Abstract
Background:
Patients with atrial fibrillation (AF) have a higher risk of fatal complications (e.g., stroke). This investigation was performed as an observational retrospective cohort study includes 137 patients (age 61 ± 15; 34.3% women) with a primary diagnosis of AF (paroxysmal, persistent, and permanent).
Methods:
We collected information about the drug therapy, comorbidities and survival of AF patients and determined their congestive heart failure, hypertension, age, diabetes mellitus, prior stroke or TIA or thromboembolism, vascular disease, age, sex category (CHA2DS2-VASc) scores. Statistical analysis identified patients with high CHA2DS2-VASc scores and defined the predictive value of individual parameters, or their combination, with regards to the outcomes of stroke and mortality.
Results:
CHA2DS2-VASc scores identified 43.8% of the patients as low to intermediate risk (score 0–1) and 56.2% of the patients as high risk (score ≥2). Increasing CHA2DS2-VASc scores were not only accompanied by an increase in the incidence of stroke (Ptrend < .001) but also by an increase in the 3 to 5 years mortality (P = .005). Comparison of anticoagulation and anti-aggregation treatment between the 3 groups of AF did not show any significant statistical difference. Highly significant predictors of death were the CHA2DS2-VASc score (OR 1.71, 95% CI 1.10–2.67, P < .017) as well as other risk factors not included in the CHA2DS2-VASc score such as valvular heart disease (OR 5.04, 95% CI 1.10-23.10, P = .037), hyperlipidemia (OR 4.82, 95% CI 1.03–22.63, P = .046) and chronic renal failure (OR 14.21, 95% CI 2.41–83.91, P = .003). The type of AF type did not affect survival (P = .158) nor the incidence of stroke (P = .466). Patients with paroxysmal AF were linked to significantly lower frequencies of ischemic heart disease (P < .0001), vascular disease (P = .002), diabetes mellitus (P = .047), valvular heart disease (P = .03) and heart failure/left ventricular dysfunction (P = .015).
Conclusion:
The CHA2DS2-VASc score correctly predicted the patients at high-risk for 3 to 5 years mortality and confirmed its significant predictive value in the patients with AF.
Keywords: atrial fibrillation, CHA2DS2-VASc, mortality, risk evaluation
1. Introduction
Atrial fibrillation (AF) is the most common cardiac rhythm disorder and is associated with an increased risk of mortality and morbidity from stroke and thromboembolism. This risk is not homogeneous and can best be estimated in individual patients using the congestive heart failure, hypertension, age, diabetes mellitus, prior stroke or TIA or thromboembolism, vascular disease, age, sex category (CHA2DS2-VASc) score.[1–3] The CHA2DS2-VASc score is an extension of the CHADS2 score and includes a number of risk factors for ischemic stroke such as age, patient history, gender and clinical risk factors and concomitant diseases.[3] The European Society of Cardiology[4] as well as the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society[5] have recommended the use of the CHA2DS2-VASc score in the management of atrial fibrillation. The aim of this score is to improve the stratification of risk of stroke in patients diagnosed with AF by identifying patients who could safely do without oral anticoagulation therapy.
There is considerable variability in published results relating to the mortality of AF patients in correlation with CHA2DS2-VASc score, especially with regards to patient history, drug treatment and clinical status.[6–13] There are several important clinical characteristics that affect the risk of such events, for example, coronary artery disease, heart failure, diabetes mellitus, and hypertension, and this could explain, at least in part, the difference between the results of the present and previous studies. Also, considerable variation exists in the publications about the risk of ischemic stroke in AF patients with CHA2DS2-VASc score of 1, focused to differences in ischemic stroke versus thromboembolism. give conflicting advice between European guidelines[4] which favor novel oral anticoagulants (NOACs) for CHA2DS2-VASc score of 1 with an alternative being warfarin, whereas the new US guidelines state that no antithrombotic therapy, aspirin, or OAC may be the appropriate treatment strategy for some in this class[5] (Table 1, Table 2).
Table 1.
CHADS2 score and Annual Stroke Risk.
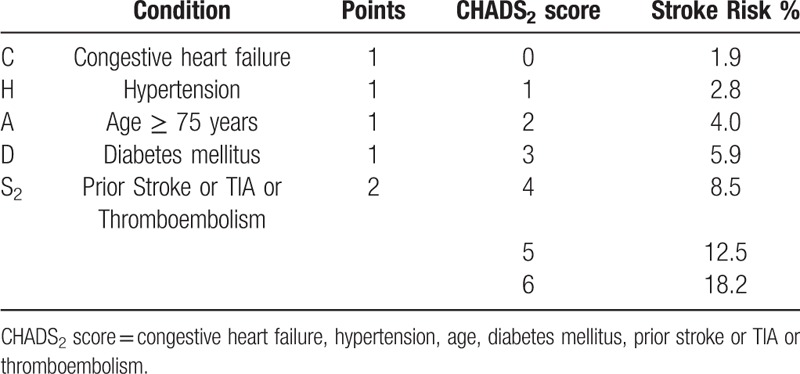
Table 2.
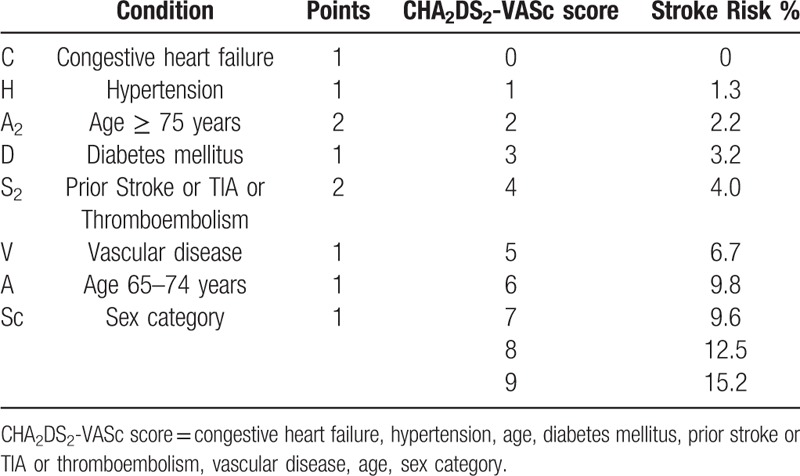
CHA2DS2-VASc score and annual stroke risk.
The purpose of this retrospective study is to further characterize the predictive value of the CHA2DS2-VASc score not only in terms of a patient's individual risk for stroke and need for anticoagulation therapy, but also for all-cause mortality in patients suffering from paroxysmal, persistent and permanent AF, because such specific data is missing in present literature.
2. Methods
2.1. Study design
We performed an observational retrospective cohort study (CONSORT compliant) of randomly selected patients with a primary diagnosis of ICD-10-CM Code I48 Atrial fibrillation that were hospitalized between 2009 and 2011 in Department of Arrhythmias and Cardiac Pacing of the National Institute of Cardiovascular Diseases in Bratislava, Slovakia. We collected data from hospital discharge documents and monitored survival on the selected patients for 3 to 5 years following discharge from the hospital. The primary outcome was to determine differences in comorbidities and the risk factors of stroke in the 3 types of atrial fibrillation. The secondary outcome was to investigate the prognostic value of CHA2DS2-VASc score and other risk factors for all-cause mortality and survival of patients with AF. The study was approved by the institutional review board.
Based on the presentation, duration, and spontaneous termination of AF episodes, the types of AF are traditionally distinguished: paroxysmal, persistent, and permanent AF.[4,5] Paroxysmal - self-terminating, in most cases within 48 hours. Some AF paroxysms may continue for up to 7 days. AF episodes that are cardioverted within 7 days should be considered paroxysmal. Persistent - AF that lasts longer than 7 days, including episodes that are terminated by cardioversion, either with drugs or by direct current cardioversion, after 7 days or more. Long-standing persistent AF Continuous AF lasting for ≥1 year when it is decided to adopt a rhythm control strategy.
Permanent - AF that is accepted by the patient (and physician). Hence, rhythm control interventions are, by AF should be adopted as a rhythm control strategy, the arrhythmia would be re-classified as “long-standing definition”, not pursued in patients with permanent persistent AF.[4,5]
2.2. Patient selection and data collection
The cohort consisted of 137 patients (mean age 61 ± 15; 34.3% women) with a confirmed diagnosis of AF. Patients were classified according to sex and type of AF – (paroxysmal, persistent or permanent fibrillation subgroups). Data relating to cardiovascular risk factors, comorbidities, etiology of AF, drug therapy, previous cardiac surgery, and clinical parameters were collected from hospital discharge documents. The mortality data was obtained from government records. During the follow-up period of our study, 121 patients survived and 16 patients died.
2.3. CHA2DS2-VASc score
The CHA2DS2-VASc score is a point-based system used to stratify the risk of stroke in AF patients. The acronym CHA2DS2-VASc stands for congestive heart failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65 to 74 and sex category (female). Two points are awarded for stroke, transient ischemic attack or thromboembolism in the medical history and for age over 75. One point is assigned for age 65 to 75, hypertension, diabetes mellitus, heart failure or left ventricular systolic dysfunction (ejection fraction <40%), vascular disease (prior myocardial infarction, peripheral artery disease, aortic plaque), and female sex. Patients were stratified into 3 groups according to CHA2DS2-VASc score as recommended by the European Society of Cardiology guidelines for Atrial Fibrillation: score 0 – low risk, score 1 – medium risk, score ≥2 – high risk (Table 1, Table 2).[4]
2.4. Statistical analysis
Statistical analysis was performed using Graph Pad Prism, v6.0c and MedCalc, v12.7.5 software. For descriptive statistics, we evaluated continuous data using the arithmetic mean ± standard deviation whereas for categorical data, results were reported as numbers and percentages. As the majority of data was categorical, we applied Chi-square test to compare subgroups and also identify the tendency for trends. Results with P value <.05 (2-sided) were considered to be statistically significant. Multivariate logistic regression analysis was used to determine the predictive strength of the risk factors regarding mortality. Survival was evaluated using Kaplan-Meier curves. Survival distribution of groups was compared using the logrank (Mantel-Cox) test.
3. Results
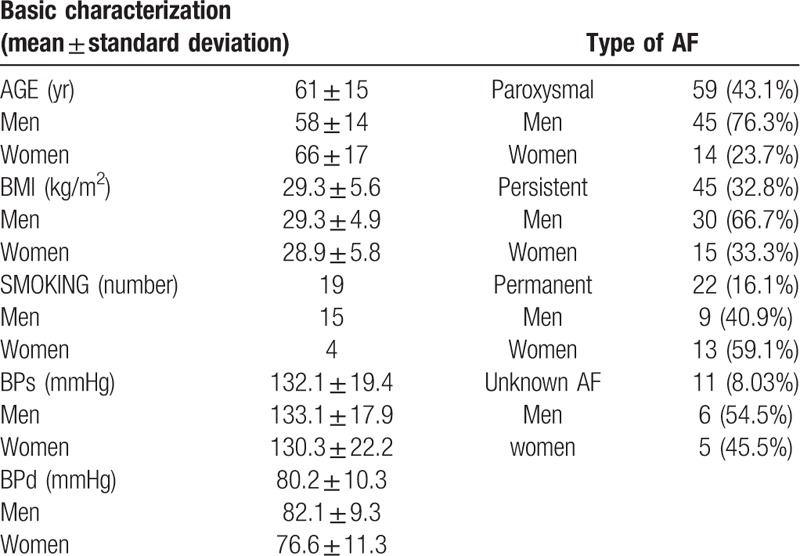
The number of patients included in the study was 137 and the follow-up time was from 3 to 5 years. The characteristics of the patients at the time of hospital discharge are described in Table 3. Patients were older with mean age of 61 and above normal weight according to the mean BMI 29.3 ± 5.6 kg/m2. The majority of patients suffered from paroxysmal (43.1%), followed by persistent (32.8%) and permanent (16.1%) AF. In 8.03% of cases, we could not determine the AF type from the hospital record.
Table 3.
Characteristics of patients at time of hospital discharge.
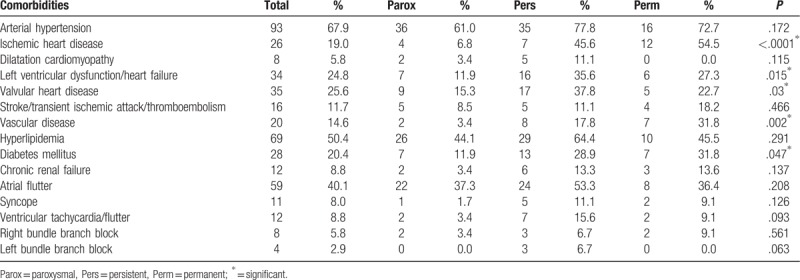
We collected data about comorbidities and related them to the various AF types, as shown in Table 4. The most common comorbidity was arterial hypertension (67.9%), followed by hyperlipidemia (50.4%) and atrial flutter (40.1%). In patients with permanent fibrillation, the most common comorbidity was ischemic heart disease (54.5%). A significant statistical difference between the incidence of some comorbidities was found between the 3 types of AF: ischemic heart disease (P < .0001), vascular diseases – as defined above (P = .002), diabetes mellitus (P = .047), valvular heart disease (P = .03) and heart failure or left ventricular dysfunction (P = .015).
Table 4.
Comorbidities in our patient group compared in different types of AF.
A statistically significant difference in age was detected when comparing the incidence of comorbidities between men and women for age≥75 (Fig. 1A), with women older than 75 having a higher incidence of comorbidities (P < .0001) (Fig. 1A). Figure 1B shows the comparison of comorbidities between the 3 types of AF.
Figure 1.
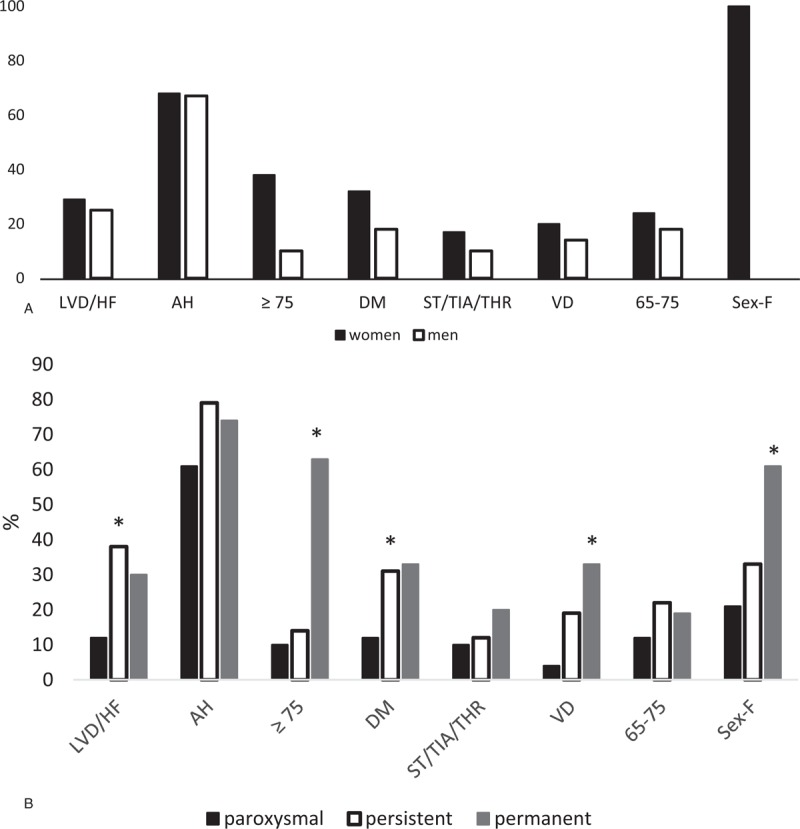
A: Comparison of comorbidities between men and women. B: Comparison of comorbidities between types of AF.
Calculated CHA2DS2-VASc scores identified 43.8% patients as low to intermediate risk (score 0–1) and 56.2% patients as high risk (score ≥2). These scores are reported in Figure 2. The most frequent was score of 1 (32.9%) and 4 (16.8%), closely followed by 3 (15.3%). In the group with the paroxysmal fibrillation, the most common score was 1 (47.46%), and in the permanent AF group it was 4 (22.73%) and 5 (27.27%). There was also an increase in the incidence of stroke with increasing CHA2DS2-VASc scores (Ptrend < .0001).
Figure 2.
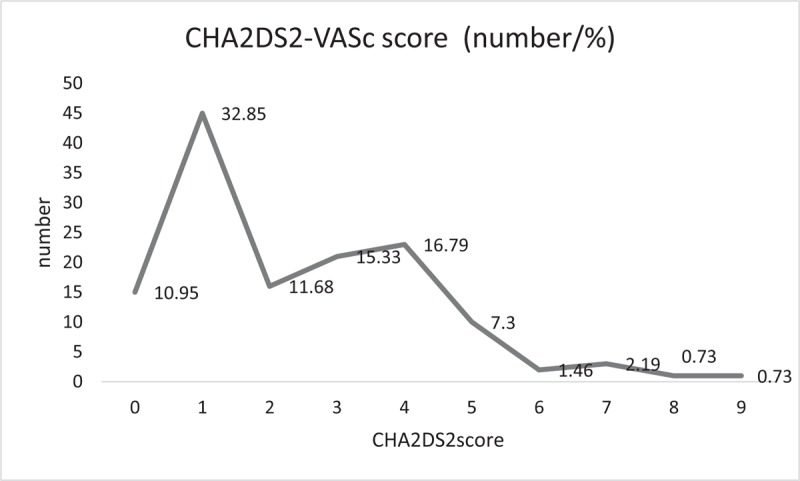
CHA2DS2-VASc score distribution. CHA2DS2-VASc score = congestive heart failure, hypertension, age, diabetes mellitus, prior stroke or TIA or thromboembolism, vascular disease, age, sex category.
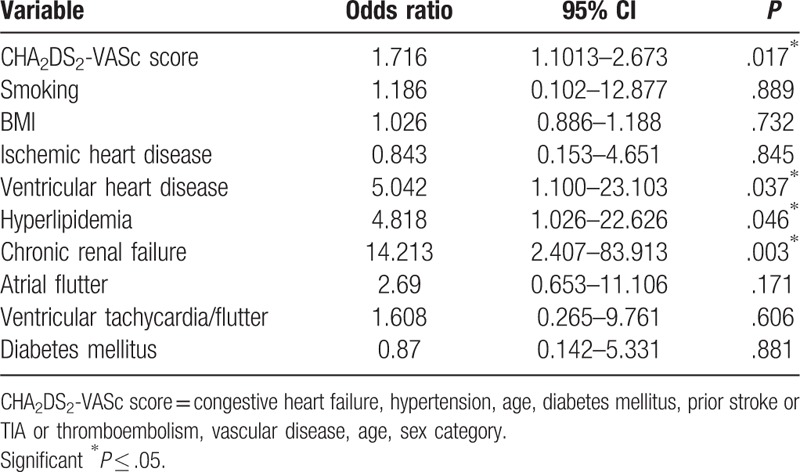
The predictive value of CHA2DS2-VASc regarding the overall, all-cause mortality was confirmed (OR 1.71, 95% CI 1.10–2.67, P < .017), as patients with higher risk scores had a survival rate of 79.1%, whereas medium risk and low risk patients had survival rates of 95.6% and 100%, respectively. Every 1 point increase in the CHA2DS2-VASc score almost doubled the probability of death. The Kaplan–Meier survival curves over the 5 years following discharge are shown in Figure 3. At the end of the follow-up period, the cohort consisted of only 121 patients (88.3% out of 137). Furthermore, we looked at other risk factors of mortality, not included in the CHA2DS2-VASc score that could be predictors of mortality in AF. Significant predictors were found to be valvular heart disease (OR 5.04, 95% CI 1.10–23.10, P = .037), hyperlipidemia (OR 4.82, 95% CI 1.03–22.63, P = .046) and chronic renal failure (OR 14.21, 95% CI 2.41–83.91, P = .003) (Table 5). AF type did not considerably affect the survival rates (paroxysmal, persistent and permanent AF: 94.9%, 86.7%, 77.3% survival, respectively, P = .158) or the incidence of stroke (P = .466). Concerning the drug therapy, 86.1% of the patients received anticoagulation treatment, combined with an anti-platelet therapy in 12.4% of cases. Only anti-platelet therapy was used in 10.2% of the patients and 3.7% of patients did not receive any therapy. Comparison between the anticoagulation and anti-aggregation treatment between the 3 groups of AF did not show any significant statistical difference (P = .208 and P = .167, respectively).
Figure 3.
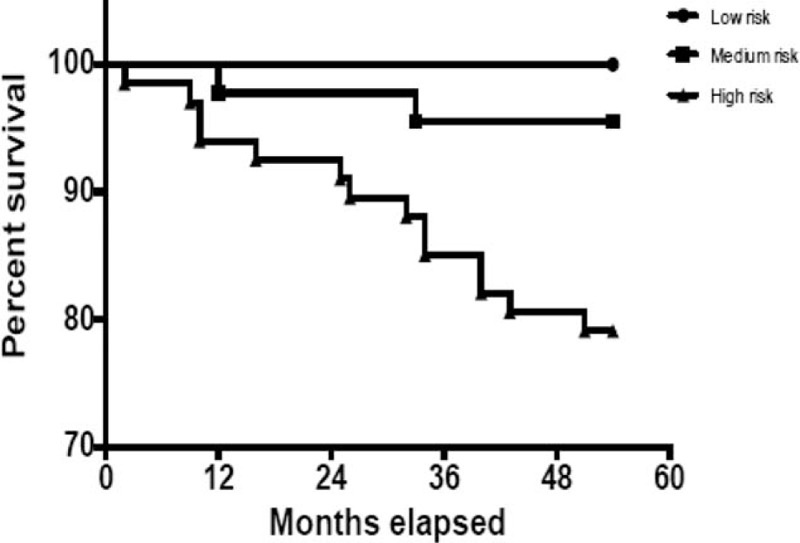
Survival curve by evaluation of risk as determined by CHA2DS2-VASc score. CHA2DS2-VASc score = congestive heart failure, hypertension, age, diabetes mellitus, prior stroke or TIA or thromboembolism, vascular disease, age, sex category.
Table 5.
Multivariate analysis of mortality predictors in AF.
4. Discussion
Although many groups have reported their findings with regards to a number of variables that are associated with outcomes of patients diagnosed with AF (such as bleeding, thrombi or embolisms), fewer reports have looked more specifically at mortality and its causes.[6–13] Because the CHA2DS2-VASc introduces new variables very useful for the clinical cardiologist, the ESC[4] as well as the ACC/AHA[5] recommended the CHA2DS2-VASc score in the management of atrial fibrillation in guidelines. The CHA2DS2-VASc introduces new variables very useful for the clinical cardiologist such as previous myocardial infarction or chronic stable coronary disease in all its clinical manifestation, and peripheral vascular disease, including aortic atherosclerosis, lower extremities arterial disease, and carotid artery disease, and also congestive heart failure, hypertension, diabetes, stroke vascular disease, age, and sex category.[6]
Our study is distinctive in investigating the variables that influence the risks of all-cause mortality for all 3 major forms of AFs. This study shows that the CHA2DS2-VASc score correctly identified high-risk patients and confirmed its significant predictive value in patients with AF as confirmed by a number of other clinical trials. In a study population of 8962 patients, Philippart et al[14] established the prognostic value of the CHA2DS2-VASc score in patients with non-valvular AF and valvular heart disease. Moreover, in the prospective Danish “diet, cancer and health” cohort study, Larsen et al[15] demonstrated the added predictive ability of the CHA2DS2-VASc score over the CHADS2 score. As the CHA2DS2-VASc score is more inclusive of common stroke risk factors, more patients with AF fulfill the criterion for anticoagulation treatment, thereby having the potential of reducing their risk of stroke and death. The mortality in our study was 11.7%, which is similar to what was reported in a Danish cohort by Olensen JB et al.[16] Our study further provided evidence that the CHA2DS2-VASc score was the most significant predictor of mortality along with other risk factors not included in the CHA2DS2-VASc score such as valvular heart disease, hyperlipidemia and chronic renal failure.
Other studies have compared a number of existing risk stratification schemes. Fox et al[8] recently compared the novel GARFIELD-AF risk tool to the CHA2DS2-VASc score and the HAS-BLED scoring systems in a cohort of 39 898 patients. This prospective study validated that the newly –constructed GARFIELD-AF integrated risk tool can be used to predict the risk of bleeding, stroke, and mortality in AF patients. The results of the GARFIELD-AF study support the data obtained from our retrospective trial showing an increase in stroke and mortality with increasing CHA2DS2-VASc scores.[17,18]
Comparing the incidence of comorbidities between men and women, we observed a statistical difference in the incidence of comorbidities between men and women for age ≥ 75, with women older than 75 showing a significantly higher incidence of comorbidities. This finding is in keeping with recent studies[19,20] showing later onset and increased severity of AF in older women. Furthermore, gender differences could be attributable to the differences that exist in the treatment recommendations and administration of oral anticoagulation therapy for female versus male patients across various guidelines. As discussed by Lip and Nielsen,[21] the American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guidelines for the management of AF recommend treatment for patients with a CHA2DS2-VASc score = 1 for men, and score = 2 women.[5] However, the European guidelines favor treatment in patients with 1 non sex-related risk factor.[22,23] The Europeans guidelines were followed for patients included in our analysis.
This study was focused on investigating the variables relating to the clinical status of AF patients for all 3 major types of AF. The type of AF type did not affect survival, nor the incidence of stroke and bleeding, nor the anticoagulation and anti-aggregation treatment. On the other hand, our findings have identified significant variations in the comorbidities between paroxysmal, persistent, and permanent AF as is stated in Table 4. Patients with paroxysmal AF were shown to suffer from significantly lower frequencies of ischemic heart disease, vascular disease, diabetes mellitus, valvular heart disease and heart failure/left ventricular dysfunction. Our data complements an increasing array of findings generated both prospective and retrospective clinical trials[6–13] that, considered together, continue to shape and improve the risk stratification of patients with AF in terms of the prediction of mortality, stroke and bleeding as well as the optimal timing of anticoagulation therapy.
4.1. Limitations
Our study included a limited number of patients as it was the first analysis of this type that was performed in the National Institute of Cardiovascular Diseases. Moreover, not all clinical information was contained in every patient's medical records. More specifically, patients that were referred to the regional cardiologists for follow-up after discharge had incomplete data.
5. Conclusion
Our data identified several important clinical variables that are associated with the risk of one or more outcomes, in terms of mortality and stroke. The type of AF type did not affect survival nor the incidence of stroke. However, patients with paroxysmal AF were linked to significantly lower frequencies of ischemic heart disease, vascular disease, diabetes mellitus, valvular heart disease and heart failure/left ventricular dysfunction. Therefore, the comprehensive management of AF should encompass, not only anticoagulation treatment, but also therapy for comorbidities strongly associated with poor outcomes, namely valvular heart disease, hyperlipidemia and chronic renal failure. The CHA2DS2-VASc score is a useful tool in identifying patients with high risk of mortality that would benefit from anticoagulation therapy.
Author contributions
Project administration: Mária Rexová, Róbert Hatala.
Supervision: John J. Leddy, Peter Hlivák, Ján Kyselovič.
Writing – original draft: Andrea Gažová.
Footnotes
Abbreviations: AF = atrial fibrillation, AH = arterial hypertension, BPd = blood pressure diastolic, BPs = blood pressure systolic, CHA2DS2-VASc score = congestive heart failure, hypertension, age, diabetes mellitus, prior stroke or TIA or thromboembolism, vascular disease, age, sex category, CHADS2 score = congestive heart failure, hypertension, age, diabetes mellitus, prior stroke or TIA or thromboembolism, DM = diabetes mellitus, LVD/HF = left ventricular dysfunction/heart failure, Parox = paroxysmal, Perm = permanent, Pers = persistent, ST/TIA/THR = stroke/transient ischemic attack/thromboembolism, VD = vascular disease.
This publication was made possible throught the support of APVV-14-0416 and MZ SR 2016/13-FAUK-1.
The authors declare no conflict of interest.
References
- [1].Wolf PA, Abbot RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–8. [DOI] [PubMed] [Google Scholar]
- [2].You JJ, Singer DE, Howard PA, et al. American College of Chest Physicians. Antithrombotic therapy for atrial fibrillation: Antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians. Evidence-based clinical practice guidelines. Chest 2012;141:e5315–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lip GY, Nieuwlaat R, Puisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. Chest 2012;137:263–327. [DOI] [PubMed] [Google Scholar]
- [4].Kirchhof P, Benussi S, Kotecha D, et al. ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. doi: 10.1093/eurheartj/ehw210. Epub 2016 Aug 27. [DOI] [PubMed] [Google Scholar]
- [5].January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–76. [DOI] [PubMed] [Google Scholar]
- [6].Jackson LR, Kim S, Fonarow GC, et al. Stroke risk and treatment in patients with atrial fibrillation and low CHA2DS2-VASc scores: findings from the ORBIT-AF I and II registries. J Am Heart Assoc 2018;7:e008764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Camm AJ, Accetta G, Ambrosio G, et al. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart 2017;103:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fox KAA, Lucas JE, Pieper KS, et al. Improved risk stratification of patients with atrial fibrillation: an integrated GARFIELD-AF tool for the prediction of mortality, stroke and bleed in patients with and without anticoagulation. BMJ Open 2017;7:e017157doi:10.1136/bmjopen-2017-017157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Andersson T, Magnuson A, Bryngelsson IL, et al. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995–2008: a Swedish nationwide long-term case-control study. Eur Heart J 2013;34:1061–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boriani G, Laroche C, Diemberger I, et al. ‘Real-world’ management and outcomes of patients with paroxysmal vs. non-paroxysmal atrial fibrillation in Europe: the EURObservational Research Programme-Atrial Fibrillation (EORP-AF) General Pilot Registry. Europace 2016;18:648–57. [DOI] [PubMed] [Google Scholar]
- [11].Fauchier L, Samson A, Chaize G, et al. Cause of death in patients with atrial fibrillation admitted to French hospitals in 2012: a nationwide database study. Open Heart 2015;2:e000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Massera D, Wang D, Vorchheimer DA, et al. Increased risk of stroke and mortality following new-onset atrial fibrillation during hospitalization. Europace 2017;19:929–36. [DOI] [PubMed] [Google Scholar]
- [13].Pokorney SD, Piccini JP, Stevens SR, et al. Cause of death and predictors of all-cause mortality in anticoagulated patients with nonvalvular atrial fibrillation: data from ROCKET AF. J Am Heart Assoc 2016;5:e002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Philippart R, Brunet-Bernard A, Clementy N, et al. Prognostic value of CHA2DS2-VASc score in patients with non-valvular atrial fibrillation and valvular heart disease: the Loire Valley Atrial Fibrillation Project. Eur Heart J 2015;36:1822–30. [DOI] [PubMed] [Google Scholar]
- [15].Larsen TB, Lip GY, Skjoth F, et al. Added predictive ability of the CHA2DS2-VASc risk score for stroke and dech in patients with atrial fibrillation. The prospective Danish diet, cancer and health cohort study. Circ Cardio-VASc Qual Outcomes 2012;5:335–42. [DOI] [PubMed] [Google Scholar]
- [16].Olensen JB, Lip GY, Hansen PR, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Inoue H, Atarashi H, Okumura K, et al. on behalf of the J-RHYTHM Registry Investigators. Impact of gender on the prognosis of patients with non-valvular atrial fibrillation. Am J Cardiol 2014;113:957–62. [DOI] [PubMed] [Google Scholar]
- [18].Perna GP. High CHA2DS2-VASc score without atrial fibrillation: ’NAO yes, NAO no’. Eur Heart J Suppl 2019;21Suppl B:B67–8. doi: 10.1093/eurheartj/suz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Magnussen C, Niiranen TJ, Ojeda FM, et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation 2017;136:1588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Emdin CA, Wong CX, Hsiao AJ, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ 2016;532:h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lip GYH, Nielsen PB. Should patients with atrial fibrillation and 1 stroke risk factor (CHA2DS2-VASc Score 1 in Men, 2 in Women) be anticoagulated? Circulation 2016;133:1498–503. [DOI] [PubMed] [Google Scholar]
- [22].Camm AJ, Lip GY, De Caterina R, et al. ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–47. [DOI] [PubMed] [Google Scholar]
- [23].Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]


