Abstract
We investigated possible associations between fatty liver and gallstone disease (GD) in a Chinese population.
This cross-sectional study included 897 people who visited the clinical center and underwent ultrasonography at The First Hospital of Jilin University between January 2018 and June 2018.
The overall prevalence of GD was 8.8%; the between-sex difference (9.3% in men, 8.4% in women) was not statistically significant. The risk of GD was similar for men and women across all age groups. GD prevalence increased steadily with increasing age, from 2.1% in patients ≤30 years of age to 15.4% in those >70 years of age. Older age (≥50 years) and fatty liver were associated with GD development. Diabetes mellitus (adjusted odds ratio [AOR]: 3.066; 95% confidence interval [CI]: 1.563–6.013) was associated with GD in female but not in male subjects. In younger patients (<50 years), fatty liver (AOR: 5.268; 95% CI: 1.832–15.147) was associated with GD development.
The factors older age and fatty liver predicted GD risk in Chinese individuals. Further studies are required to explore differences in lithogenesis according to sex.
Keywords: fatty liver, gallstones, insulin resistance
1. Introduction
Gallstone disease (GD) and its associated complications (eg, cholecystitis, pancreatitis, and cholangitis) have major public health significance worldwide.[1–3] Since 2010, 20% to 30% of adults in developed countries have had a diagnosis of GD. The prevalence of GD has also been increasing in China in recent years.[4] The main risk factors associated with gallstone formation are sex, advanced age, obesity, alcohol consumption, diabetes mellitus (DM), hypertriglyceridemia, and metabolic syndrome.[5,6]
Fatty liver and gallstones have common risk factors (eg, obesity, DM, dyslipidemia, and hyperinsulinemia).[7–9] Patients with gallstones may be susceptible to developing fatty liver as a result of impaired gallbladder motility and increased bile lysogenicity.[10] GD may represent another component of metabolic syndrome, as defined by the National Cholesterol Education Program's Adult Treatment Panel III report.[11–14]
The association between fatty liver and gallstones has been evaluated in populations in the United States, Italy, Pakistan, Korea, and Taiwan.[15–17,4,18,9,19] No association has been found between fatty liver and GD in US or Korean populations.[15,9] Fatty liver is associated with increased gallstone risk in Pakistani and Taiwanese individuals.[16,20,19] Few studies have investigated whether fatty liver is associated with the risk of gallstone development in a Chinese population.[4,21]
The objective of this study was to identify risk factors for gallstone development and the association between fatty liver and GD in Chinese patients. Adjustment for age and gender was included in the analysis.
2. Patients and methods
2.1. Sample collection
This cross-sectional study included subjects who visited the clinical center and underwent ultrasonography at The First Hospital of Jilin University between January 2018 and June 2018. All methods were performed in accordance with approved guidelines.
The independent Institutional Review Board of The First Hospital of Jilin University approved the study protocol and the recruitment of human participants. Written informed consent was obtained from each participant before enrollment in the study.
2.2. Diagnosis of gallbladder disease and fatty liver
A confirmed GD diagnosis was defined as ultrasonographic detection of an echogenic area within the gallbladder lumen that produced a posterior acoustic shadow[22] or a history of cholecystectomy for GD, or both.
Fatty liver was diagnosed by first excluding other etiologies of chronic liver disease (eg, positive for hepatitis C virus antibody or hepatitis B antigen, autoimmune hepatitis). Abdominal ultrasonographic evidence of brightness of the liver and of the presence of diffuse echogenicity in the liver parenchyma was then used to identify fatty liver.[23]
2.3. Diagnosis of DM
A diagnosis of DM was defined as a history of diabetes treated using an antidiabetic therapy, or the presence of at least one of the following:
-
(1)
fasting glucose concentration ≥7.0 mmol/L,
-
(2)
randomly-measured glucose concentration ≥11.1 mmol/L, or
-
(3)
2-hour postprandial plasma glucose concentration ≥11.1 mmol/L.[24]
2.4. Study variables
Demographic (ie, sex, age) and clinical presentation variables (ie, history of excessive alcohol consumption, the presence of DM, fatty liver, or hypertension) were evaluated in this study. Biochemical parameters (ie, glutamyl transpeptidase [GGT] and alkaline phosphatase [ALP]) were also analyzed and abdominal ultrasonography results were reviewed to detect the presence of gallstones or fatty liver.
2.5. Statistical analysis
Two-tailed, independent sample t tests and Chi-square analyses were used to evaluate continuous and categorical variables, respectively. The results for continuous variables were presented as median (25th and 75th percentile) values. The results for categorical variables were presented as numbers and percentages. Multivariate logistic regression analysis was used to adjust for potential confounding effects among variables. The results were reported as adjusted odds ratios (AORs) and 95% confidence intervals (CIs). P values < .05 were considered to indicate statistically significant results. SPSS software (version 13.0; SPSS Inc, Chicago, IL) was used for the data analysis.
3. Results
3.1. Demographic and clinical characteristics
The results for baseline demographic and clinical characteristics of the study population (N = 897) are presented in Table 1. The case group consisted of 79 patients with gallstones. There were 35 (44.3%) male and 44 female patients in this group. The median age was 59.0 years. In this group, 24 (30.4%) patients had DM, 27 (34.2%) had hypertension, and 43 (54.4%) had fatty liver. There were 818 patients in the control group (ie, patients without gallstones). The median age of this group was 53.0 years (P < .001 vs the case group); 41.6% were male. A total of 147 (18.0%) patients in this group had DM, 189 (23.1%) had hypertension, and 324 (39.6%) had fatty liver. Compared with the case group, the percentages for all 3 diseases were significantly lower in the control group. GGT and ALP levels were significantly higher in the case group compared with the control group.
Table 1.
Demographic and clinical characteristics of cases and controls.
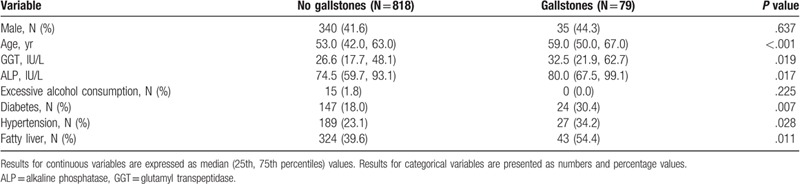
The results for the analysis of GD prevalence rates in different age groups, according to sex, are presented in Table 2. The overall prevalence of GD was 8.8% and was similar in men and women (9.3% vs 8.4%, P = .637). For the entire study population, the rate of GD increased with age. It was 2.1% for the ≤30 years group and 15.4% for the >70 years group (Fig. 1). This trend was present in both sexes. Prevalence was 2.3% in the group of men ≤30 years of age and increased to 15.1% in the group of men >70 years of age. It was 2.0% in the group of women ≤30 years of age and increased to 15.7% in the group of women >70 years of age.
Table 2.
Prevalence of gallstone disease in different age groups, by sex.
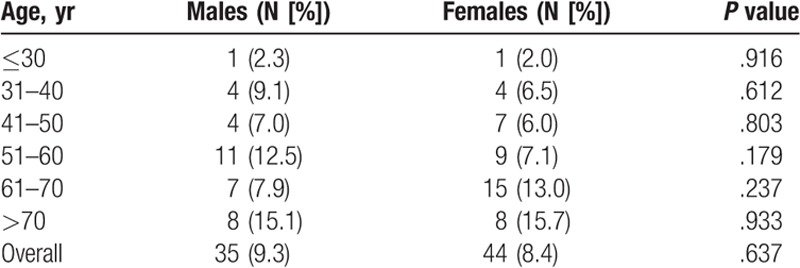
Figure 1.
Gallstone prevalence for the entire population, and by sex. For all participants, the rate of GD increased with age, from 2.1% for those ≤30 yr to 15.4% for those >70 yr. This trend was observed in both sexes; prevalence increased from 2.3% in men ≤30 yr to 15.1% in men >70 yr and from 2.0% in women ≤30 yr to 15.7% in women >70 yr. GD = gallstone disease.
3.2. Univariate and multivariate analyses of variables associated with the presence of gallstones
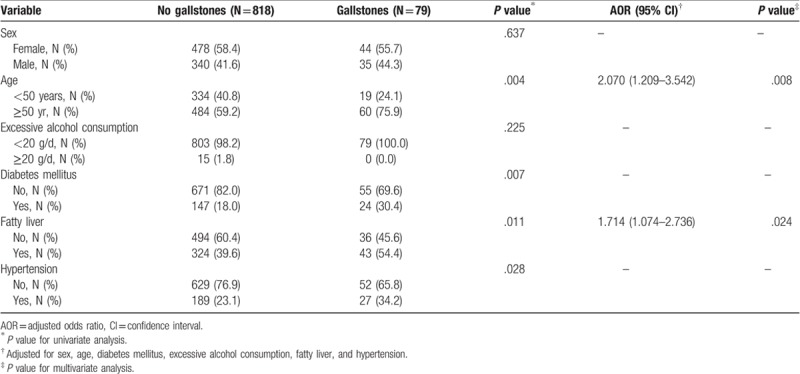
The univariate analysis revealed statistically significant differences between the case and control groups for the distributions for age, DM, fatty liver, and hypertension (Table 3). Sex, age, excessive alcohol consumption, and the presence of DM, fatty liver, or hypertension were thus considered for multivariate analysis. The AOR for the comparison between the group of patients without fatty liver and the group of patients with fatty liver was 1.714 (95% CI: 1.074–2.736; P = .024) (Table 3). The AOR for the group of patients ≥50 years of age, compared with younger patients (<50 years), was 2.070 (95% CI: 1.209–3.541; P = .008). Sex, excessive alcohol consumption, hypertension, and DM were not significantly associated with gallstone formation.
Table 3.
Results for univariate and multivariate analyses of variables associated with gallstone disease.
3.3. Effects of sex and age on risk factors associated with the presence of gallstones
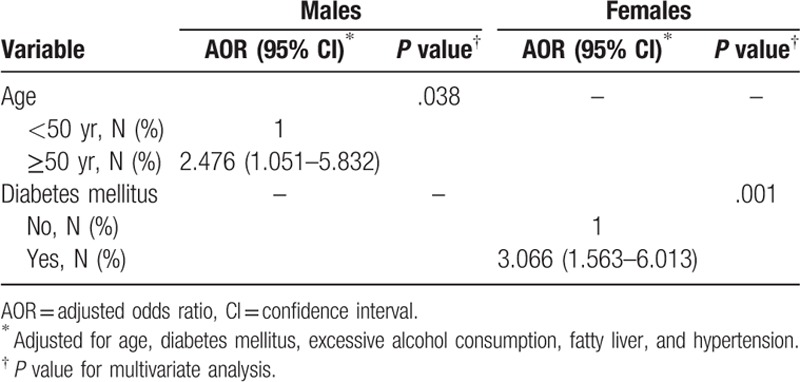
The sex-adjusted logistic regression analysis found that older age (AOR: 2.476, 95% CI: 1.051–5.832) was associated with GD in men. The clinical characteristics DM, fatty liver, and hypertension were not associated with GD in males. In females, DM was significantly associated with the risk of gallstone development (AOR: 3.066, 95% CI: 1.563–6.013) (Table 4). Age, excessive alcohol consumption, fatty liver, and hypertension were not significantly associated with gallstone formation in females.
Table 4.
Risk factors for gallbladder disease in males and females.
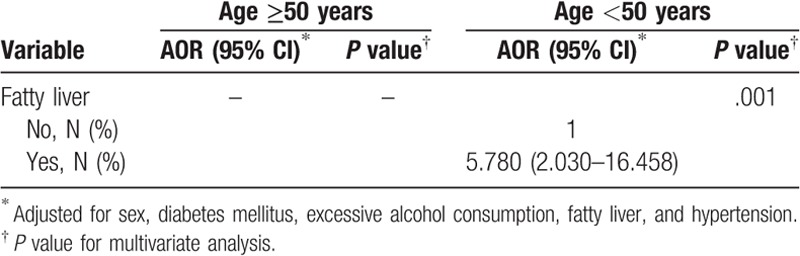
The results for the analysis of associations between gallstone risk and sex, DM, excessive alcohol consumption, fatty liver, and hypertension in patients of different ages are presented in Table 5. In younger patients (<50 years), the AOR for developing GD was 5.268 (95% CI: 1.832–15.147; P = .002) in patients with fatty liver, compared with patients without fatty liver. The association between fatty liver and gallstone formation in patients ≥50 years of age was not statistically significant. Sex, DM, excessive alcohol consumption, and hypertension were not associated with GD in younger or older subjects.
Table 5.
Logistic regression analysis, adjusted odds of gallbladder disease in different age groups.
4. Discussion
We found that Chinese people with gallstones were more likely to have a fatty liver; this association occurred mainly in patients <50 years of age. This finding is consistent with the findings of other studies.[25,7–9,21] Lee et al[20] assessed the relationship between GD and severity of fatty liver. They found that moderate to severe nonalcoholic fatty liver disease (NAFLD) is associated with an elevated risk of GD. In a prospective observational study, Qiao et al[21] found that gallstones were strongly associated with NAFLD in a Chinese population. However, other studies found no statistically significant associations between NAFLD and GD.[26–28] The discrepant results between studies may be due to differences between study populations, such as differences in income or eating habits.[21]
The results of previous studies suggest there is an association between GD and fatty liver.[8,29,30] The risk of both disorders is especially high in patients with obesity, hyperlipidemia, type 2 DM, and hypertension.[7,31,32] Therefore, this association may reflect that gallstones and fatty liver are caused by common pathogenic factors. Obesity, hyperlipidemia, type 2 DM, and hypertension are all components of metabolic syndrome, and the presence of fatty liver significantly increases the risk of development of metabolic syndrome.[33–35] After we adjusted for metabolic factors, the association between fatty liver and GD remained statistically significant.
Insulin resistance may contribute to the association between fatty liver and GD.[36,37] Sekine et al[38] found that accumulation of abdominal visceral fat has an important role in the development of GD, and that visceral adiposity can promote insulin resistance and hyperinsulinemia. Hyperinsulinemia reduces the gallbladder's response to cholecystokinin, the hormone responsible for effective gallbladder contraction.[38] Therefore, fatty liver may lead to the formation of gallstones through gallbladder dysmotility induced by insulin resistance and high insulin levels. Insulin resistance also promotes gallstone formation via activation of hydroxymethylglutaryl CoA reductase. This enzyme increases bile secretion and cholesterol content.[39] Insulin resistance stimulates the lipolysis in adipose tissue and influx of free fatty acids into the liver that potentially aggravates hepatic insulin resistance. Thus, systemic and hepatic insulin resistance are interrelated. An animal model found that hepatic insulin resistance increased bile cholesterol secretion by stimulating the expression of biliary cholesterol transporter proteins, reduced bile acid synthesis by suppressing bile acid synthetic enzymes, and impaired gallbladder motility.[40]
In Taiwan, a community-based study examined associations between GD and hyperinsulinemia, insulin resistance, and pancreatic beta-cell function in subjects with type 2 DM.[41] Compared with females without GD, females with GD had significantly higher serum insulin and HOMA–insulin resistance values, and worse beta-cell function.[41] None of these 3 variables differed between males with or without GD.[41] Our study also found that females with DM have a 3-fold higher likelihood of gallstones than females without DM, but there was no similar association for males. Taken together, these results suggest that there are sex-based differences in lithogenesis that may be related to the effects of estrogen on glucose metabolism in females.[4,21] More research to investigate this hypothesis and the underlying molecular mechanisms.
We found that older age was a significant risk factor for GD; this result is consistent with the results of previous studies.[42,43] The prevalence of gallstones and fatty liver increases with age.[7,4] The possible explanations for the higher prevalence of GD with increasing age include that the prevalence of metabolic syndrome rises with increasing age, and the risk of GD is associated with metabolic syndrome.[44] Another possibility is that older people are often exposed to sedentary lifestyles and other risk factors for GD for longer periods than younger individuals. These exposures may increase the risk of gallstone development.[45,46] GD is a chronic disorder, so prevalence increases as age increases.[47]
This study had some limitations. The retrospective design prevented us from obtaining detailed information about triglyceride and cholesterol levels or the results of other investigations useful for exploring associations between metabolic factors and GD. The case and control subjects were selected from patients seeking medical care at our hospital, which permitted us to obtain a sufficient number of subjects. However, this study population did not necessarily reflect the general Chinese population (eg, incidence of fatty liver in both case group and control group are higher than in general Chinese population). We also did not examine the type of GD or fatty liver (eg, nonalcoholic vs alcoholic fatty liver disease). Consequently, we were unable to examine risk factors for the different types of GD or the effect of alcohol on the development of GD.
In conclusion, we found that fatty liver was an independent risk factor for the presence of gallstones in our study population of Chinese patients, particularly for the group of subjects <50 years of age. Future studies that examine the relationships between metabolic factors and the risk of developing GD should include adjustment for sex.
Author contributions
Conceptualization: Pujun Gao.
Data curation: Xu Li, Pujun Gao.
Formal analysis: Xu Li.
Investigation: Xu Li.
Project administration: Xu Li, Pujun Gao.
Writing – original draft: Xu Li.
Writing – review and editing: Pujun Gao.
Footnotes
Abbreviations: ALP = alkaline phosphatase, AOR = adjusted odds ratios, CI = confidence intervals, DM = diabetes mellitus, GD = gallstone disease, GGT = gamma-glutamyl transpeptidase, HBV = hepatitis B virus, HCV = hepatitis C virus, NAFLD = nonalcoholic fatty liver disease.
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- [1].Barker DJ, Gardner MJ, Power C, et al. Prevalence of gall stones at necropsy in nine British towns: a collaborative study. Br Med J 1979;2:1389–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Godrey PJ, Bates T, Harrison M, et al. Gall stones and mortality: a study of all gall stone related deaths in a single health district. Gut 1984;25:1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gibney EJ. Asymptomatic gallstones. Br J Surg 1990;77:368–72. [DOI] [PubMed] [Google Scholar]
- [4].Liu J, Lin H, Zhang C, et al. Non-alcoholic fatty liver disease associated with gallstones in females rather than males: a longitudinal cohort study in Chinese urban population. BMC Gastroenterol 2014;14:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bennion LJ, Grundy SM. Risk factors for the development of cholelithiasis in man (first of two parts). N Engl J Med 1978;299:1161–7. [DOI] [PubMed] [Google Scholar]
- [6].Attili AF, Capocaccia R, Carulli N, et al. Factors associated with gallstone disease in the MICOL experience. Multicenter Italian Study on Epidemiology of Cholelithiasis. Hepatology (Baltimore, Md) 1997;26:809–18. [DOI] [PubMed] [Google Scholar]
- [7].Loria P, Lonardo A, Lombardini S, et al. Gallstone disease in non-alcoholic fatty liver: prevalence and associated factors. J Gastroenterol Hepatol 2005;20:1176–84. [DOI] [PubMed] [Google Scholar]
- [8].Fracanzani AL, Valenti L, Russello M, et al. Gallstone disease is associated with more severe liver damage in patients with non-alcoholic fatty liver disease. PloS One 2012;7:e41183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kwak MS, Kim D, Chung GE, et al. Cholecystectomy is independently associated with nonalcoholic fatty liver disease in an Asian population. World J Gastroenterol 2015;21:6287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li X, Guo X. Gallstones in patients with chronic liver diseases. Biomed Res Int 2017;2017:9749802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Diehl AK. Cholelithiasis and the insulin resistance syndrome. Hepatology (Baltimore, Md) 2000;31:528–30. [DOI] [PubMed] [Google Scholar]
- [12].Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–421. [PubMed] [Google Scholar]
- [13].Grundy SM. Cholesterol gallstones: a fellow traveler with metabolic syndrome? Am J Clin Nutr 2004;80:1–2. [DOI] [PubMed] [Google Scholar]
- [14].Mendez-Sanchez N, Chavez-Tapia NC, Motola-Kuba D, et al. Metabolic syndrome as a risk factor for gallstone disease. World J Gastroenterol 2005;11:1653–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ruhl CE, Everhart JE. Relationship of non-alcoholic fatty liver disease with cholecystectomy in the US population. Am J Gastroenterol 2013;108:952–8. [DOI] [PubMed] [Google Scholar]
- [16].Chen YC, Chiou C, Lin MN, et al. The prevalence and risk factors for gallstone disease in taiwanese vegetarians. PloS One 2014;9:e115145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lee YC, Wu JS, Yang YC, et al. Hepatitis B and hepatitis C associated with risk of gallstone disease in elderly adults. J Am Geriatr Soc 2014;62:1600–2. [DOI] [PubMed] [Google Scholar]
- [18].García-Monzón C, Vargas-Castrillón J, Porrero JL, et al. Prevalence and risk factors for biopsy-proven non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in a prospective cohort of adult patients with gallstones. Liver Int 2015;35:1983–91. [DOI] [PubMed] [Google Scholar]
- [19].Ahmed F, Baloch Q, Memon ZA, et al. An observational study on the association of nonalcoholic fatty liver disease and metabolic syndrome with gall stone disease requiring cholecystectomy. Ann Med Surg 2017;17:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee YC, Wu JS, Yang YC, et al. Moderate to severe, but not mild, nonalcoholic fatty liver disease associated with increased risk of gallstone disease. Scand J Gastroenterol 2014;49:1001–6. [DOI] [PubMed] [Google Scholar]
- [21].Qiao QH, Zhu WH, Yu YX, et al. Nonalcoholic fatty liver was associated with asymptomatic gallstones in a Chinese population. Medicine 2017;96:e7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cooperberg PL, Burhenne HJ. Real-time ultrasonography. Diagnostic technique of choice in calculous gallbladder disease. New Engl J Med 1980;302:1277–9. [DOI] [PubMed] [Google Scholar]
- [23].Ballestri S, Romagnoli D, Nascimbeni F, et al. Role of ultrasound in the diagnosis and treatment of nonalcoholic fatty liver disease and its complications. Expert Rev Gastroenterol Hepatol 2015;9:1–25. [DOI] [PubMed] [Google Scholar]
- [24].Alberti KG, Zimmet PZ. Definition diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–53. [DOI] [PubMed] [Google Scholar]
- [25].Nomura H, Kashiwagi S, Hayashi J, et al. Prevalence of gallstone disease in a general population of Okinawa, Japan. Am J Epidemiol 1988;128:598–605. [DOI] [PubMed] [Google Scholar]
- [26].Chen CH, Huang MH, Yang JC, et al. Prevalence and risk factors of gallstone disease in an adult population of Taiwan: an epidemiological survey. J Gastroenterol Hepatol 2006;21:1737–43. [DOI] [PubMed] [Google Scholar]
- [27].Nervi F, Miquel JF, Alvarez M, et al. Gallbladder disease is associated with insulin resistance in a high risk Hispanic population. J Hepatol 2006;45:299–305. [DOI] [PubMed] [Google Scholar]
- [28].Koller T, Kollerova J, Hlavaty T, et al. Cholelithiasis and markers of nonalcoholic fatty liver disease in patients with metabolic risk factors. Scand J Gastroenterol 2012;47:197–203. [DOI] [PubMed] [Google Scholar]
- [29].Roesch-Dietlen F, Perez-Morales A, Melo-Santisteban G, et al. Frequency and clinical, biochemical and histological characteristics of nonalcoholic fatty liver disease in patients with gallstone disease. Cir Cir 2008;76:37–42. [PubMed] [Google Scholar]
- [30].Chen JY, Hsu CT, Liu JH, et al. Clinical predictors of incident gallstone disease in a Chinese population in Taipei, Taiwan. BMC Gastroenterol 2014;14:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen LY, Qiao QH, Zhang SC, et al. Metabolic syndrome and gallstone disease. World J Gastroenterol 2012;18:4215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ahmed MH, Ali A. Nonalcoholic fatty liver disease and cholesterol gallstones: which comes first? Scand J Gastroenterol 2014;49:521–7. [DOI] [PubMed] [Google Scholar]
- [33].Lonardo A, Ballestri S, Marchesini G, et al. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis 2015;47:181–90. [DOI] [PubMed] [Google Scholar]
- [34].Ballestri S, Nascimbeni F, Romagnoli D, et al. The independent predictors of non-alcoholic steatohepatitis and its individual histological features: insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol Res 2016;46:1074–87. [DOI] [PubMed] [Google Scholar]
- [35].Ballestri S, Zona S, Targher G, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis,. J Gastroenterol Hepatol 2016;31:936–44. [DOI] [PubMed] [Google Scholar]
- [36].Smith BW, Adams LA. Nonalcoholic fatty liver disease and diabetes mellitus: pathogenesis and treatment. Nat Rev Endocrinol 2011;7:456–65. [DOI] [PubMed] [Google Scholar]
- [37].Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit rev Clin Lab Sci 2011;48:97–113. [DOI] [PubMed] [Google Scholar]
- [38].Sekine K, Nagata N, Sakamoto K, et al. Abdominal visceral fat accumulation measured by computed tomography associated with an increased risk of gallstone disease. J Gastroenterol Hepatol 2015;30:1325–31. [DOI] [PubMed] [Google Scholar]
- [39].Tsai CJ, Leitzmann MF, Willett WC, et al. Macronutrients and insulin resistance in cholesterol gallstone disease. Am J Gastroenterol 2008;103:2932–9. [DOI] [PubMed] [Google Scholar]
- [40].Biddinger SB, Haas JT, Yu BB, et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med 2008;14:778–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liu CM, Tung TH, Tsai ST, et al. Serum insulin, insulin resistance, beta-cell dysfunction, and gallstone disease among type 2 diabetics in Chinese population: a community-based study in Kinmen, Taiwan. World J Gastroenterol 2005;11:7159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Festi D, Dormi A, Capodicasa S, et al. Incidence of gallstone disease in Italy: results from a multicenter, population-based Italian study (the MICOL project). World J Gastroenterol 2008;14:5282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liew PL, Lee WJ, Wang W, et al. Fatty liver disease: predictors of nonalcoholic steatohepatitis and gallbladder disease in morbid obesity. Obes Surg 2008;18:847–53. [DOI] [PubMed] [Google Scholar]
- [44].Sun H, Tang H, Jiang S, et al. Gender and metabolic differences of gallstone diseases. World J Gastroenterol 2009;15:1886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Volzke H, Baumeister SE, Alte D, et al. Independent risk factors for gallstone formation in a region with high cholelithiasis prevalence. Digestion 2005;71:97–105. [DOI] [PubMed] [Google Scholar]
- [46].Kriska AM, Brach JS, Jarvis BJ, et al. Physical activity and gallbladder disease determined by ultrasonography. Med Sci Sports Exerc 2007;39:1927–32. [DOI] [PubMed] [Google Scholar]
- [47].Liu CM, Tung TH, Liu JH, et al. A community-based epidemiologic study on gallstone disease among type 2 diabetics in Kinmen, Taiwan. Dig Dis 2004;22:87–91. [DOI] [PubMed] [Google Scholar]


