advance.
2.2. Anesthesia treatment
All patients were subjected to the induction of general anesthesia via intravenous administration of fentanyl (5 μg/kg), propofol (target plasma concentration 4 μg/mL), and rocuronium (0.8 mg/kg). Esophageal temperature was monitored and maintained at >36°C throughout the operation.
Epidural anesthesia was administrated for the patients in groups C and D. Epidural anesthesia implementation included epidural puncture between T10 and T11 on the left lateral position, followed by the insertion of an epidural catheter using the paramedian approach and loss-of-resistance technique. The patients would receive the test adopting 3 mL of 2% lidocaine through the epidural catheter after showing negative results in aspiration test for blood and cerebrospinal fluid. Each patient took 8 mL of 0.375% bupivacaine after the induction of anesthesia. In addition, 4 mL of bupivacaine was also administered for the patients every 50 minutes until the end of surgery.
Then, all of the patients received general anesthesia. Subsequent to orotracheal intubation, they were provided with 1.5% to 3.5% sevoflurane for the maintenance of anesthesia.
During the operation, Narcotrend monitoring electric electrode was placed in the forehead of the patients while opened Narcotrend single channel detection observed NI level. Narcotrend D1 level was controlled within 47 to 56, and Narcotrend D2 level within 37 to 46. Anesthesia depths were maintained through controlling fentanyl and propofol infusion speed. Except for anesthesia methods, all groups received the same perioperative care protocols.
2.3. Sample collection and indicator tests
Peripheral venous blood samples were collected in sodium heparin anticoagulant tubes before anesthesia (T0), 4 hours after the surgery (T1), 24 hours after surgery (T2), and 72 hours after surgery (T3). Separated serum samples were stored at −80°C until analysis. TNF-α, interleukin (IL)-6, and IL-10 levels were measured using commercial enzyme-linked immunosorbent assay kits (Invitrogen Corporation, Camarillo, CA). The levels of cortisol (Cor), adrenocorticotropic hormone (ACTH), and endothelin-1 (ET-1) were measured through radioimmunoassay with commercial kits provided by Beijing Chinatown force biotechnology research institute.
2.4. Statistical analysis
SPSS 18.0 software was employed to analyze statistical data, and data were expressed as mean ± standard deviation. Parametric data were compared using t test or analysis of variance. Categorical data were assessed using Chi-square test. P < .05 was considered statistically significant.
3. Results
3.1. General information of the patients
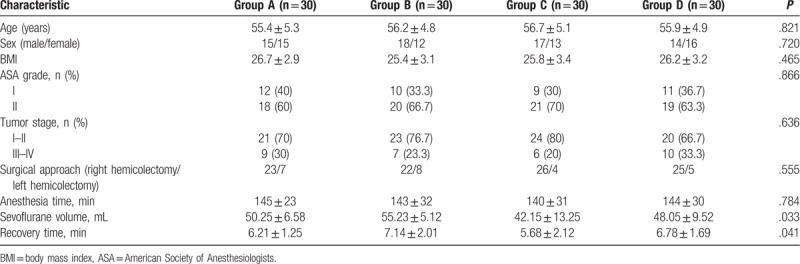
A total of 120 patients were recruited in the study and their clinical characteristics are summarized in Table 1, including age, sex, body mass index, American Society of Anesthesiologists score, tumor stage, surgical approach, and anesthesia time. Analysis results showed that all basic clinical data were similar in the 4 groups (A, B, C, D) with no statistical difference (all, P > .05). Adopted sevoflurane volume and recovery time were, however, significantly different between the 4 groups (P = .033 and P = .041, respectively) (Table 1). At the same anesthesia depth, combined application of epidural anesthesia and general anesthesia could significantly reduce anesthetic dosage and shorten recovery time.
Table 1.
The clinical characteristics of the colon patients in 4 groups.
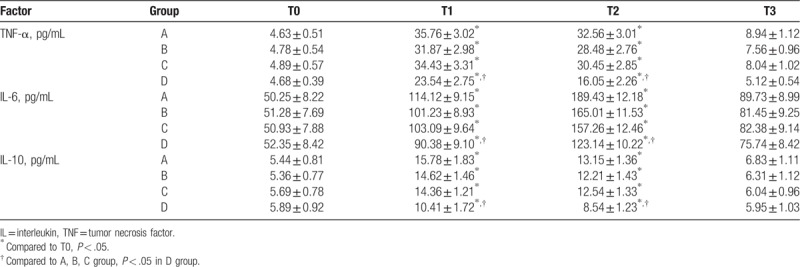
3.2. Comparison on inflammatory cytokines between 4 groups
At the points of 4 hours after surgery (T1) and 24 hours after surgery (T2), the levels of TNF-α, IL-6, and IL-10 were all significantly higher than those at T0 in the 4 groups (all, P < .05 Table 2). Furthermore, at T1 and T2, the levels of TNF-α, IL-6, and IL-10 in group D were significantly lower than those in groups A, B, and C, and the difference was statistically significant (all, P < .05, Table 2). The levels of TNF-α, IL-6, and IL-10 were all lower in groups B and C than in group A, but with no statistical difference. At T3, the cytokines levels were near to those at T0. Remaining values were not significantly different between the groups.
Table 2.
Comparison of inflammatory cytokines levels in the 4 groups.
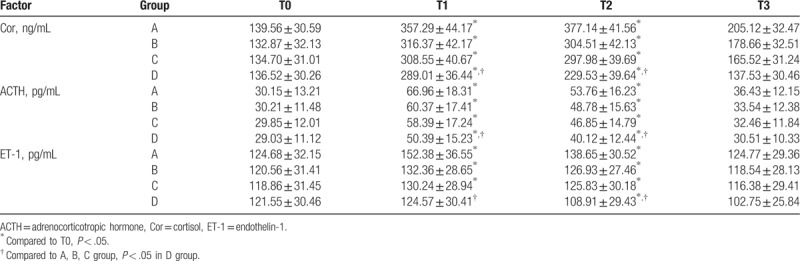
3.3. Comparison on stress hormone levels between 4 groups
As shown in Table 3, the levels of stress hormone Cor, ACTH, and ET-1 were significantly different between the 4 groups. Compared to those at T0, the levels of Cor, ACTH, and ET-1 were all obviously increased at T1 and T2 in the 4 groups (all, P < .05 Table 3), except ET-1 in D group, which was slightly increased at T1 and decreased at T2. Moreover, the levels of Cor, ACTH, and ET-1 were significantly lower in group D than in other 3 groups at T1 and T2, and the difference was statistically significant (all, P < .05 Table 3). At the 4 time points, hormone levels exhibited maximum changes in group A and minimum changes in group D, with levels at T3 near to preoperative ones. No significant difference was found for the remaining values.
Table 3.
Comparison of stress hormone levels in the 4 groups.
4. Discussion
Surgical resection is the only possible cure for colon cancer. Surgical stresses may, however, induce immunosuppressive effects and other oncogenic mechanisms, thus contributing to tumor growth at local or distant sites.[17–19] Anesthesia technique is a key factor for surgical stress.[20] Common anesthesia techniques include general anesthesia and radical anesthesia. Epidural anesthesia belongs to commonly used radical anesthesia. Published clinical trial demonstrated that general anesthesia combined with epidural anesthesia mitigated surgical stress-related impairment of antitumor immune responses and hastened the recovery of intestinal function.[21] Appropriate anesthesia may help relieve surgical stress. Anesthesia depth also affects surgical stress. Currently, such depth could be monitored through perioperative clinical signs. Usually, it is difficult to detect most clinical signs; moreover, surgery and anesthesia will make clinical signs complicated, which in turn makes the monitoring more difficult.[22,23] Certainly, intraoperative monitoring of anesthesia depth is vital to maintain signs, preserve stable hemodynamics, reduce side effects, achieve optimal anesthesia, and realize favorable analgesic and muscle relaxing.
Over the past few years, cytokines and more accepted stress hormones like cortisol and catecholamines have been reported to be involved in perioperative responses to surgery.[4,24,25] TNF-α, one of the main cytokines, mediates early response to tissue injury, and stimulates the production of IL-6. The concentrations of IL-6 is rapidly increased within 30 to 90 minutes after skin incision in elective surgery, which may be a sensitive marker for early tissue damage.[26] IL-10 exhibits antitumor and antimetastatic activity through enhancing nature kill cell lysis targeting tumor cells.[27,28]
In this study, we detected the levels of inflammatory cytokines and surgical stress response hormones among colon patients so as to compare the effect of different anesthesia methods. During perioperative period, the levels of inflammation cytokines and stress hormone significantly increased at postsurgery points T1 and T2 in the 4 groups, demonstrating that surgical trauma and surgical stress could trigger systemic inflammation and suppress immune defense mechanisms in postoperative period. In addition, the levels of cytokines and stress hormones were significantly lower in group D at time points T1 and T2 than in other groups, which indicated faster recovery of the immune response in Group D. Although there was no significant difference between groups A and B or between groups A and C, the levels of cytokines and stress hormone were decreased at T1 and T2. Our results demonstrated that general anesthesia combined with epidural anesthesia with Narcotrend monitor at D2 level could protect postoperative antitumor immune response among colon cancer patients.
In conclusion, general anesthesia combined with epidural anesthesia with Narcotrend D2 depth monitoring reduces inflammatory activation response to colon carcinoma surgery and may hasten the recovery of intestinal function. Additional large-scale prospective trials are required to determine the significance of these observations.
Author contributions
Conceptualization: Bao-Jun Hou.
Data curation: Bao-Jun Hou.
Formal analysis: Bao-Jun Hou, Yan-Ying Wang.
Funding acquisition: Ying Du, Yan-Ying Wang.
Investigation: Ying Du, Yan-Ying Wang.
Methodology: Ying Du, Yan-Ying Wang.
Project administration: Shu-Xin Gu.
Resources: Shu-Xin Gu, Hong Deng.
Software: Shu-Xin Gu, Hong Deng.
Supervision: Jie Fan, Hong Deng.
Validation: Jie Fan, Ran Wang, Hong Deng, Dan-Xia Guo, Li Wang.
Visualization: Jie Fan, Ran Wang, Hong Deng, Dan-Xia Guo, Li Wang.
Writing – original draft: Jie Fan, Ran Wang, Hong Deng, Dan-Xia Guo, Li Wang.
Writing – review and editing: Ran Wang, Hong Deng, Dan-Xia Guo, Li Wang.
Footnotes
Abbreviations: ACTH = adrenocorticotropic hormone, Cor = cortisol, ET-1 = endothelin-1, IL-6 = interleukin-6, NI = Narcotrend Index, TNF-α = tumor necrosis factor α.
The study was supported by 2016 annual Hebei provincial science and technology project (No. 16277708D).
The authors have no conflicts of interest to disclose.
References
- [1].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [2].Center MM, Jemal A, Smith RA, et al. Worldwide variations in colorectal cancer. CA Cancer J Clin 2009;59:366–78. [DOI] [PubMed] [Google Scholar]
- [3].Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann Surg Oncol 2003;10:972–92. [DOI] [PubMed] [Google Scholar]
- [4].Soop M, Nygren J, Thorell A, et al. Stress-induced insulin resistance: recent developments. Curr Opin Clin Nutr Metabol Care 2007;10:181–6. [DOI] [PubMed] [Google Scholar]
- [5].Mari G, Costanzi A, Crippa J, et al. Surgical stress reduction in elderly patients undergoing elective colorectal laparoscopic surgery within an ERAS protocol. Chirurgia (Bucur) 2016;111:476–80. [DOI] [PubMed] [Google Scholar]
- [6].Tohme S, Simmons RL, Tsung A. Surgery for cancer: a trigger for metastases. Cancer Res 2017;77:1548–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cata JP. Outcomes of regional anesthesia in cancer patients. Curr Opin Anaesthesiol 2018;31:593–600. [DOI] [PubMed] [Google Scholar]
- [8].Levene JL, Weinstein EJ, Cohen MS, et al. Local anesthetics and regional anesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children: a Cochrane systematic review and meta-analysis update. J Clin Anesth 2019;55:116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth 2008;22:263–77. [DOI] [PubMed] [Google Scholar]
- [10].Chen WK, Miao CH. The effect of anesthetic technique on survival in human cancers: a meta-analysis of retrospective and prospective studies. PLoS One 2013;8:e56540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kent CD, Domino KB. Depth of anesthesia. Curr Opin Anaesthesiol 2009;22:782–7. [DOI] [PubMed] [Google Scholar]
- [12].Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev 2008;222:129–44. [DOI] [PubMed] [Google Scholar]
- [13].Geginat J, Larghi P, Paroni M, et al. The light and the dark sides of Interleukin-10 in immune-mediated diseases and cancer. Cytokine Growth Factor Rev 2016;30:87–93. [DOI] [PubMed] [Google Scholar]
- [14].Matsuzaki J, Tsuji T, Luescher IF, et al. Direct tumor recognition by a human CD4(+) T-cell subset potently mediates tumor growth inhibition and orchestrates anti-tumor immune responses. Sci Rep 2015;5:14896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lai RC, Lu YL, Huang W, et al. Application of a narcotrend-assisted anesthesia in-depth monitor in the microwave coagulation for liver cancer during total intravenous anesthesia with propofol and fentanyl [in Chinese]. Chin J Cancer 2010;29:117–20. [DOI] [PubMed] [Google Scholar]
- [16].Amornyotin S, Chalayonnawin W, Kongphlay S. Deep sedation for endoscopic retrograde cholangiopancreatography: a comparison between clinical assessment and Narcotrend(TM) monitoring. Med Devices (Auckl) 2011;4:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Heaney A, Buggy DJ. Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis? Br J Anaesth 2012;109 suppl 1:i17–28. [DOI] [PubMed] [Google Scholar]
- [18].Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science 2011;331:44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth 2010;105:106–15. [DOI] [PubMed] [Google Scholar]
- [20].Dubowitz JA, Sloan EK, Riedel BJ. Implicating anaesthesia and the perioperative period in cancer recurrence and metastasis. Clin Exp Metastasis 2018;35:347–58. [DOI] [PubMed] [Google Scholar]
- [21].Chen WK, Ren L, Wei Y, et al. General anesthesia combined with epidural anesthesia ameliorates the effect of fast-track surgery by mitigating immunosuppression and facilitating intestinal functional recovery in colon cancer patients. Int J Colorectal Dis 2015;30:475–81. [DOI] [PubMed] [Google Scholar]
- [22].Panousis P, Heller AR, Burghardt M, et al. The effects of electromyographic activity on the accuracy of the Narcotrend monitor compared with the Bispectral Index during combined anaesthesia. Anaesthesia 2007;62:868–74. [DOI] [PubMed] [Google Scholar]
- [23].Tian K, Kang Y, Deng L, et al. Effects of different anesthesia depth on stress response in elderly patients undergoing elective laparoscopic surgery for colorectal cancer [in Chinese]. Nan Fang Yi Ke Da Xue Xue Bao 2014;34:694–8. [PubMed] [Google Scholar]
- [24].Porter C, Tompkins RG, Finnerty CC, et al. The metabolic stress response to burn trauma: current understanding and therapies. Lancet 2016;388:1417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hill AG. Initiators and propagators of the metabolic response to injury. World J Surg 2000;24:624–9. [DOI] [PubMed] [Google Scholar]
- [26].Minetto MA, Oprandi G, Saba L, et al. Serum interleukin-6 response to elective total hip replacement surgery. Int Orthop 2006;30:172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brito-Melo GE, Peruhype-Magalhaes V, Teixeira-Carvalho A, et al. IL-10 produced by CD4+ and CD8+ T cells emerge as a putative immunoregulatory mechanism to counterbalance the monocyte-derived TNF-alpha and guarantee asymptomatic clinical status during chronic HTLV-I infection. Clin Exp Immunol 2007;147:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Deegan CA, Murray D, Doran P, et al. Anesthetic technique and the cytokine and matrix metalloproteinase response to primary breast cancer surgery. Reg Anesth Pain Med 2010;35:490–5. [DOI] [PubMed] [Google Scholar]


