Supplemental Digital Content is available in the text
Keywords: elderly patient, hepatocellular carcinoma, overall survival, surgical resection
Abstract
We evaluated the post-treatment overall survival (OS) of elderly hepatocellular carcinoma (HCC) patients.
The archived records of 10,578 HCC patients registered at the Korean Central Cancer Registry from 2008 through 2014 were retrospectively analyzed. In this registry, we selected Barcelona Clinic Liver Cancer (BCLC) 0, A, or B staged HCC patients (n = 4744) treated by surgical resection (SR), local ablation therapy (LAT), or locoregional therapy (LRT). OSs in nonelderly (<70 years) and elderly (≥70 years) patients were compared after propensity score matching (PSM).
In BCLC 0-A staged HCC, the cumulative OS rates of elderly patients were poorer than those of nonelderly patients after PSM (P < .001), but not in those with BCLC stage B (P > .05). In BCLC 0-A staged elderly patients, OS after SR was significantly better than after LAT (P = .005) or LRT (P < .001). In BCLC B staged elderly patients, SR achieved better OS than LRT (P = .006). Multivariable analysis showed that LAT (hazard ratio [HR] 1.52, P = .048) or LRT (HR, 2.01, P < .001) as compared with SR, and large (>3 cm) tumor size (HR1.49, P = .018) were poor predictors of OS for elderly patients with BCLC stage 0-A, and that LRT (HR, 2.64, P = .042) was a poor predictor for those with BCLC stage B.
SR provided a better OS rate than LAT or LRT in elderly HCC patients with BCLC stage 0–A, than LRT in those with BCLC stage B. SR should be considered the first therapeutic option even in elderly HCC patients with these stages.
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second most common cause of cancer mortality worldwide.[1] It has been predicted 60% of all cancers will be detected in elderly patients.[2] The number of elderly HCC patients has been increasing in-line with increasing average life expectancy[3,4]; societal aging elevates the importance of health age as well as physical age. Accordingly, a systematic approach is required to determine which therapeutic strategy to adopt for elderly HCC patients, and whether palliative treatment is the better option. However, the most suitable therapeutic options for these patients are controversial, and the management of elderly HCC patients had become a global issue.
In general, treatments for HCC are chosen based on several indications, such as, tumor size, number, and location, and reserved liver function.[5–7] According to the Barcelona Clinical Liver Cancer (BCLC) staging system for a single, small (≤3)-sized HCC, curable options, such as, surgical resection (SR), radiofrequency ablation (RFA), or liver transplantation (LT), can be recommended.[5] However, elderly (≥70 years) HCC patients, even those of BCLC stage 0-A, tend to receive less-invasive therapies, such as, RFA or transarterial chemoembolization (TACE),[8] possibly because of concerns about lack of survival gain after surgery or a lack of clinical evidence concerning treatment outcomes. Recently, it was reported that SR is safe in elderly patients,[9–13] but appropriate treatment options for elderly HCC patients have not been clarified. Furthermore, few studies have compared treatment outcomes or thoroughly analyzed effective treatment options in elderly HCC patients. Given that means of providing effective treatment to elderly patients are becoming increasingly important, research on this topic is essential.
Therefore, we conducted a nationwide cancer registry-based cohort study to evaluate the effectiveness of treatment options in elderly HCC patients with BCLC stage 0, A or B using the database of the Korea Central Cancer Registry (KCCR) in South Korea. Survival outcomes of elderly (≥70 years) HCC patients were evaluated with respect to treatment type, classified as, SR, local ablation therapy (LAT), or locoregional therapy (LRT), and compared with those of nonelderly (<70 years) patients. Propensity score matching (PSM) was used to adjust for differences between the 2 age groups.
2. Materials and methods
2.1. Extraction of database
A nationwide cancer registry called the Korea Central Cancer Registry (KCCR) was established by the Ministry of Health and Welfare, South Korea in 1980. HCC patients were abstracted from the KCCR registry using C22.0 as defined by the International Classification of Disease 10th edition (ICD-10) coding system. The National Cancer Center and Korean Liver Cancer Study group have systemically organized the KCCR database annually from establishment using the random sample audit method. We initially investigated the clinical data of 83,231 patients registered in the KCCR during the period 2008 to 2014. Of these, 10,811 (13%) patient records, which contained an additional 3% considering sample errors, were randomly abstracted. Thus, clinical data of 10,578 HCC patients were finally considered for inclusion in this study.
Mortality data were obtained from the Korean National Statistics Office (KNSO), and initial treatment dates were determined using KCCR records. For survival analysis, follow-up durations were calculated from date of initial treatment to date of death or to December 31, 2016. The study was approved by the Institutional Review Board of Inha University Hospital, Incheon, South Korea (Approval number: INHAUH 2018-09-003-001).
2.2. Study subjects
A schematic flowsheet of study subjects is provided in Figure 1. Of the 10,578 patients, those with incomplete data for BCLC stage (n = 1196) or with an age of <18 years (n = 6) were excluded. Of the remaining 9376 patients, those with BCLC stage C (n = 3397) or D (n = 662), and those who received LT (n = 52) or chemotherapy, radiotherapy, or sorafenib therapy (n = 72) were excluded. In addition, patients with no data for treatment type or follow-up loss (n = 449) were also excluded. Finally, the data of 4744 patients with BCLC 0, A, or B staged HCC patients treated by SR, LAT, or LRT were analyzed in this retrospective cohort study.
Figure 1.
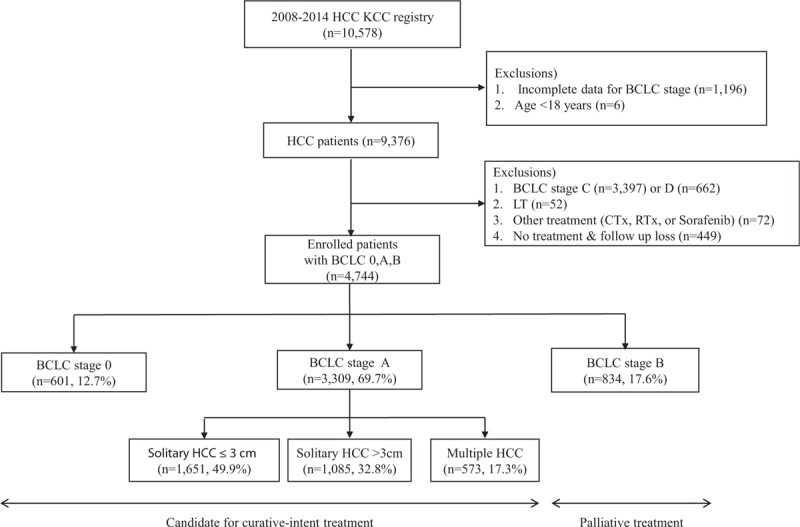
Flowsheet of enrolled all patients (n = 4744). BCLC = , HCC = , KCC = , LT = .
Of these 4744 patients, 601, 3309, and 834 had BCLC 0, A, and B staged HCCs, respectively. In 3309 patients with BCLC A staged HCC, 1651 and 1085 patients had a solitary HCC with 3 cm and >3 cm in tumor size, respectively, and 573 patients had 2 or 3 numbered HCCs with ≤3 cm (Fig. 1). In this study, LAT included RFA or percutaneous ethanol injection, and LRT included TACE or transarterial chemoinfusion.
2.3. Statistical analyses
The primary study endpoint was overall survival (OS) rate in elderly or nonelderly HCC patients. The secondary endpoints were the OS differences and OS-related factors according to the treatment methods selected for HCC patients and the BCLC stage.
Using the KCCR database, we acquired the following variables: age, sex, weight and height, smoking history, comorbidity of hypertension (HTN) or diabetes mellitus (DM), HCC etiology, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, total bilirubin, prothrombin time (PT), serum sodium (Na), creatinine, total cholesterol, alpha-fetoprotein (AFP), Child-Turcotte-Pugh (CTP) class, model for end-stage liver disease (MELD) scores, MELD-sodium (MELD-Na) scores, tumor numbers and sizes, BCLC stages, and treatment types. We investigated whether relationships existed between these factors and primary and secondary outcomes.
Clinical characteristics of study subjects and HCCs are expressed as medians (ranges) for continuous variables, and numbers (percentages) for categorical variables. Differences between categorical or continuous variables were analyzed using the Student t test, the χ2 test, or Fisher exact test.
To investigate the association between treatment selection and clinical outcomes for an observational, nonrandomized study, we performed PSM) analysis to reduce imbalance in distributions of demographic and clinical characteristics in two patients groups, that is, nonelderly (<70 years’ old) and elderly (≥70 years’ old) patients. Given that the characteristics and prognosis of HCC are generally different between BCLC stage 0–A and stage B, patients with these BCLC stages were separately matched and analyzed. Propensity scores for the 2 age groups were estimated in the multiple logistic regression model of demographic and clinical variables, such as, sex, body mass index (BMI), smoking, HTN, DM, cause of underlying liver disease (chronic hepatitis B, chronic hepatitis C, alcohol, unknown), serum albumin, serum total bilirubin, PT (international normalized ratio), serum creatinine, serum sodium, AFP level, CTP class, MELD score, tumor number, tumor size, and BCLC stage. PSM was implemented using the 1:1 nearest algorithm with a caliper width of 0.03 multiplied by the standard deviation of values. PSM analysis was performed using R software v. 3.5.0 (https://www.r-project.org/, ‘MatchIt’ package).
Post-treatment OS rates were estimated using the Kaplan–Meier method. Differences between group OS curves among groups were tested using the log-rank test. Cox proportional hazards models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for mortality. Two-tailed P values of <.05 were considered statistically significant. P values were corrected by the Bonferroni method for multiple comparisons. The statistical analysis was performed using SPSS v19.0 (SPSS Inc, Chicago, IL).
3. Results
3.1. Baseline characteristics
Baseline clinical characteristics of nonelderly and elderly patients are provided in Table 1. Of the 4744 study patients, about one-fifth (n = 978, 20.6%) were elderly patients. The median ages of elderly and nonelderly patients were 75 (range, 70–91 years) and 56 years (range, 18–69 years), respectively. The proportion of males was smaller in the elderly group than (65.6% vs 80.0%, P < .001), and percentages of patients with accompanying DM (33.8% vs 22.5%, P < .001) or HTN (54.3% vs 29.1%, P < .001) were also greater in the elderly group. Remnant liver function, such as, CTP class, was not significantly different between the 2 age groups (P = .189).
Table 1.
Baseline clinical characteristics of total study subjects.
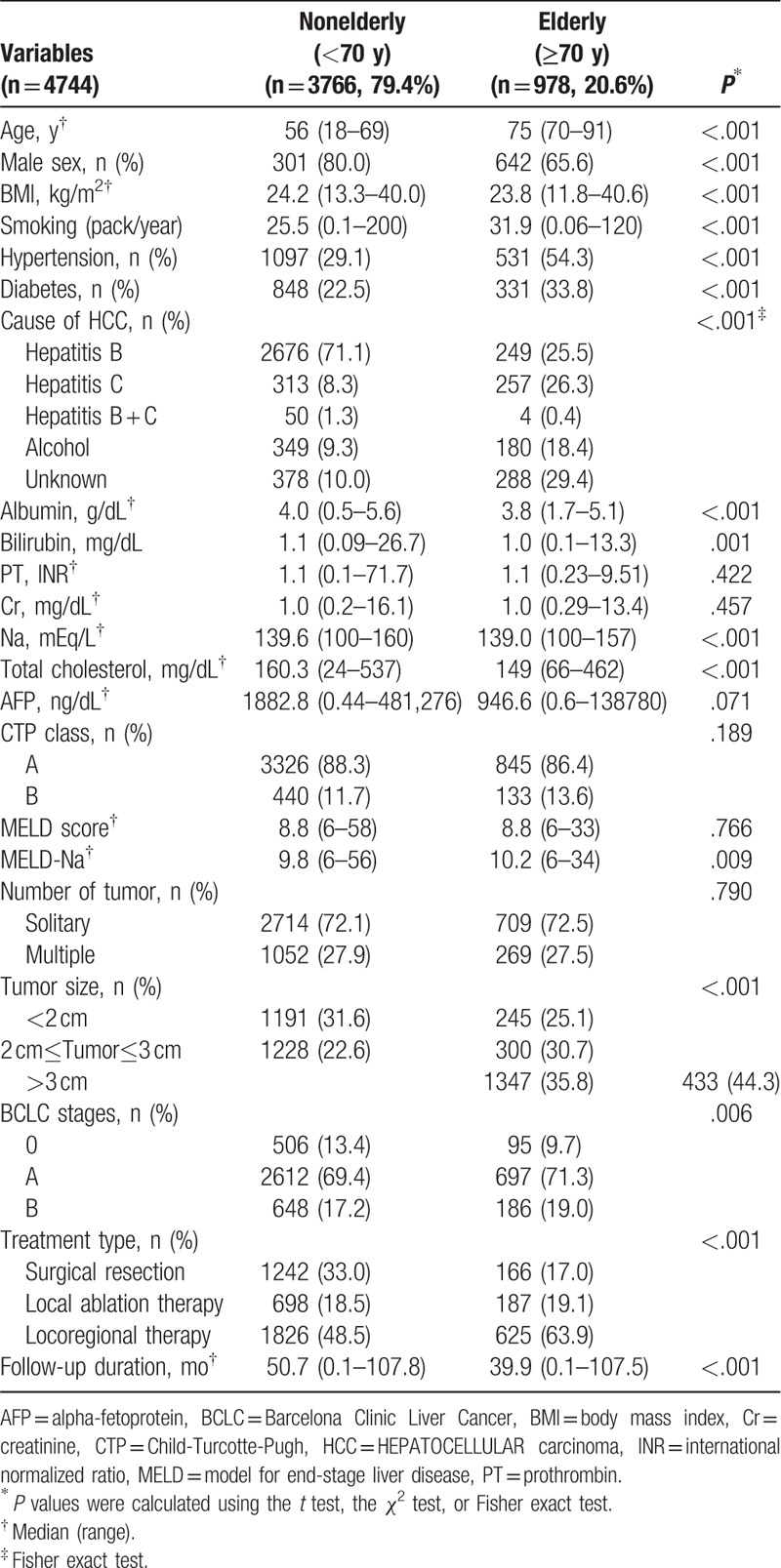
In terms of HCC etiology, the frequency of hepatitis C virus (HCV) infection (26.3% vs. 8.3%, P < .001) was greater in elderly patients, but the frequency of hepatitis B virus (HBV) infection (25.4% vs 71.1%, P < .001) and alcohol consumption (0.4% vs 9.3%, P < .001) were higher in nonelderly patients (Table 1). In terms of tumor features, no significant intergroup difference was found between solitary HCC rates in the 2 groups (P = .790). However, a greater proportion of elderly patients had a tumor of ≥2 cm (75.0% vs 68.4%, P < .001). The percentage of patients treated by SR was lower for elderly patients (17.0% vs. 33.0%), but the percentage of patients treated by LAT or LRT (63.9% vs 48.5%) was higher (P < .001) (Table 1). Similar results for the percentage of elderly patients treated by SR were obtained when separately analyzed in BCLC stage 0 (P = .012) and A (P < .001), but not in BCLC stage B (P = .336) (Supplementary Table 1).
3.2. OS rates of HCC patients according to age before and after PSM
After PSM, 1210 HCC patients with BCLC stage 0-A were allocated equally to nonelderly and elderly groups (Table 2), and similarly, 272 patients with BCLC stage B were allocated to nonelderly and elderly groups (Supplementary Table 2). The 1-, 3-, and 5- year cumulative OS rates of elderly patients with BCLC stage 0-A were significantly lower than those of nonelderly patients before PSM (P < .001) (Fig. 2A) and after PSM (86.4%, 59.6%, and 43.9% vs. 90.7%, 74.7%, and 63.8%, respectively, p < 0.001) (Fig. 2C). However, the 1-, 3-, and 5- year cumulative OS rates were not significantly different between non-elderly and elderly patients with BCLC stage B before (p = 0.070) (Fig. 2B), or after PSM (76.5%, 46.8%, and 28.5% vs. 77.9%, 46.8%, and 37.1%, respectively, p < 0.448) (Fig. 2D).
Table 2.
Baseline clinical characteristics of BCLC 0–A patients who underwent either surgical resection or local ablation therapy or locoregional therapy, after propensity score matching analysis.
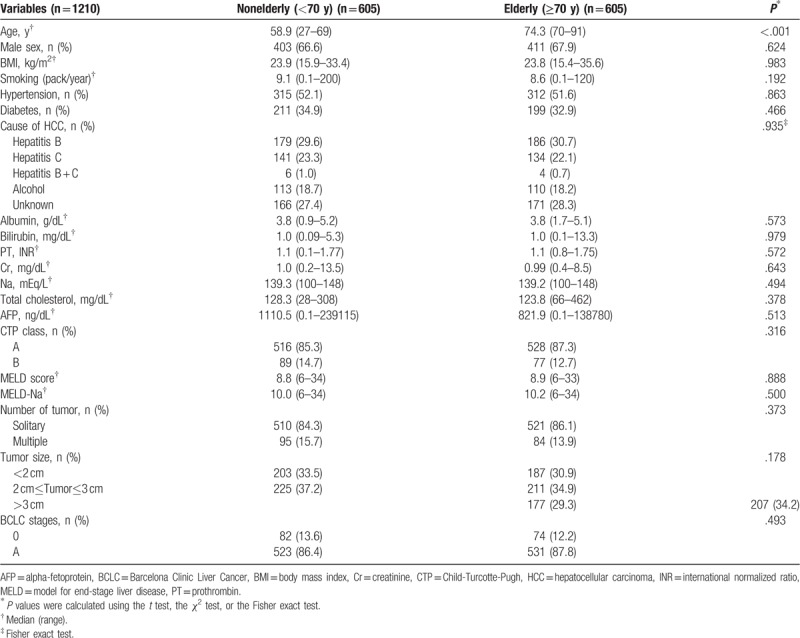
Figure 2.
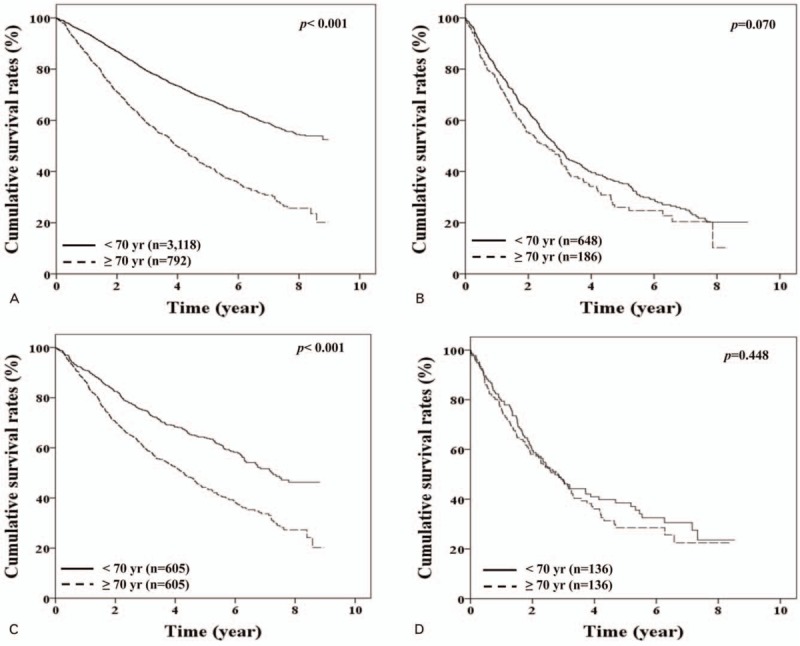
Cumulative overall survival rates of patients with Barcelona Clinic Liver Cancer (BCLC) stages 0–A and B according to patient age. (A) and (C) Showed cumulative OS rates of patients with BCLC stage 0–A before (n = 3910) and after (n = 1210) propensity score matching (PSM), respectively. (B) and (D) Showed cumulative OS rates of patients with BCLC stage B before (n = 834) and after (n = 272) PSM, respectively.
When patients with BCLC stages 0 or A were analyzed separately (Supplementary Figure 1), cumulative OS rates were significantly lower for elderly than nonelderly patients before PSM (P values for all <.05) (Supplementary Figures 2A and 2B). However, after PSM for HCC patients with BCLC stage 0, cumulative OS rates were not significantly different between elderly and nonelderly patients despite the tendency (P = .074) (Supplementary Figure 2C), unlike for those with BCLC stage A (P < .001) (Supplementary Figure 2D).
3.3. OS rates of HCC patients with BCLC stage 0-A by treatment type
In HCC patients with BCLC stage 0-A, the cumulative OSs were significantly better in SR, LAT, and LRT order both in elderly (P value for all <.05) and nonelderly patients (P value for all <.05), regardless of PSM (Fig. 3A and D). Furthermore, similar results were obtained for tumors of ≤3 cm and >3 cm (Fig. 3B, C, E, and F). Elderly patients had lower OSs than nonelderly patients after SR, LAT, or LRT, regardless of PSM (Fig. 3A and D). However, elderly and non-elderly patients treated by SR for tumor(s) ≤3 cm had similar OS rates after PSM (P = .575), but significantly different OS rates before PSM (P = .002) (Fig. 3B and E). However, elderly and nonelderly patients with BCLC 0-A staged HCC that underwent SR for tumor(s) >3 cm had significant different OSs, regardless of PSM (all P values <.05) (Fig. 3C and F).
Figure 3.
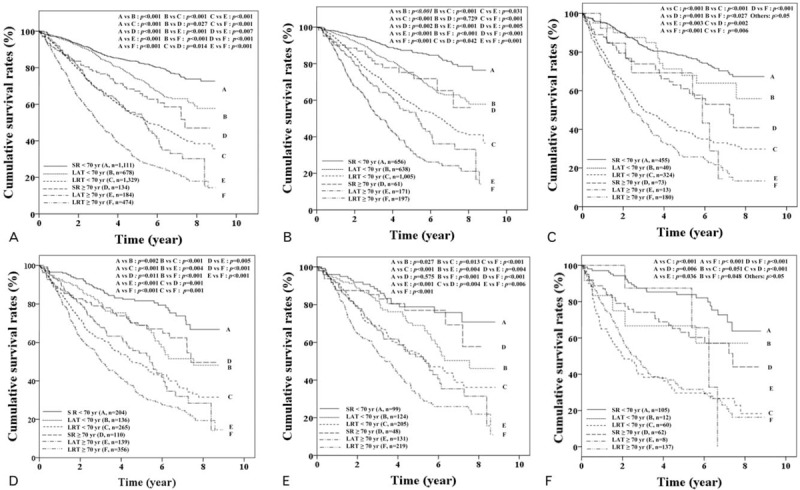
Cumulative overall survival rates of patients with Barcelona Clinic Liver Cancer (BCLC) stage 0–A with a tumor size of ≤3 cm or >3 cm according to age and treatment type. (A) and (D) Showed overall survival (OS) in patients with BCLC stage 0–A before (n = 3910) and after (n = 1210) propensity score matching (PSM), respectively. (B) and (E) Showed OS in patients with BCLC 0–A staged hepatocellular carcinoma (HCC) of ≤3 cm in tumor size before (n = 2825) and after PSM (n = 826), respectively. (C) and (F) Showed OS in patients with BCLC 0–A staged HCC of >3 cm in tumor size before (n = 1085) and after PSM (n = 384), respectively. LAT = local ablation therapy, LRT = locoregional therapy, SR = surgical resection.
3.4. OS rates of HCC patients by BCLC stage according to age and treatment type
We analyzed patients with BCLC stage 0 and A separately after PSM (Supplementary Figure 1). Between elderly and nonelderly HCC patients with BCLC stage 0, the OSs of SR and LAT were not significantly different (P > .05) (Fig. 4A). Furthermore, OSs of these 2 treatment types were not significantly different for elderly patients with BCLC stage 0 (P > .05), and SR and LAT provided better survival rates than LRT, respectively (P < .05) (Fig. 4A). For HCC patients with BCLC stage A, cumulative OSs were significantly better in SR, LAT, and LRT order both in elderly and nonelderly patients (P values for all <.05), but were not significantly different between LAT and LRT in elderly patients (P = .058) (Fig. 4B).
Figure 4.
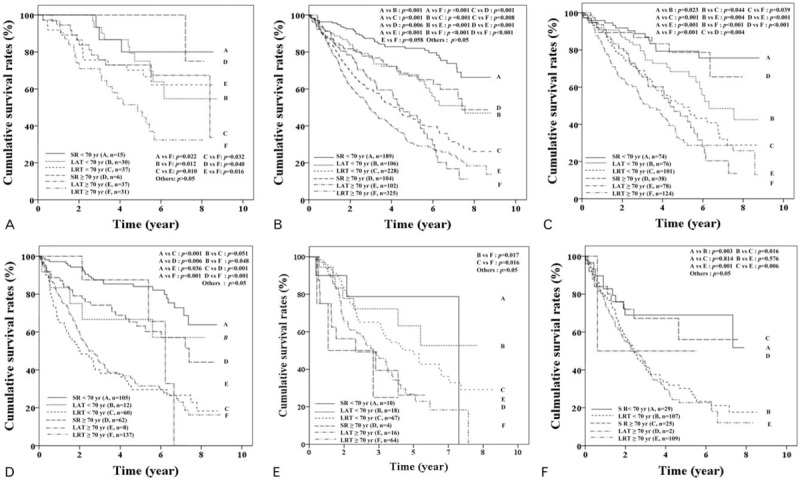
Cumulative overall survival rates of patients with Barcelona Clinic Liver Cancer (BCLC) stages 0, A, and B according to age and treatment type after propensity score matching. (A) and (B) Showed overall survival (OS) of patients with BCLC stage 0 (n = 156) and A (n = 1054), respectively. (C) and (D) Showed OS of patients with BCLC A staged solitary hepatocellular carcinoma (HCC) with ≤3 cm in tumor size (n = 491) and with >3 cm in tumor size (n = 384), respectively. (E) Showed OS of patients with BCLC A staged multiple HCCs (n = 179) and (F) showed OS in patients with BCLC stage B (n = 272). LAT = local ablation therapy, LRT = locoregional therapy, SR = surgical resection.
For HCC patients with solitary BCLC stage A (Fig. 4C and 4D), OSs were not different between elderly and nonelderly patients who underwent SR for a tumor of ≤3 cm in size (P > .05) (Fig. 4C), but OSs were lower in elderly patients than nonelderly patients for tumor of >3 cm in size (P = .006) (Fig. 4D). In addition, SR showed better OS rates than LRT, regardless of tumor size (≤3 cm or >3 cm), even in elderly patients (Fig. 4C and 4D). However, for BCLC A staged elderly patients with multiple HCCs (Fig. 4E), no significant difference was observed between the OSs of different treatment types.
Subgroup analysis of patients with solitary BCLC A staged HCC of <2 cm or 2 to 3 cm (Supplementary Figures 1 and 3) showed OSs were not significantly different between elderly and nonelderly patients that underwent SR (P > .05). For these elderly patients, SR and LAT had better OSs than LRT, respectively (P < .05) (Supplementary Figures 3A and 3B). However, the OSs of SR and LAT were significantly different in elderly and nonelderly patients with a <2 cm sized solitary HCC, respectively (Supplementary Figure 3A), but SR had a better OS than LAT in elderly and nonelderly patients with a 2 to 3 cm sized solitary HCC, respectively (Supplementary Figure 3B).
For elderly and nonelderly patients with BCLC stage B, OSs of SR (P = .814) and LRT (P = .576) were not significantly different. However, SR had a better OS than LRT in elderly patients (P = .006) (Fig. 4F).
3.5. Significant predictors of OS in elderly HCC patients
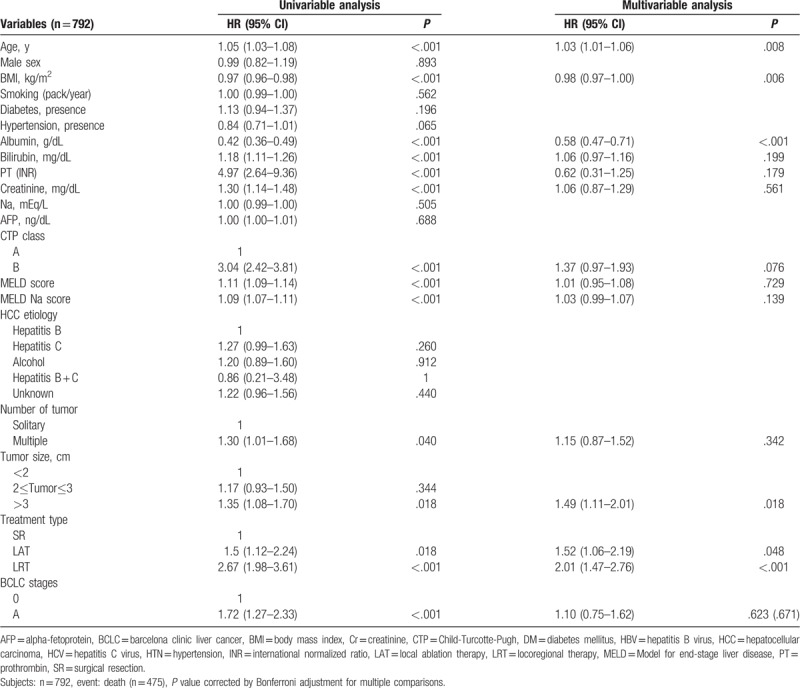
In elderly patients with BCLC stage 0-A (n = 792), multivariable analysis showed that age (hazard ratio [HR] 1.03, P = .008), BMI (HR 0.98, P = .006), serum albumin level (HR 0.58, P < .001), a tumor size >3 cm (HR 1.49, P = .018) (as compared with a tumor size of <2 cm), LAT (HR 1.52, P = .048) or LRT (HR 2.01, P < .001) as compared with SR were poor predictors of OS (Table 3). In elderly patients with BCLC stage B (n = 186), multivariable analysis showed that LRT (HR 2.64, P = .042) as compared with SR was a poor predictor of OS, and that multiple tumors (HR 2.64, P = .072) tended to indicate a poor prognosis as compared with solitary tumor (Supplementary Table 3).
Table 3.
Significant predictors of overall survival of in old age (≥70) HCC patients with BCLC 0-A.
4. Discussion
In this large-scaled study, we found that the OS rate of elderly patients (≥70 years) with BCLC A staged HCC was lower than that of nonelderly patients (≤70 years), regardless of PSM. However, no significant difference was found between the OSs of elderly and nonelderly patients with BCLC stage 0 or B, after PSM. As was expected, elderly patients underwent less-invasive treatments than nonelderly patients. However, interestingly, SR was observed to have better OS rates than LRT in elderly patients with BCLC stage 0, A, and B, respectively, even in those with solitary BCLC stage A, regardless of tumor size (<2 cm, 2–3 cm, or >3 cm), after PSM. Furthermore, multivariable analyses showed that SR was a better prognostic factor of OS than LRT in elderly patients with BCLC stage 0-A and B, respectively. The main strength of the present study is that results were derived by a comprehensive analysis, which included PSM of a considerable amount of data obtained by random sample audit in a nationwide database. Furthermore, mortality data were obtained from the KNSO, and thus, is likely to be more accurate than data used in previous studies.
The phenomenon of population aging means that clinicians are being more frequently presented with elderly HCC patients with age of ≥70 , and the number of them has been increasing.[14–16] According to a report of statistics of South Korea in 2017, our country has already become an aging society as the rate of the population aged >70 years was 8.2% and the average life expectancy has reached about 80.5 years. Therefore, we used 70 years as the cutoff value to divide the patients into the elderly and younger to clarify the characteristics of older HCC patients, considering the population structure owing to aging or the increase in elderly HCC patients. Until now, elderly patients, even those with BCLC 0-A staged HCC, tend to be administered palliative treatments, such as, TACE, because it is less invasive than SR.[8] In this study, we also found elderly patients received LRT more frequently than SR. We ascribed this situation to a lack of sufficient evidence-based studies on survival rates by treatment methods and tumor stage, and because post-treatment follow-up for them continuously is more difficult. Thus, in our opinion, elderly HCC patients require more attention, especially with regard to effective management and survival.
Recently, several studies have reported that elderly patients with HCC have survival rates similar to those of nonelderly patients,[8,11,17] but, these studies were limited by single-center designs,[11,16] small subjects numbers,[8,11,17] short follow-up durations,[8,11,17] and no assessment of survival rates by age at each BCLC stage.[8,11,17] The other recent study reported that the OS rate of elderly patients was similar to that of nonelderly patients at the same tumor stage,[8] but patients with BCLC stage 0 or A were not analyzed separately, and no comparisons were made by age combined with treatment type at each tumor stage.[18] The other previous study also reported OSs were not different between elderly and noelderly patients who received same treatments,[17] but survival outcomes among treatment types, such as, SR, RFA, and TACE in each tumor stage were not evaluated in elderly HCC patients. Interestingly, we found the OS rate of elderly patients with BCLC stage 0-A was lower than that of non-elderly patients, regardless of PSM. Furthermore, when we performed the analysis for different BCLC stages, the OS rates of elderly and nonelderly patients were found to differ significantly for BCLC stage A, but not for BCLC stage 0 or B. This finding probably explains discrepancies between studies, as the previous studies did not compare the OS by each BCLC stage. Furthermore, it appears to be more reasonable that OSs of HCC patients be analyzed at each BCLC stage, owing to the relation between stage and prognosis. Notably, the present study has several strengths as compared with the previous studies.[8,11,17,18] First, it has more statistical power because of the large number of elderly patients with BCLC 0-B staged HCC enrolled. Second, accurate mortality data were obtained from the KNSO, and thus, the median post-treatment follow-up duration was around 40 months. Third, selection bias was minimized despite the retrospective study design because study subjects were enrolled by random audit from KCCR database, and furthermore, the analysis was conducted using PSM and multivariable analysis. Fourth, survival rates of elderly patients were comprehensively assessed by age and treatment types for each BCLC stage. In particular, patients with BCLC stage A were analyzed in more detail with respect to tumor number and size. These strengths of the present study indicate its results are more reliable than those of the previous studies.
The prognoses of HCC patients that receive curative treatment are better than those of patients that receive palliative treatment, and BCLC 0-A staged HCC patients are a good candidate for curative-intent treatments, such as, LT, SR, or RFA. In a previous study,[8] elderly patients with BCLC stage 0-A were reported to have survival outcomes similar to those of nonelderly patients, which may have been caused by similar proportions of curative treatments (SR or RFA) for elderly and nonelderly patients.[8] However, in the present study, elderly patients with BCLC stage 0-A staged HCC had poorer survival outcomes than nonelderly patients. In addition, when proportions of treatment types were analyzed at BCLC stage 0 and A, respectively, the SR proportion was found to be very low in BCLC stage 0, and to increase slightly in BCLC stage A. Interestingly, in elderly patients with BCLC stage 0 or A with a tumor size of ≤3 cm, we found OS rates decreased in the order of SR, LAT, and LRT, which was not observed in nonelderly patients. Furthermore, SR produced better OS rates than LAT or LRT both in elderly and nonelderly patients with BCLC stage 0 or A stages, and multivariable analysis showed SR was a good predictor for OS in elderly patients with these stages. These findings suggest that survival difference between elderly and nonelderly patients with BCLC stage 0 or A may depend on how many patients received curative-intent SR. However, in the present study, elderly patients with these stages were mainly treated by LAT or LRT rather than SR, especially in those with BCLC stage 0, which suggests that elderly patients are being treated using less risky rather than more effective treatment methods. Taken together, our findings indicate that even in elderly HCC patients with BCLC stage 0-A, curative-intent SR needs to be considered as the preferred treatment option.
Previous studies have also concluded SR is effective even in elderly HCC patients.[9,11,13,17] Although elderly patients are more likely to experience complications after surgery, previous studies have reported no significant difference between elderly and nonelderly patients in terms of the incidence of postoperative complications or hospitalization stay after surgery.[9,10,19,20] Although we did not assess post-treatment complications because they were not detailed in KCCR database, SR may be safely applied to elderly patients with good performance status or well-preserved liver function based on the results of these previous studies.[9,10,19,20] In fact, in the present study, reserved liver function was good in most BCLC 0-A staged patients. Furthermore, although DM and HTN were more common in elderly patients, multivariable analysis showed they were not significant predictors of OS. These observations suggest that chronological age per se does not contraindicate SR, in elderly HCC patients, and that the performance status and physiological age are more important.
With regard to the definition of BCLC B staged HCC, we defined it as multinodular HCC base on the recently issued clinical practice guidelines for HCC management.[6,21] Generally, TACE is the standard therapy for patients with BCLC stage B, and in the present study, the majority of these patients received TACE. Until recently, TACE indications for BCLC stage B were based on the results of studies that compared TACE with conservative treatments,[21–24] but not with SR. In the present study, SR was associated with better survival outcome than TACE regardless of age in BCLC B staged HCC patients. Recent studies also support our results by reporting SR provides the greater long-term OS than TACE in BCLC stage.[25–27] Another studies reported the safeties of SR and TACE are comparable for this staged patients.[28,29] Therefore, despite the relatively small number of elderly patients with SR in BCLC stage B, our results suggest SR needs to be recommended even to those patients, if they are good surgical candidates. Moreover, given the small proportion of BCLC stage B patients who underwent SR and the absence of data for elderly patients with this stage, we believe the present study provides a reasonable basis for future prospective studies.
In the present study, elderly patients with HCC had lower BMIs, a higher HCV infection rate, and larger tumors, and a higher proportion of women than nonelderly patients. In elderly patients, lower BMIs are largely explained by age-associated muscle and bone density decreases, and the higher proportion of women by longer life expectancies of female, as have been previously reported.[8,29,30] Furthermore, HCV infection is a more common cause of HCC in elderly patients despite the endemic nature of HBV in South Korea. This observation was also made in a recent study,[18] and is probably explained by HBV infection via vertical transmission during the perinatal period, and the higher risk of HBV-associated HCC even in the absence of LC.[22] Second, most HCV is infected through blood transmission at a later life rather than HBV, and HCV-associated HCC risk usually increases slowly in the background of LC.[22] Thus, mean age of onset of HCV-related HCC may be greater than that of HBV-related HCC. In terms of tumor size, elderly patients may be insensitive to clinical symptoms, and the majority are not eligible for workplace health screening because of retirement from work. Therefore, HCC diagnosis may be delayed in them and their tumor sizes may be large. In fact, in the present study, HCC stage tended to be more advanced in elderly patients than nonelderly patients, which indicates the importance of early screening for HCC nationwide, even in elderly patients.
Some limitations of the present study warrant consideration. First, we could not completely eliminate inherent selection bias associated with the retrospective study design. However, we tried to minimize potential confounding factors using the random sampling audit method and a large-scale nationwide cancer registry, and by performing PSM and multivariable analysis. Moreover, to our knowledgement, this is the first large-scale study to evaluate and compare the OS of elderly and nonelderly HCC patients with respect to treatment types for each BCLC stage 0, A, and B. Second, we could not evaluate the effects of all comorbidities on survival of elderly patients because of the insufficient data for patient's comorbidity in KCCR database. However, a medical history of DM or HTN, and smoking history were analyzed, but not found to predict OS. Nonetheless, elderly patient's comorbidity, such as, heart or cerebrovascular disease and lung or thyroid disease, needs to be considered in the future randomized controlled study. Third, unfortunately, post-treatment complications could not be evaluated in this study because they are not included in the KCCR database. However, comorbidities and liver functions were adjusted using PSM, and remnant liver function in most of the enrolled patients was as good as CTP class A, and MELD or MELD-Na score was also satisfactory, which suggested post-treatment complications may not be different in between elderly and nonelderly patients. Fourth, we did not evaluate the effects of chemotherapy or sorafenib therapy. However, because of the small number of them in patients with BCLC stage 0-B, it may be meaningless to evaluate them in these stages. Furthermore, the survival outcomes of patients with BCLC stage C or D were not compared, because SR and RFA are not generally recommended for these stages according to the current HCC treatment guidelines.[5–7] In addition, LT was not assessed because advanced age has been regarded as a relative contraindication for LT,[31] and no elderly patients in the KCCR database received LT.
In conclusion, in this large-scale nationwide cancer registry-based study, we found that OS rate was significantly lower in elderly HCC patients than in nonelderly patients with BCLC stage 0-A after PSM. However, when we performed the analysis on patients with BCLC stage 0 and A, respectively, OSs were not found to be significantly different between elderly and nonelderly patients with BCLC stage 0, but to be significantly different between those with BCLC stage A. In elderly HCC patients with BCLC stage 0-A, SR provided better OS rates than LAT or LRT, and in those with BCLC stage B, SR produced a better OS rate than LRT. Therefore, our results show SR is an effective treatment option that provides survival benefit for elderly HCC patients with BCLC stages 0-A and B, and suggest that SR be considered the first therapeutic option even in elderly HCC patients with these stages.
Acknowledgments
The authors thank the Korea Central Cancer Registry (KCCR)-affiliated Hospitals and non KCCR-affiliated Hospitals.
Author contributions
Conceptualization: Young-Joo Jin, Jongbeom Shin, Jung Hwan Yu, Jin-Woo Lee.
Data curation: Young-Joo Jin, Jongbeom Shin, Jung Hwan Yu.
Formal analysis: Young-Joo Jin, Young Ju Suh, Deuck Hwa Kim, Seyoun Byun.
Investigation: Young-Joo Jin, Jongbeom Shin, Jung Hwan Yu.
Methodology: Young-Joo Jin, Jongbeom Shin, Jung Hwan Yu, Young Ju Suh, Deuck Hwa Kim, Seyoun Byun.
Project administration: Jongbeom Shin, Jung Hwan Yu.
Supervision: Jin-Woo Lee.
Writing – original draft: Young-Joo Jin, Jongbeom Shin, Jung Hwan Yu.
Supplementary Material
Footnotes
Abbreviations: AFP = Alpha-fetoprotein, BCLC = Barcelona Clinic Liver Cancer, BMI = body mass index, CI = confidence intervals, Cr = Creatinine, CTP = Child-Turcotte-Pugh, DM = diabetes mellitus, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HR = hazard ratio, HTN = hypertension, INR = international normalized ratio, KASL = the Korean Association for the study of liver, KCC = Korea Central Cancer, LAT = local ablation therapy, LRT = loco-regional therapy, LT = Liver transplantation, MELD = model for end-stage liver disease, OS = overall survival, PSM = Propensity Score Matching, PT = prothrombin time, RFA = Radiofrequency ablation, SR = Surgical resection, TACE = transarterial chemoembolization.
These two authors (JS and JHY) are co-first authors.
This study was supported by The Research Supporting Program of The Korean Association for the Study of the Liver and The Korean Liver Foundation.
Data were provided by the Korean Central Cancer Registry, Ministry of Health and Welfare, South Korea, and the Korean Liver Cancer Study Group (KLCSG).
The authors report no conflicts of interest:.
Supplemental Digital Content is available for this article.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–386. [DOI] [PubMed] [Google Scholar]
- [2].Pallis AG, Fortpied C, Wedding U, et al. EORTC elderly task force position paper: approach to the older cancer patient. Eur J Cancer 2010;46:1502–13. [DOI] [PubMed] [Google Scholar]
- [3].Ozakyol A. Global epidemiology of hepatocellular carcinoma (HCC epidemiology). Journal of gastrointestinal cancer 2017;48:238–40. [DOI] [PubMed] [Google Scholar]
- [4].Mittal S, El-Serag HB. Epidemiology of HCC: Consider the population. J Clin Gastroenterol 2013;47:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358–80. [DOI] [PubMed] [Google Scholar]
- [6].European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- [7].Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kozyreva ON, Chi D, Clark JW, et al. A multicenter retrospective study on clinical characteristics, treatment patterns, and outcome in elderly patients with hepatocellular carcinoma. Oncologist 2011;16:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tsujita E, Utsunomiya T, Ohta M, et al. Outcome of repeat hepatectomy in patients with hepatocellular carcinoma aged 75 years and older. Surgery 2010;147:696–703. [DOI] [PubMed] [Google Scholar]
- [10].Zhao LY, Huo RR, Xiang X, et al. Hepatic resection for elderly patients with hepatocellular carcinoma: a systematic review of more than 17,000 patients. Expert Rev Gastroenterol Hepatol 2018;1–0. [DOI] [PubMed] [Google Scholar]
- [11].Kaibori M, Matsui K, Ishizaki M, et al. Hepatic resection for hepatocellular carcinoma in the elderly. J Surg Oncol 2009;99:154–60. [DOI] [PubMed] [Google Scholar]
- [12].Santambrogio R, Barabino M, Scifo G, et al. Effect of age (over 75 years) on postoperative complications and survival in patients undergoing hepatic resection for hepatocellular carcinoma. J Gastrointest Surg 2017;21:657–65. [DOI] [PubMed] [Google Scholar]
- [13].Huang J, Li BK, Chen GH, et al. Long-term outcomes and prognostic factors of elderly patients with hepatocellular carcinoma undergoing hepatectomy. J Gastrointest Surg 2009;13:1627–35. [DOI] [PubMed] [Google Scholar]
- [14].McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev 2006;15:1198–203. [DOI] [PubMed] [Google Scholar]
- [15].Dohmen K, Shigematsu H, Irie K, et al. Trends in clinical characteristics, treatment and prognosis of hepatocellular carcinoma. Hepatogastroenterology 2003;50:1872–7. [PubMed] [Google Scholar]
- [16].Kim BH, Park JW. Epidemiology of liver cancer in South Korea. Clin Mol Hepatol 2018;24:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mirici-Cappa F, Gramenzi A, Santi V, et al. Treatments for hepatocellular carcinoma in elderly patients are as effective as in younger patients: A 20-year multicentre experience. Gut 2010;59:387–96. [DOI] [PubMed] [Google Scholar]
- [18].Guo H, Wu T, Lu Q, et al. Hepatocellular carcinoma in elderly: clinical characteristics, treatments and outcomes compared with younger adults. PLoS One 2017;12:e0184160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nanashima A, Abo T, Nonaka T, et al. Prognosis of patients with hepatocellular carcinoma after hepatic resection: Are elderly patients suitable for surgery? J Surg Oncol 2011;104:284–91. [DOI] [PubMed] [Google Scholar]
- [20].Inoue Y, Tanaka R, Fujii K, et al. Surgical outcome and hepatic regeneration after hepatic resection for hepatocellular carcinoma in elderly patients. Dig Surg 2018;36:289–301. [DOI] [PubMed] [Google Scholar]
- [21].Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003;37:429–42. [DOI] [PubMed] [Google Scholar]
- [22].Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734–9. [DOI] [PubMed] [Google Scholar]
- [24].Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164–71. [DOI] [PubMed] [Google Scholar]
- [25].Yin L, Li H, Li AJ, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol 2014;61:82–8. [DOI] [PubMed] [Google Scholar]
- [26].Liu PH, Hsia CY, Lee YH, et al. Surgical resection versus transarterial chemoembolization for BCLC stage C hepatocellular carcinoma. J Surg Oncol 2015;111:404–9. [DOI] [PubMed] [Google Scholar]
- [27].Hyun MH, Lee YS, Kim JH, et al. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: a meta-analysis of high-quality studies. Hepatology 2018;68:977–93. [DOI] [PubMed] [Google Scholar]
- [28].Zhong JH, Xiang BD, Gong WF, et al. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One 2013;8:e68193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Reddy SK, Barbas AS, Turley RS, et al. Major liver resection in elderly patients: a multi-institutional analysis. J Am Coll Surg 2011;212:787–95. [DOI] [PubMed] [Google Scholar]
- [30].Yau T, Yao TJ, Chan P, et al. The outcomes of elderly patients with hepatocellular carcinoma treated with transarterial chemoembolization. Cancer 2009;115:5507–15. [DOI] [PubMed] [Google Scholar]
- [31].Farkas S, Hackl C, Schlitt HJ. Overview of the indications and contraindications for liver transplantation. Cold Spring Harb Perspect Med 2014;4:a015602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


