Abstract
Background:
The role of Helicobacter pylori eradication is still not clear in endoscopic submucosal dissection (ESD)-induced artificial ulcer. This study investigates the therapeutic effects of H. pylori eradication on ESD-induced artificial ulcers.
Methods:
Eighty-four patients with ESD-induced artificial ulcers were enrolled. H. pylori eradication success subgroup (Group A1) and H. pylori eradication failure subgroups (Group A2) received standard triple therapy orally for 7 days, followed by esomeprazole 20 mg bis in die (bid) orally for the remainder of the treatment period (4 weeks in total). The H. pylori positive (Group B1) and H. pylori negative subgroups (Group B2) received esomeprazole 20 mg bid orally for 4 weeks. Ulcer healing was evaluated by gastroscopy, and H. pylori was identified by a C13 breath test or an Hp-RUT 2 and 6 months after treatment.
Results:
Successful eradication of H. pylori can promote healing of ESD-induced artificial ulcers. The ESD-induced artificial ulcer healing rate in Group A1 was statistically higher than that in Groups A2, B1, and B2.
Conclusion:
Our results indicated that early H. pylori eradication therapy can promote ESD-induced artificial ulcer healing in H. pylori positive patients with ESD-induced artificial ulcers.
Keywords: ESD-induced artificial ulcer, H. pylori, healing
1. Introduction
Endoscopic submucosal dissection (ESD) has been widely used for endoscopic treatment, especially for early digestive tract tumors, submucosal lesions, giant polyps, and carcinoids. This technique has the advantages of minor trauma, decreased complications, fast recovery, and decreased impact on the patient's physical condition. However, different-sized artificial ulcers can begin to form after ESD.[1] Some artificial ulcers fail to heal within 8 weeks after ESD. Quality of life for patients with unhealed artificial ulcers may be impeded because of symptoms such as abdominal pain, indigestion, and other complications such as bleeding and perforation.[2] Many factors may influence postoperative ulcer healing, such as smoking, drinking, treatment compliance, Helicobacter pylori (H. pylori), nutritional status, and other drugs that damage the digestive tract mucosa. Some previous studies have indicated that H. pylori eradication can promote ulcer healing, but other studies have found contradictory results.[3–5] In the present study, we aimed to assess the therapeutic effects of H. pylori eradication on ESD-induced artificial ulcers.
2. Methods
2.1. Study subjects
This study was a retrospective study. H. pylori eradication therapy, ESD, and gastroscopy are already used in clinical practice. Given that the medical information of the patients was recorded necessarily and anonymously as part of the case history, which would not cause any risk to the participants, the Ethics Committee of Weihai Municipal Hospital approved this retrospective study with a waiver of informed consent from the patients.
Inpatients who underwent ESD treatment from January 2010 to May 2018 at Weihai Municipal Hospital were included. Patients were excluded according to the following criteria: severe organ dysfunction, anticoagulant, nonsteroidal anti-inflammatory drugs, or other gastric mucosal damaging drug use, or unhealthy living habits such as smoking, drinking, poor sleeping habits, or addictive/poor eating behaviors. H. pylori was measured in all patients. All patients were divided into the H. pylori eradication treatment group (Group A) and the non-H. pylori eradication treatment group (Group B). According to the H. pylori eradication results, Group A was divided into the H. pylori eradication success subgroup (Group A1) and the H. pylori eradication failure subgroup (Group A2). Group B was divided into the H. pylori positive subgroup (Group B1) and the H. pylori negative subgroup (Group B2).
After patients were treated with ESD, Group A received standard triple therapy (esomeprazole 20 mg bis in die (bid), amoxicillin 1 g bid, and clarithromycin 0.5 g bid or levofloxacin 0.5 g quaque die (qd)) orally for 7 days, followed esomeprazole 20 mg bid orally for the remainder of the treatment period (4 weeks in total). Ulcer healing was evaluated by gastroscopy, and H. pylori was identified by a C13 breath test or an H. pylori rapid urease test (Hp-RUT) 2 and 6 months after treatment.
2.2. Calculating the ulcer area
The ulcer area was calculated with the traditional formula: the endoscope measurement ruler was placed in the stomach via a biopsy port that was close to the ulcer lesions.[6] The long diameter (d1) and short diameter (d2) were measured. Ulcer area = π (d1/2) (d2/2).
2.3. Evaluation criteria for ulcer healing
Gastric ulcer stages were classified using a 6-stage system[7]:
(1) A1 stage: Ulcer that contains a mucus coating with marginal elevation because of edema.
(2) A2 stage: Mucus-coated ulcers with discrete margins and less edema than stage A1.
(3) H1 stage: Unhealed ulcer covered by less than 50% regenerating epithelium with or without converging folds.
(4) H2 stage: Ulcer with a mucosal break but almost covered with regenerating epithelium.
(5) S1 stage: Red scar with rough epithelialization without a mucosal break.
(6) S2 stage: White scar with complete re-epithelialization.
2.4. Statistics
Statistical comparisons of the patients were performed using the χ2 test for categorical data and Student t test and analysis of variance (ANOVA) for numerical data. Data are expressed as the mean ± standard deviation. Differences in the categorical variables between the 2 groups were examined with the χ2 test. A 2-tailed P value less than .05 was considered statistically significant.
3. Results
3.1. Patient characteristics
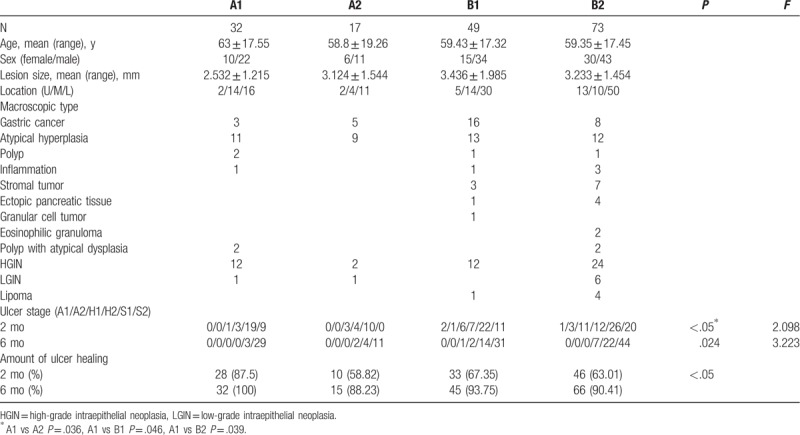
Group A and Group B had no significant differences in factors, such as sex ratio, age, and ulcer area (Table 1). In each group, most of the lesions were located in the lower part of the stomach, followed by the middle and upper parts of the stomach. The pathological types were classified as gastric cancer, atypical hyperplasia, stromal tumors, polyps with atypical dysplasia, high-grade intraepithelial neoplasia (HGIN), polyps, ectopic pancreatic tissue, granular cell tumors, eosinophilic granulomas, lipomas, and inflammation (Tables 1 and 2).
Table 1.
Baseline characteristics of patients in Groups A and B.
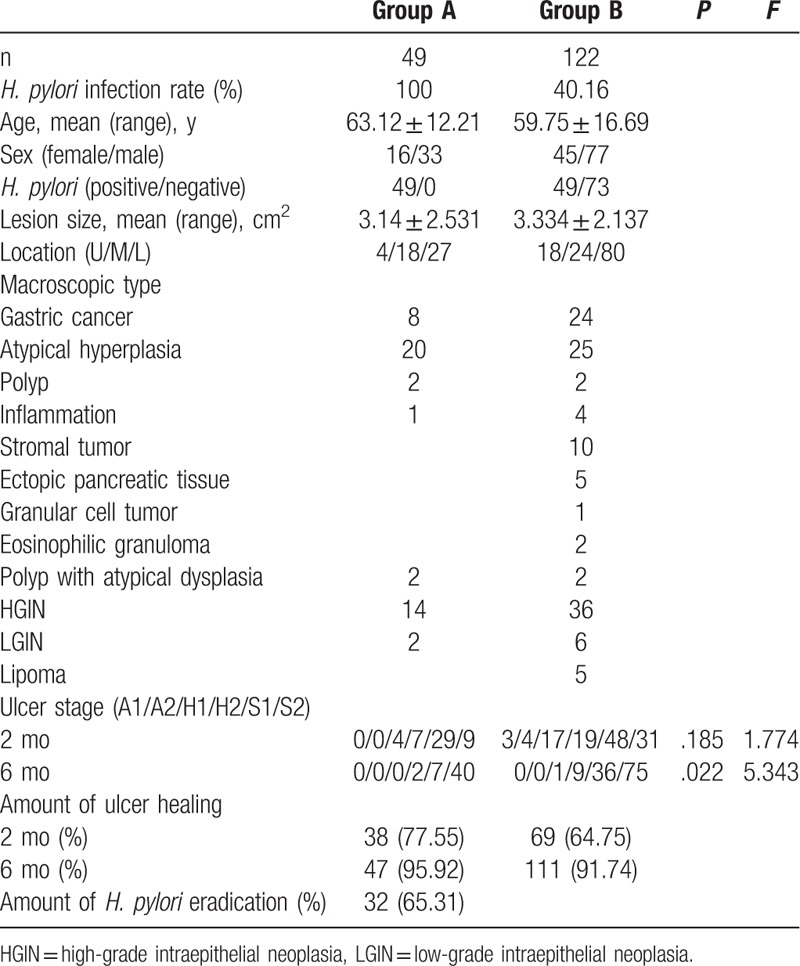
Table 2.
Baseline characteristics of patients in Groups A1, A2, B1, B2.
3.2. Successful H. pylori eradication can promote healing of ESD-induced artificial ulcers
Two months after ESD, the healing rate in Group A1 was significantly higher than those in Groups A2, B1, and B2 (87.50% vs 58.82%, 67.35%, and 63.01%, respectively), but that in Group A2 was slower but not significantly different than those in Groups B1 and B2 (58.82% vs 67.35% and 63.01%, respectively). Six months after ESD, the healing rate in Group A1 (100.00%) and was higher but not significantly different than those in Group A2 (88.23%), Group B1 (93.75%), and Group B2 (90.41%) (Table 1). Two months later, there was no statistically significant difference in ulcer stage between Group A and Group B (Fig. 1) (P > .05). Six months later, only 2 patients were in stage H2 and the others in Group A were in stage S1 or S2; however, in Group B, 1 patient was in stage H1, 9 patients were in stage H2, and the rest were in stage S1 or S2. Therefore, there was significant difference between the two group (Table 2, Fig. 2) (P = .022). In terms of ulcer stage, Group A1 had better stages than Groups A2, B1, and B2 (Table 2, Figs. 3 and 4). In addition, 1 case of HGIN with local carcinogenesis in small curvature of gastric antrum was treated with ESD in Group B 1.2 months later, the artificial ulcer was in H2 stage. But 6 months later, HGIN was identified at the site of the scar. Therefore, the patient was excluded from the statistical data for 6 months after ESD.
Figure 1.
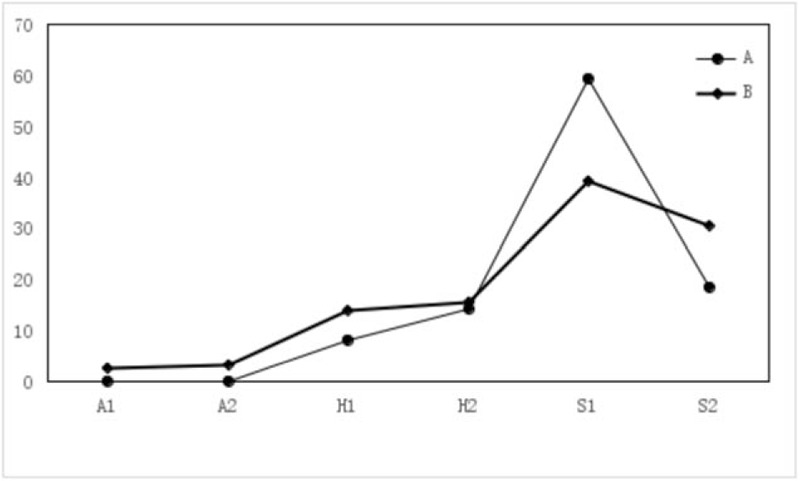
The F-figure for ulcer staging ratio of 2 months after ESD in Groups A, B. There was not statistically significant difference in ulcer stage between Group A and Group B (P > .05).
Figure 2.
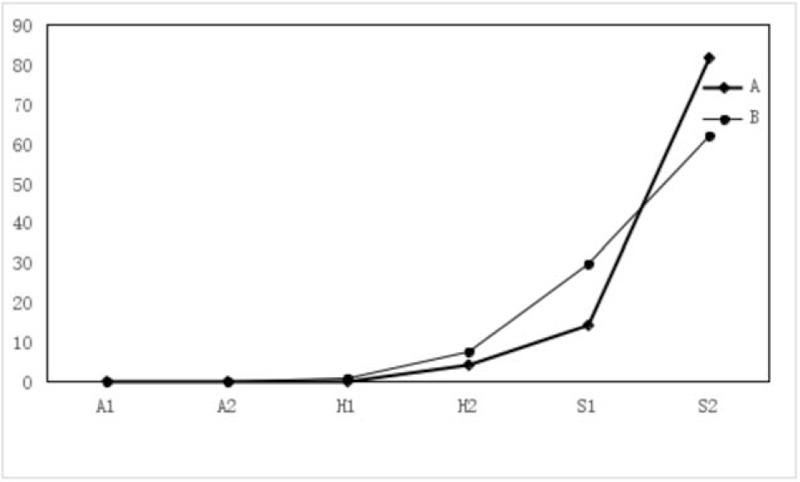
The F-figure for ulcer staging ratio of 6 months after ESD in Groups A, B. Only 4.08% were in stage H2 and 95.92% were in stage S1 or S2 in Group A; however, in Group B, 0.82% were in stage H1, 7.38% were in stage H2, and 91.74% were in stage S1 or S2. Therefore, there was significant difference between the 2 group (P = .022).
Figure 3.
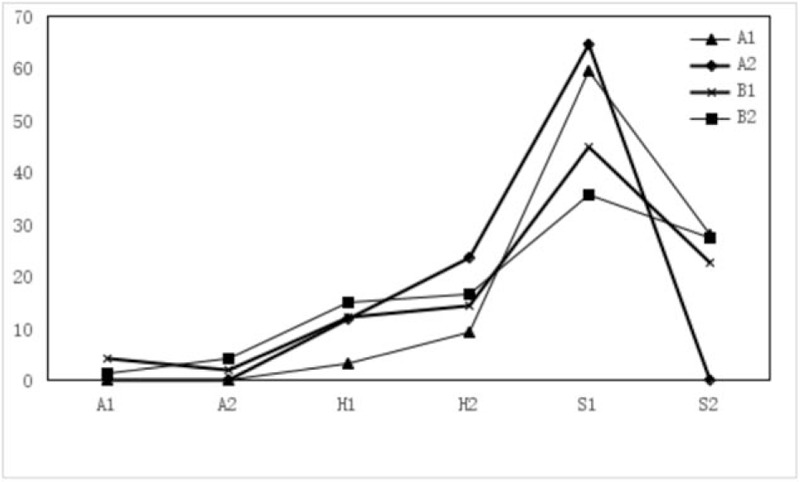
The F-figure for ulcer staging ratio of 2 months after ESD in Groups A1, A2, B1, B2. There was significant difference in ulcer stage between Group A1 and Group A2 [stage H1: 1 vs 3 case (3.13% vs 17.65%), stage H2: 3 vs 4 case (9.38% vs 23.53%)] (P < .05), but there was a statistically significant difference in the stage S2 between Group A1 and Group A2 (P < .05). In Groups B1 and B2, it still had few patients in stage A1 (4.08%, 1.37%), A2 (2.04%, 4.11%). But Group A1 and Group A2 did not have patients in stage A. Group A1 had better stages than Group A2 (P = .036), Groups B1 (P = .046), and B2 (P = .039).
Figure 4.
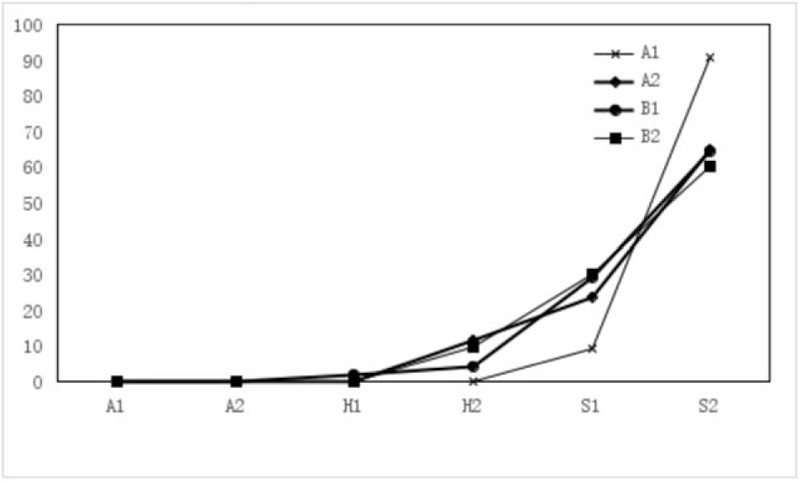
The F-figure for ulcer staging ratio of 6 months after ESD in Groups A1, A2, B1, B2. About 11.76% were in stage H2 and the others were in stage S1 (23.53%) or S2 (64.71%) in Group A2, but all patients in Group A1 were in stage S1 (8.38%) or S2 (91.62%). In Group B1, 2.04% were in stage H1, 4.08% were in stage H2, 28.57% were in stage S1, and 63.27% were in stage S2. In Group B2, 9.59% were in stage H2, 30.14% were in stage S1, and 60.27% were in stage S2. Group A1 had better stages than Group A2, Groups B1, and B2 (P = .024).
3.3. Ulcer healing in patients with successful H. pylori eradication was significantly faster than that in patients with failed H. pylori eradication
The eradication rate of H. pylori in Group A was 65.31%. Ulcer healing in patients with successful H. pylori eradication was faster than that in those with failed H. pylori eradication. However, there was significant difference in the healing rate of 2 months between Group A1 and Group A2 (87.50% vs 58.82%) (Table 2, Fig. 3) (P < .05). But there was not significant difference in that of 6 months (100.00% vs 88.23%) (Table 2); the rate of group A1 was faster but Group A2 was slower than those of Groups B1 and B2 (Figs. 3 and 4). Endoscopic findings 2 months later revealed that there was a significant difference in ulcer stage between Group A1 and Group A2 [stage H1: 1 vs 3 case (3.13% vs 17.65%), stage H2: 3 vs 4 case (9.38% vs 23.53%)] (P < .05), but there was a statistically significant difference in the number of patients with stage S2 between Group A1 and Group A2 (9 vs 0 cases) (Table 2, Fig. 3) (P < .05). Regarding endoscopic findings 6 months later, only 2 patients were in stage H2 and the others were in stage S1 or S2 in Group A2, but all patients in Group A1 were in stage S1 or S2 (Table 2, Fig. 4).
4. Discussion
ESD is the primary treatment for early digestive tract carcinoma, precancerous lesions, giant polyps, and submucous eminence lesions. Because this technique is minimally invasive, associated with rapid recovery, decreased pain, and en bloc lesion resection, it has been rapidly popularized since it was introduced in China 10 years ago. However, ESD also has some complications, such as postoperative ulceration, bleeding, perforation, postoperative digestive tract stenosis, and lesion recurrence. Among them, artificial ulceration is the most common complication that can affect the quality of life of patients because it might cause them to suffer from abdominal pain, bloating, indigestion, hematemesis, hemafecia, or weakness. The pathogenesis of ESD-induced artificial ulceration is not the same as that of nonartificial gastric ulceration: Lack of enhanced gastric mucosal damage and impaired defensive function; Most gastric ulcers are deeper than ESD-induced artificial ulcers; H. pylori does not play a dominant role in ulcer formation. It is believed that H. pylori may be involved during the early stages of ulcer formation and interfere with ulcer healing, but it has no effect during the later stages of ulcer formation. Previous studies have reported that H. pylori eradication facilitated ulcer healing at 4 weeks after endoscopic resection but not at 8 weeks.[5,8–10] The role of H. pylori eradication is still not clear in ESD-induced artificial ulcers. Previous studies have confirmed that H. pylori eradication can accelerate ESD-induced artificial ulcer healing and reduce the recurrence of gastric cancer and atypical hyperplasia. However, other studies have drawn the opposite conclusion.[4,5] Proton pump inhibitors (PPIs) are the first-choice treatment for ESD-induced artificial ulcers. The remaining methods are based on PPIs, which include H2 receptor antagonists or gastric mucosal protective agents. These medications can promote healing of artificial ulcers and reduce complications, such as bleeding and perforation. Most courses of treatment last for 4 to 8 weeks. Previous studies have shown that there is no significant difference in the ulcer healing rate after 4 and 8 weeks of treatment.[11]
Because the factors that affect healing of ESD-induced artificial ulcers are still not completely clear, many factors may affect ulcer healing, such as smoking, the location and extent of resection, positive or negative cutting margins, presence or absence of atrophy, H. pylori infection, and ulcer size.[12] To confirm whether eradication of H. pylori can accelerate ESD-induced artificial ulcer healing, this study compared the outcomes after successful H. pylori eradication in ESD-induced artificial ulcer patients with those in H. pylori negative patients, non-H. pylori eradication treatment patients, and H. pylori eradication failure patients. Patients were treated with standard triple therapy for H. pylori eradication 1 week after ESD and then took PPIs until the end of the course (4 weeks). All patients were followed up with endoscopy at 2 and 6 months after the treatment. The results indicate that successful H. pylori eradication can promote healing of ESD-induced artificial ulcers. This finding is consistent with some of the results from relevant literature but contrary to those of others. For example, Higuchi et al[3] performed a control study in patients with post-ESD artificial ulcers treated with triple therapy for 1 week and rebamipide for 7 weeks and a single PPI for 8 weeks. This study found that the healing rate of small ulcers was similar between the 2 groups and that of large ulcers (over 565.5 mm2) in the PPI group was significantly higher than that in the H. pylori eradication group. Kakushima et al[5] suggested that preoperative H. pylori eradication and the degree of atrophic gastritis had no obvious effect on post-ESD ulcer healing. Yoshizawa et al[13] found that the post-ESD ulcer healing rates between preoperative and postoperative triple therapy for H. pylori eradication with the addition of PPIs for 8 weeks were similar and not significantly different. However, our results show that early postoperative H. pylori eradication and PPI treatment can significantly accelerate ESD-induced artificial ulcer healing. Failed eradication might prolong the healing of these ulcers, because the healing rate in Group A2 was slower but not significantly different than those in Groups B1 and B2. This finding suggests that H. pylori may play a certain role in ESD-induced artificial ulcer formation. The mechanism of action of H. pylori in ESD-induced artificial ulcer formation may be different from that in gastric ulcer formation, which is more complicated. The sample size of this study was relatively small. In addition, the study is retrospective that may have the potential to introduce bias in the results. Therefore, we will further increase the sample size and extend the follow-up time to further summarize some relatively reasonable conclusions and to further explore the specific mechanism of action of H. pylori in ESD-induced artificial ulcer formation.
The study shows that the ESD artificial ulcer healing rate of the successful eradication group was faster than the others. Some related literature also shows that H. pylori eradication failure is still a risk factor for artificial ulcer recurrence. Furthermore, H. pylori is a major risk factor for the recurrence of early gastric cancer and atypical hyperplasia, and age is also one of the main risk factors.[14–16] Some studies have shown that eradication of H. pylori can effectively reduce the recurrence of gastric cancer and the incidence of metachronous gastric neoplasias and can promote the healing rate of artificial ulcers.[4,17,18] Therefore, for elderly and H. pylori eradication failure patients, successful H. pylori eradication should also be the main therapeutic target to prevent lesion and artificial ulcer recurrence.
5. Conclusion
Early H. pylori eradication therapy can promote H. pylori positive ESD-induced artificial ulcer healing and can be used as the preferred treatment after ESD. But it also needs to further explore the specific mechanism of action of H. pylori in ESD-induced artificial ulcer formation.
Acknowledgment
We would like to extend our thanks to all participants of this study.
Author contributions
Conceptualization: Wen chong Song.
Data curation: Wen chong Song, Xiao feng Wang, Wei wei Lv, De min Tian.
Formal analysis: Wen chong Song, Xiao feng Wang, Xiao yan Xu.
Supervision: Wen chong Song.
Writing – original draft: Wen chong Song, Xiao feng Wang.
Writing – review & editing: Wen chong Song, De min Tian.
Footnotes
Abbreviations: ESD = endoscopic submucosal dissection, H. pylori = Helicobacter pylori, HGIN = high-grade intraepithelial neoplasia, Hp-RUT = H. pylori rapid urease test, PPIs = proton pump inhibitors.
The authors have no conflicts of interest to disclose.
References
- [1].Sugano K. Detection and management of early gastric cancer. Curr Treat Options Gastroenterol 2015;13:398–408. [DOI] [PubMed] [Google Scholar]
- [2].Lim JH, Kim SG, Choi J, et al. Risk factors of delayed ulcer healing after gastric endoscopic submucosal dissection. Surg Endosc 2015;29:3666–73. [DOI] [PubMed] [Google Scholar]
- [3].Higuchi K, Takeuchi T, Uedo N, et al. Efficacy and safety of 1-week Helicobacter pylori eradication therapy and 7-week rebamipide treatment after endoscopic submucosal dissection of early gastric cancer in comparison with 8-week PPI standard treatment: a randomized, controlled, prospective, multicenter study. Gastric Cancer 2015;18:612–7. [DOI] [PubMed] [Google Scholar]
- [4].Bornschein J, Rokkas T, Selgrad M, et al. Helicobacter pylori and clinical aspects of gastric cancer. Helicobacter 2009;14 suppl 1:41–5. [DOI] [PubMed] [Google Scholar]
- [5].Kakushima N, Fujishiro M, Yahagi N, et al. Helicobacter pylori status and the extent of gastric atrophy do not affect ulcer healing after endoscopic submucosal dissection. J Gastroenterol Hepatol 2006;21:1586–9. [DOI] [PubMed] [Google Scholar]
- [6].Ji S, Kim HS, Kim JW, et al. Comparison of the efficacy of rabeprazole 10 mg and omeprazole 20 mg for the healing rapidity of peptic ulcer diseases. J Gastroenteml Hepatol 2006;21:1381–7. [DOI] [PubMed] [Google Scholar]
- [7].Sakita T, Fukutomi H. Endoscopic diagnosis. Ulcer of Stomach and Duodenum 1971;Tokyo: Nankodo, 198–208. [Google Scholar]
- [8].Cheon JH, Kim JH, Lee SK, et al. Helicobacter pylori eradication therapy may facilitate gastric ulcer healing after endoscopic mucosal resection: a prospective randomized study. Helicobacter 2008;13:564–71. [DOI] [PubMed] [Google Scholar]
- [9].Ueda H, Ito M, Tanaka S, et al. The effect of Helicobacter pylori eradication therapy on gastric ulcer healing after endoscopic mucosal resection. J Clin Gastroenterol 2006;40:293–6. [DOI] [PubMed] [Google Scholar]
- [10].Adachi K, Suetsugu H, Moriyama N, et al. Influence of Helicobacter pylori infection and cetraxate on gastric mucosal blood flow during healing of endoscopic mucosal resection-induced ulcers. J Gastroenterol Hepatol 2001;16:1211–6. [DOI] [PubMed] [Google Scholar]
- [11].Kajiura S, Hosokawa A, Ueda A, et al. Effective healing of endoscopic submucosal dissection-induced ulcers by a single week of proton pump inhibitor treatment: a retrospective study. BMC Res Notes 2015;8:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huang Y, Kakushima N, Takizawa K, et al. Risk factors for recurrence of artificial gastric ulcers after endoscopic submucosal dissection. Endoscopy 2011;43:236–9. [DOI] [PubMed] [Google Scholar]
- [13].Yoshizawa Y, Sugimoto M, Sato Y, et al. Factors associated with healing of artificial ulcer after endoscopic submucosal dissection with reference to Helicobacter pylori infection, CYP2C19 genotype, and tumor location: multicenter randomized trial. Dig Endosc 2016;28:162–72. [DOI] [PubMed] [Google Scholar]
- [14].Kwon YH, Heo J, Lee HS, et al. Failure of Helicobacter pylori eradication and age are independent risk factors for recurrent neoplasia after endoscopic resection of early gastric cancer in 283 patients. Aliment Pharmacol Ther 2014;39:609–18. [DOI] [PubMed] [Google Scholar]
- [15].Shu L, Zheng PF, Zhang XY, et al. Dietary patterns and Helicobacter pylori infection in a group of Chinese adults ages between 45 and 59 years old: an observational study. Medicine (Baltimore) 2019;98:e14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zamani M, Zamani V, Derakhshan MH, et al. The efficacy of first-line regimens for Helicobacter pylori eradication in different continents: a systematic review and network meta-analysis protocol. Medicine (Baltimore) 2018;97:e13682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shehata MA, Talaat R, Soliman S, et al. Randomized controlled study of a novel triple nitazoxanide (NTZ)-containing therapeutic regimen versus the traditional regimen for eradication of Helicobacter pylori infection. Helicobacter 2017;22:e12395. [DOI] [PubMed] [Google Scholar]
- [18].Abd-Elsalam S, Kobtan A, El-Kalla F, et al. A 2-week nitazoxanide-based quadruple treatment as a rescue therapy for Helicobacter pylori eradication: a single center experience. Medicine (Baltimore) 2016;95:e3879. [DOI] [PMC free article] [PubMed] [Google Scholar]


