Supplemental Digital Content is available in the text
Keywords: β-catenin, chromosome 8 open reading frame 4, cervical cancer, Wnt
Abstract
Chromosome 8 open reading frame 4 (C8orf4) is an activator of Wnt signaling pathway, and participates in the tumorigenesis and progression of many tumors. The expression levels of C8orf4 and β-catenin were assessed via immunohistochemical staining in 100 cervical squamous cell carcinoma (CSCC) tissues, 50 high-grade squamous intraepithelial lesions (HSILs), 50 low-grade squamous intraepithelial lesions (LSILs), and 50 normal cervical tissues. Bisulfite sequencing polymerase chain reaction analysis was used to examine the methylation status of the C8orf4 locus in CSCC and normal cervical tissues. The expression rates of C8orf4 and β-catenin were significantly higher in CSCCs or HSILs than in LSILs or normal cervical tissues (P < .05). C8orf4 expression was positively correlated with the poor differentiation of CSCCs (P = .009), and with aberrant expression of β-catenin in CSCCs (P = .002) and squamous intraepithelial lesions (P < .001). The methylation rate of C8orf4 in CSCCs was significantly lower than that in normal cervical tissues (P = .001). The Cancer Genome Atlas genomics data also confirmed that the mRNA expression of C8orf4 was positively associated with the copy number alteration of C8orf4 (correlation coefficient = 0.213, P < .001), and negatively correlated with the methylation level of C8orf4 (correlation coefficient = −0.408, P < .001). In conclusion, the expressions of C8orf4 and β-catenin were synergistically increased in CSCCs and HSILs and higher than those in LSILs and normal cervical tissues. The methylation level of C8orf4 is decreased in CSCCs and is responsible for the increased expression of C8orf4.
1. Introduction
Cervical cancer is the fourth leading cause of cancer-related death among women worldwide. More than 560,000 new cases of cervical cancer were detected, and more than 310,000 people died of cervical cancer in 2018.[1] Cervical squamous intraepithelial lesions (SIL) are a group of precancerous lesions closely associated to cervical cancer, reflecting the continuous process of the occurrence and development of cervical cancer. Many signaling pathways participate in this process and promote the transformation of cervical cancer. Wnt signaling pathway is one of the key pathways involved in cervical cancer transformation. The aberrant activation of Wnt signaling contributes to tumor initiation, progression, invasion, and therapeutic resistance of cervical cancer.[2]
Chromosome 8 open reading frame 4 (C8orf4, also named thyroid cancer 1) is a positive regulator of the Wnt signaling pathway.[3] C8orf4 interacts with Chibby via its transient helical structure, and, in turn, releases β-catenin from Chibby.[3–5] Free β-catenin then forms a complex with transcription factors of the T-cell factor/lymphoid enhancer factor (TCF/LEF) family, leading to the activation of Wnt target genes.[6] Besides regulation of the Wnt signaling pathway, C8orf4 promotes the G1 to S-phase transition of the cell cycle by regulating the mitogen-activated extracellular signal-regulated kinase1/2 signaling pathway.[7] C8orf4 is also a target gene of nuclear factor-κB, and serves as an endothelial inflammatory regulator enhancing nuclear factor-κB activity.[8] C8orf4 can be upregulated by transforming growth factor β pathway and the Interleukin-1β/tumor necrosis factor-α and fibroblast growth factor receptor 2 pathways.[9–11] C8orf4 also serves as a novel heat shock response regulator and a novel hematopoietic regulator in mice.[12,13]
C8orf4 was originally detected to be overexpressed in thyroid cancer.[14,15] Then, accumulating reports showed that C8orf4 acts as an oncogene, and its overexpression was correlated with the progression of many cancers, including thyroid cancer,[14] lung cancer,[16,17] gastric cancer,[4] breast cancer,[11] ovarian carcinomas,[18] oral tongue squamous cell carcinomas,[19] and hematological malignancies.[20,21] However, a recent report indicated that C8orf4 suppressed Notch2 signaling and negatively regulated self-renewal of liver cancer stem cells.[22] So, the role of C8orf4 in cancers is still unclear and needs further investigation.
The expression and role of C8orf4 in cervical cancer was unclear till now. In this study, we examined the expression levels of C8orf4 and β-catenin in cervical cancer, SILs and normal cervical tissues, and investigated the correlations among C8orf4, β-catenin, and the development of cervical cancers. We also examined the methylation status of C8orf4 in cervical cancer and normal cervical tissues, and analyzed its role in cervical cancer.
2. Materials
2.1. Patients and tissue samples
A total of 100 cervical squamous cell carcinoma (CSCC) tissues, 50 high-grade squamous intraepithelial lesions (HSILs), 50 low-grade squamous intraepithelial lesions (LSILs), and 50 normal cervical tissue samples were obtained randomly from patients who underwent surgery or biopsy at Shenyang Women's and Children's Hospital and the First Hospital of China Medical University between 2016 and 2018. Patients included in the study ranged from 19 to 70 years of age (mean: 39 years). The histological diagnosis and grade of differentiation were assessed by examination of hematoxylin and eosin-stained sections. The 100 CSCC tissues were classified as well (n = 54), moderately (n = 33), or poorly (n = 13) differentiated tumors according to the classification system of the World Health Organization. None of the CSCC patients exhibited lymphatic metastasis. The tumor stage was classified as stage Ia, Ib, and IIa (n = 23, 59, and 18, respectively) according to the International Federation of Gynecology and Obstetrics (FIGO) staging system (2009). Thirty fresh CSCC samples and corresponding normal cervical tissues were also obtained from patients who underwent surgery at the First Hospital of China Medical University in 2017 and stored at −70°C immediately after resection. These samples were used for DNA extraction. The study was conducted according to the regulations stipulated by the institutional review boards at the China Medical University and Shenyang Women's and Children's Hospital.
2.2. Immunohistochemistry
Specimens were fixed in 10% neutral-buffered formalin for 24 hours and embedded in paraffin blocks. Tissue blocks were cut into 4-μm sections. These sections were deparaffinized, rehydrated, and processed by pressure cooking in a citrate buffer (pH 6) for 1.5 min. Then, the sections were incubated with polyclonal rabbit anti-C8orf4 antibody (1:200; ab133885, Abcam, Cambridge, MA) and monoclonal mouse anti-β-catenin antibody (1:200; 610154; BD Transduction Laboratories, Lexington, KY) at 4°C overnight. The streptavidin–peroxidase method was used to detect the staining by antibodies. Some slides were stained in the absence of primary antibodies and served as negative controls.
2.3. Evaluation of immunostaining
Two investigators evaluated the immunostained sections without knowing the clinical data of enrolled patients. Five representative views per slide were examined, and 100 tumor cells were observed per view at 400× magnification. The positivity of each case was obtained by calculating the percentage of positively stained cells. Positive rate of each case was scored as follows: 1 (1% to 25%), 2 (26% to 50%), 3 (51% to 75%), and 4 (76% to 100%). The intensity of immunostaining was scored as 0, 1, 2, or 3 in case of negative, weak, moderate, or strong, respectively. Scores from each sample were multiplied to give a final score ranging from 0 to 12, and the cases were categorized based on scores as having low (≤4), moderate (>4 and ≤8), or high (>8) expression, respectively.
2.4. DNA extraction and bisulfite sequencing polymerase chain reaction analysis
We analyzed the gene sequence of C8orf4 as described previously.[16] The CpG island of C8orf4 was located closer to the 5′ end of the exon, near the start codon. The extraction of genomic DNA and bisulfite conversion of DNA was performed using the tissue/cell DNA extraction reagent kit (Bioteke, Beijing, China) and the EZ DNA Methylation kit (Zymo Research, Beijing, China) according to the manufacturer's instructions. The primers used for bisulfite sequencing PCR (BSP) of C8orf4 were as follows: forward, 5′-GGAGTTGAATTTCGGAAGAT-3′; reverse, 5′-ATTACCCACGACTTTCTTAC-3′ (product length: 144 bp). The bisulfite-treated DNA was amplified for 30 cycles: 95°C for 5 min, followed by cycling at 95°C for 10 s, 52°C for 20 s, and 72°C for 30 s, with a final step at 4°C for 5 min. Polymerase chain reaction (PCR) products were electrophoresed on 1.5% agarose gels, and then, observed using a Bio-Imaging system (UVP, Upland, CA). Purified PCR products were used for sequencing.
2.5. Statistical analysis
Pearson's chi-squared and likelihood ratio tests were used to examine correlations among the expression of C8orf4, β-catenin, and cervical lesions. Pearson's and Spearman's correlation tests were used to assay correlations between expression levels of C8orf4 and β-catenin. Nonparametric Wilcoxon signed ranks test was used to compare the C8orf4 methylation status of cervical cancers with that of the corresponding normal cervical tissues. Statistical significance was established at P < .05.
3. Results
3.1. Expression levels of C8orf4 in different tissues and its association with differentiation of CSCCs
The expression of C8orf4 was primarily localized in cytoplasm. As summarized in Table 1, the high and moderate expression rates of C8orf4 were 30.0% (30/100) and 35.0% (35/100) in CSCCs, respectively, which were significantly higher than those in LSILs (10.0% and 30.0%, P = .005) and normal cervical tissues (6.0% and 40.0%, P = .003). Similarly, in HISLs, the high and moderate expression rates of C8orf4 were 46.0% (23/50) and 30.0% (15/50), which were also significantly higher than those in LSILs (P < .001) and normal cervical tissues (P < .001) (Fig. 1). Furthermore, enhanced C8orf4 expression was positively correlated with the poor differentiation of CSCCs (P = .009), but was not correlated with the FIGO stage of CSCCs (P = .107) (Table 2).
Table 1.
The expressions of chromosome 8 open reading frame 4 and β-catenin in cervical lesions and normal cervical tissues.
Figure 1.
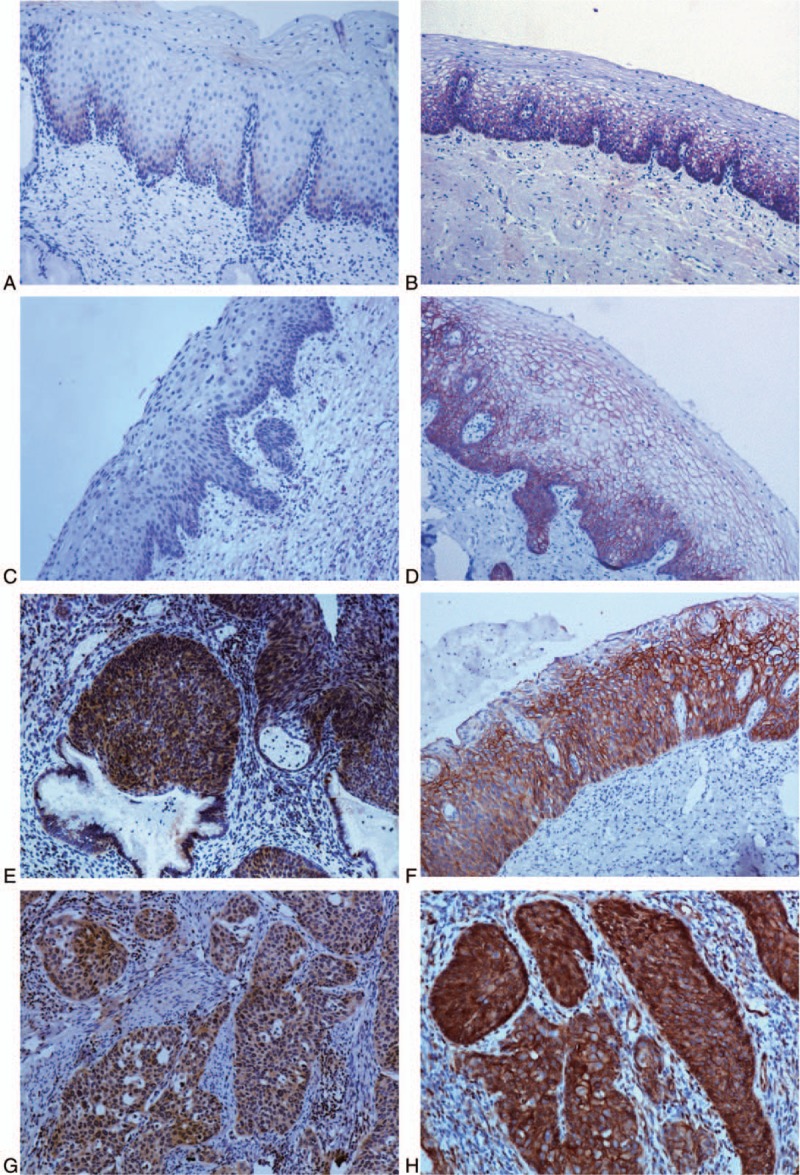
Expressions of C8orf4 and β-catenin in normal cervical epithelia, squamous intraepithelial lesions, and cervical squamous cell carcinomas. The expression of C8orf4 was weak in normal cervical epithelia (A) and low-grade squamous intraepithelial lesion (C), but significantly strong in high-grade squamous intraepithelial lesions (E) and cervical squamous cell carcinomas (G). The expression of β-catenin was primarily at the membrane of cells in normal cervical epithelia (B) and low-grade squamous intraepithelial lesion (D), the cytoplasmic expression of β-catenin was weak. But, in high-grade squamous intraepithelial lesion (F) and cervical squamous cell carcinoma (H), the expression of β-catenin was significantly strong in the cytoplasm. (Original magnification, 100×; streptavidin–peroxidase immunohistochemistry method). C8orf4 = chromosome 8 open reading frame 4.
Table 2.
The relationships between the expressions of chromosome 8 open reading frame 4 and β-catenin and clinicopathological factors in cervical squamous cell carcinomas.
3.2. Abnormal expression of β-catenin in different tissues, and its correlation with poor differentiation and advanced FIGO stage of CSCCs
The high and moderate abnormal expression rates of β-catenin were 36.0% (36/100) and 33.0% (33/100) in CSCCs, respectively, which were significantly higher than those in LSILs (8.0% and 38.0%, P = .001) and normal cervical tissues (8.0% and 38.0%, P = .001). Meanwhile, in HISLs, the high and moderate abnormal expression rates of β-catenin were 46.0% (23/50) and 34.0% (17/50), which were significantly higher than those in LSILs (P < .001) and normal cervical tissues (P < .001) (Table 1) (Fig. 1). Furthermore, the abnormal expression of β-catenin was positively correlated with the poor differentiation (P = .004) and advanced FIGO stage of CSCCs (P < .001) (Table 2).
3.3. Methylation levels of C8orf4 in CSCCs were lower than those in corresponding normal cervical tissues
As observed after BSP and sequencing analysis, the methylation rate of C8orf4 in CSCCs (73.10% ± 2.32%) were significantly lower than that in normal cervical tissues (84.76% ± 1.84%) (P = .001, n = 30) (Fig. 2). But, the methylation status of C8orf4 was not correlated with the differentiation (correlation coefficient = −0.111, P = .559) and FIGO stage of CSCCs (correlation coefficient = −0.043, P = .820).
Figure 2.
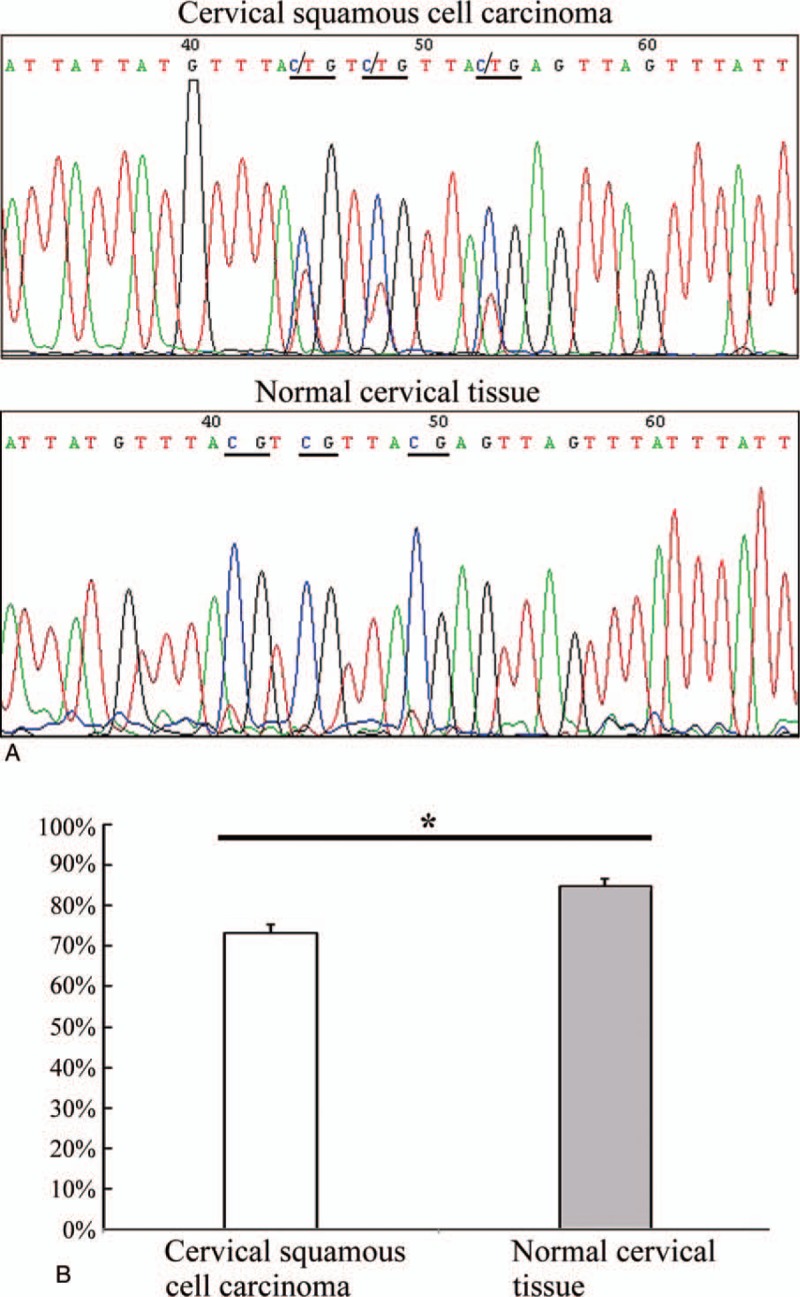
Bisulfite sequencing PCR analysis of cervical squamous cell carcinomas and corresponding normal cervical tissues. (A) In a representative case of cervical squamous cell carcinoma, the CpG sites (underlined) were semi-methylated, whereas their corresponding sites in normal cervical tissue were completely methylated (underlined). (B) The methylation rate of C8orf4 gene in cervical squamous cell carcinomas (73.10% ± 2.32%) were significantly lower than that in normal cervical tissues (84.76% ± 1.84%) (P = .001, n = 30). C8orf4 = chromosome 8 open reading frame 4, PCR = polymerase chain reaction, ∗P < .05.
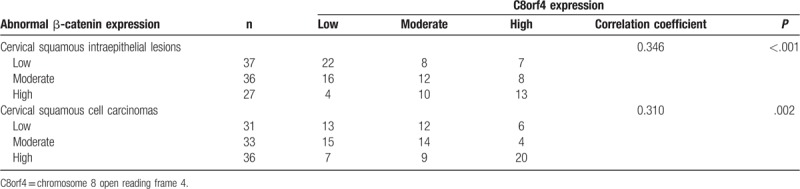
3.4. The correlations between C8orf4 and β-catenin in SILs and CSCCs
Spearman correlation analysis showed that C8orf4 expression was correlated with the aberrant expression of β-catenin in CSCCs (correlation coefficient = 0.310, P = .002) and SILs (correlation coefficient = 0.346, P < .001) (Table 3). By analyzing The Cancer Genome Atlas (TCGA) genomics data of cervical cancers, which were obtained from cBioPortal database,[23,24] we disclosed that the mRNA expression of C8orf4 was positively correlated with the copy number alteration of C8orf4 gene (correlation coefficient = 0.213, P < .001), and negatively correlated with the methylation level of C8orf4 (correlation coefficient = −0.408, P < .001) and methylation level of β-catenin (correlation coefficient = −0.121, P = .038). Moreover, the methylation level of C8orf4 was positively correlated with the methylation level of β-catenin (correlation coefficient = 0.122, P = .037), but negatively correlated with mRNA expression of β-catenin (correlation coefficient = −0.223, P < .001) (supplementary Table).
Table 3.
The correlation of chromosome 8 open reading frame 4 and β-catenin in cervical squamous intraepithelial lesions and cervical squamous cell carcinomas.
4. Discussion
Wnt signaling pathway is involved in the multistep process of cervical carcinogenesis, including tumor initiation, progression, and invasion, suggesting its role as a potential biomarker or therapeutic target.[2,25,26] Activation of Wnt signaling pathway promotes cell growth and tumorigenicity, and inhibits apoptosis of cervical cancer cells.[27,28] Downregulating the activity of Wnt signaling pathway suppresses cell proliferation and tumor formation of cervical cancer.[29] As a member of Wnt signaling pathway, the aberrant expression of β-catenin has been reported in many tumors, and its expression is correlated with the proliferation and invasion of tumor cells.[30–34] Previous studies on cervical cancers also indicate that the aberrant expression of β-catenin promotes cell migration, invasion, and epithelial–mesenchymal transition, and is associated with poor cancer-specific survival and overall recurrence rate.[32,33,35] Our results showed that aberrant expression of β-catenin was significantly enhanced with increase in grade of SILs and CSCCs, and was correlated with the FIGO stage of CSCCs, which confirms that aberrant expression of β-catenin promotes the carcinogenesis and progression of CSCCs.
C8orf4 is a novel activator of β-catenin and Wnt signaling pathway.[3–5] Recent studies have indicated that C8orf4 is overexpressed and correlated with the development of many cancers.[4,11,14,16–21] But the expression and role of C8orf4 in CSCC and SIL was unclear till now. Our study demonstrated that the expression level of C8orf4 in CSCCs and HSILs was significantly higher than that in LSILs and normal cervical tissues, which was similar to the trend of β-catenin expression. Moreover, the expression of C8orf4 was positively correlated with the aberrant β-catenin expression and poor differentiation of CSCCs. So, C8orf4 plays a synergistic role with β-catenin and enhances the activation of Wnt signaling pathway, and, in turn, promotes the carcinogenesis and development of CSCCs. To investigate the underlying mechanisms of upregulation of C8orf4 expression in CSCCs, we, for the first time, examined the methylation level of C8orf4 in CSCCs and corresponding normal cervical tissues. The results revealed that the methylation level of C8orf4 in CSCCs was much lower than that in normal cervical tissues, which indicated that reduction of methylation level was one of the key reasons for increased expression of C8orf4. We also found the reduced methylation level and increased expression of C8orf4 in lung cancer tissues previously, which supported this view.[16] But we did not find any correlation between the methylation levels of C8orf4 and clinicopathological factors, which might be due to the small sample size. By analyzing the TCGA genomics data obtained from cBioPortal database, it was also confirmed that the mRNA expression of C8orf4 was negatively correlated with its methylation level, and positively correlated with the copy number alteration of C8orf4. In addition, mutation in C8orf4 was not found in 607 cases of TCGA genomic data.[23,24] So, reduction of methylation level and copy number alteration are considered to be the key causes for increased expression of C8orf4 in CSCCs.
In conclusion, the expressions of C8orf4 and β-catenin were synergistically increased in CSCCs and HSILs and higher than those in LSILs and normal cervical tissues, which promoted the carcinogenesis and development of CSCCs. The methylation level of C8orf4 is decreased in CSCCs and is responsible for the increased expression of C8orf4.
Author contributions
Funding acquisition: Chong Lan, Hong-Tao Xu.
Methodology: Chong Lan, Da-Wei Huan, Ju-Min Niu, Jian-Hua Sun, Wen-Jing Huang, Zhi-Han Li.
Writing – original draft: Chong Lan, Hong-Tao Xu.
Writing – review & editing: Chong Lan, Xiao-Cui Nie, Hong-Tao Xu.
Hong-Tao Xu orcid: 0000-0001-5062-3108.
Supplementary Material
Footnotes
Abbreviations: BSP = bisulfite sequencing PCR, C8orf4 = chromosome 8 open reading frame 4, CSCC = cervical squamous cell carcinoma, FIGO = the International Federation of Gynecology and Obstetrics, HSIL = high-grade squamous intraepithelial lesion, LSIL = low-grade squamous intraepithelial lesion, PCR = polymerase chain reaction, SIL = squamous intraepithelial lesions, TCF/LEF = T-cell factor/lymphoid enhancer factor, TCGA = The Cancer Genome Atlas.
This study was supported by the Program for Liaoning Excellent Talents in University (Grant No. LR2015067 to H.-T.X.) and Natural Science Foundation of Liaoning Province (Grant No. 20170540833 to C.L.).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Yang M, Wang M, Li X, et al. Wnt signaling in cervical cancer? J Cancer 2018;9:1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jung Y, Bang S, Choi K, et al. TC1 (C8orf4) enhances the Wnt/beta-catenin pathway by relieving antagonistic activity of Chibby. Cancer Res 2006;66:723–8. [DOI] [PubMed] [Google Scholar]
- [4].Kim B, Koo H, Yang S, et al. TC1(C8orf4) correlates with Wnt/beta-catenin target genes and aggressive biological behavior in gastric cancer. Clin Cancer Res 2006;12:3541–8. [DOI] [PubMed] [Google Scholar]
- [5].Gall C, Xu H, Brickenden A, et al. The intrinsically disordered TC-1 interacts with Chibby via regions with high helical propensity. Protein Sci 2007;16:2510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kretzschmar K, Clevers H. Wnt/beta-catenin signaling in adult mammalian epithelial stem cells. Dev Biol 2017;428:273–82. [DOI] [PubMed] [Google Scholar]
- [7].Wang YD, Bian GH, Lv XY, et al. TC1 (C8orf4) is involved in ERK1/2 pathway-regulated G(1)- to S-phase transition. BMB Rep 2008;41:733–8. [DOI] [PubMed] [Google Scholar]
- [8].Kim J, Kim Y, Kim HT, et al. TC1(C8orf4) is a novel endothelial inflammatory regulator enhancing NF-kappaB activity. J Immunol 2009;183:3996–4002. [DOI] [PubMed] [Google Scholar]
- [9].Friedman JB, Brunschwig EB, Platzer P, et al. C8orf4 is a transforming growth factor B induced transcript downregulated in metastatic colon cancer. Int J Cancer 2004;111:72–5. [DOI] [PubMed] [Google Scholar]
- [10].Kim Y, Kim J, Park J, et al. TC1(C8orf4) is upregulated by IL-1beta/TNF-alpha and enhances proliferation of human follicular dendritic cells. FEBS Lett 2006;580:3519–24. [DOI] [PubMed] [Google Scholar]
- [11].Yang ZQ, Moffa AB, Haddad R, et al. Transforming properties of TC-1 in human breast cancer: interaction with FGFR2 and beta-catenin signaling pathways. Int J Cancer 2007;121:1265–73. [DOI] [PubMed] [Google Scholar]
- [12].Park J, Jung Y, Kim J, et al. TC1 (C8orf4) is upregulated by cellular stress and mediates heat shock response. Biochem Biophys Res Commun 2007;360:447–52. [DOI] [PubMed] [Google Scholar]
- [13].Jung Y, Kim M, Soh H, et al. TC1(C8orf4) regulates hematopoietic stem/progenitor cells and hematopoiesis. PLoS One 2014;9:e100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sunde M, McGrath KC, Young L, et al. TC-1 is a novel tumorigenic and natively disordered protein associated with thyroid cancer. Cancer Res 2004;64:2766–73. [DOI] [PubMed] [Google Scholar]
- [15].Chua EL, Young L, Wu WM, et al. Cloning of TC-1 (C8orf4), a novel gene found to be overexpressed in thyroid cancer. Genomics 2000;69:342–7. [DOI] [PubMed] [Google Scholar]
- [16].Zheng YW, Zhang L, Wang Y, et al. Thyroid cancer 1 (C8orf4) shows high expression, no mutation and reduced methylation level in lung cancers, and its expression correlates with beta-catenin and DNMT1 expression and poor prognosis. Oncotarget 2017;8:62880–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Su K, Huang L, Li W, et al. TC-1 (C8orf4) enhances aggressive biologic behavior in lung cancer through the Wnt/beta-catenin pathway. J Surg Res 2013;185:255–63. [DOI] [PubMed] [Google Scholar]
- [18].Xu HT, Liu Y, Liu SL, et al. (C8orf4) expression is correlated with differentiation in ovarian carcinomas and might distinguish metastatic ovarian from metastatic colorectal carcinomas. Virchows Arch 2013;462:281–7. [DOI] [PubMed] [Google Scholar]
- [19].Zhang P, Cao HY, Bai LL, et al. The high expression of TC1 (C8orf4) was correlated with the expression of beta-catenin and cyclin D1 and the progression of squamous cell carcinomas of the tongue. Tumour Biol 2015;36:7061–7. [DOI] [PubMed] [Google Scholar]
- [20].Zhang J, Gao Y, Zhao X, et al. Investigation of copy-number variations of C8orf4 in hematological malignancies. Med Oncol 2011;28Suppl 1:S647–52. [DOI] [PubMed] [Google Scholar]
- [21].Walter MJ, Payton JE, Ries RE, et al. Acquired copy number alterations in adult acute myeloid leukemia genomes. Proc Natl Acad Sci U S A 2009;106:12950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhu P, Wang Y, Du Y, et al. C8orf4 negatively regulates self-renewal of liver cancer stem cells via suppression of NOTCH2 signalling. Nat Commun 2015;6:7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bahrami A, Hasanzadeh M, ShahidSales S, et al. Clinical significance and prognosis value of Wnt signaling pathway in cervical cancer. J Cell Biochem 2017;118:3028–33. [DOI] [PubMed] [Google Scholar]
- [26].Wang Y, Lei L, Zheng YW, et al. Odd-skipped related 1 inhibits lung cancer proliferation and invasion by reducing Wnt signaling through the suppression of SOX9 and beta-catenin. Cancer Sci 2018;109:1799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu XF, Li XY, Zheng PS, et al. DAX1 promotes cervical cancer cell growth and tumorigenicity through activation of Wnt/beta-catenin pathway via GSK3beta. Cell Death Dis 2018;9:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang J, Gao Y. CCAT-1 promotes proliferation and inhibits apoptosis of cervical cancer cells via the Wnt signaling pathway. Oncotarget 2017;8:68059–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li L, Yang WT, Zheng PS, et al. SOX17 restrains proliferation and tumor formation by down-regulating activity of the Wnt/beta-catenin signaling pathway via trans-suppressing beta-catenin in cervical cancer. Cell Death Dis 2018;9:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Xu X, Kim JE, Sun PL, et al. Immunohistochemical demonstration of alteration of beta-catenin during tumor metastasis by different mechanisms according to histology in lung cancer. Exp Ther Med 2015;9:311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Saukkonen K, Hagstrom J, Mustonen H, et al. PROX1 and beta-catenin are prognostic markers in pancreatic ductal adenocarcinoma. BMC Cancer 2016;16:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bohr Mordhorst L, Ahlin C, Sorbe B. Prognostic impact of the expression of Wnt-signaling proteins in cervical carcinoma FIGO stage I-IV treated with radiotherapy or chemoradiotherapy. Oncotarget 2016;7:63042–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liang J, Zhou H, Peng Y, et al. beta-catenin expression negatively correlates with WIF1 and predicts poor clinical outcomes in patients with cervical cancer. Biomed Res Int 2016;2016:4923903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Xu HT, Wang L, Lin D, et al. Abnormal beta-catenin and reduced axin expression are associated with poor differentiation and progression in non-small cell lung cancer. Am J Clin Pathol 2006;125:534–41. [DOI] [PubMed] [Google Scholar]
- [35].Zhang LZ, Huang LY, Huang AL, et al. CRIP1 promotes cell migration, invasion and epithelial-mesenchymal transition of cervical cancer by activating the Wnt/betacatenin signaling pathway. Life Sci 2018;207:420–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


