Supplemental Digital Content is available in the text
Keywords: CD4+CD25+Foxp3+ Treg cells, cytotoxicity, NK cells, NKG2D, suppression
Abstract
Background:
Studies have shown that CD4+CD25+Foxp3+Treg cells suppress NKG2D expression on NK cells via a cell contact-dependent mechanism and increased TGF-β and IL-10 production in some cancer models. We herein aimed to explore whether CD4+CD25+Foxp3+Tregs suppress NKG2D-mediated NK cell cytotoxicity in peripheral blood and elucidate the exact mechanism underlying this phenomenon.
Methods:
To explore the function of NKG2D, NK cell cultures were treated with an NKG2D-blocking antibody to block these receptors. Additionally, TGF-β- and IL-10-blocking antibodies were added to NK and CD4+CD25+Foxp3+Treg cell cocultures to evaluate whether the latter cells suppress NKG2D expression of NK cells via increasing the production of TGF-β and IL-10. The expression of NKG2D on NK cells was detected by 3-color flow cytometry, and NK cell activity was assessed by 3 assays: a nonradioactive cytotoxicity assay, an ELISA measuring IFN-γ production and a flow cytometry assay to evaluate CD107a expression.
Results:
Blocking NKG2D decreased NK cell cytotoxicity, IFN-γ production and CD107a expression. Moreover, blocking TGF-β and IL-10 substantially increased the NKG2D expression in NK and CD4+CD25+Foxp3+Treg cell cocultures. Similarly, blocking TGF-β and IL-10 enhanced NK cell cytotoxicity, IFN-γ production and CD107a expression; Transwell insert assays also revealed increased IFN-γ production and CD107a and NKG2D expression.
Conclusion:
CD4+CD25+Foxp3+Tregs suppress NKG2D-mediated NK cell cytotoxicity in peripheral blood via a cell contact-dependent mechanism and increased TGF-β and IL-10 production.
1. Introduction
Immune escape and tumor immunosurveillance are the main mechanisms underlying cancer development.[1] Treg cells (Tregs) play a crucial role in immunological self-tolerance and suppression on effector cells, such as NK cells, in an effort to evade the immune system.[2] The most typical Tregs is CD4+CD25+Foxp3+ Tregs defined as nTregs (natural Tregs), which is vital to maintain peripheral tolerance and limit beneficial responses. Genetic anomaly of Foxp3 controlling CD4+CD25+Tregs differentiation and function causes autoimmune and inflammatory disease, especially cancer. In general, NK cells have high antitumor activity with no prior stimulation, function as the major immunosurveillance component and act as the first line of defense.[3] NKG2D is an NK cell receptor that mediates the NK cell cytotoxicity by recognizing and binding to NKG2D ligands (NKG2DLs), which are upregulated within the context of cancer.[4]
To date, considerable evidence shows that the number of Tregs is increased while the number of NK cells is decreased in the peripheral blood of cancer patients. Mirjačić et al reported that the percentage of CD4+CD25+Tregs is negatively correlated with NK cell cytotoxicity in metastatic melanoma patients as well as with the expression of NKG2D in peripheral blood.[5] Furthermore, CD4+CD25+ Tregs may inhibit NKG2D-mediated NK cell cytotoxicity in mice with melanoma [6] and in human squamous cell carcinoma in vitro.[7] Our previous studies showed that the number of CD4+CD25+ Tregs was significantly increased in the peripheral blood of patients with colorectal cancer but that NKG2D expression was significantly decreased.[8,9] Nonetheless, whether CD4+CD25+Foxp3+Tregs can suppress NK cytotoxicity via downregulating NKG2D expression in peripheral blood and the potential underlying mechanism are not well understood.
Various cytokines, such as TGF-β and IL-10, and a cell contact-dependent mechanism mediate the suppressive effect of Tregs.[10] TGF-β is regarded as an inhibitory cytokine that regulates Treg-mediated suppression.[11] In cervical cancer patients and a mouse melanoma model, Tregs can abrogate NK cell cytotoxicity via the TGF-β pathway,[6,12] and IL-10, a key anti-inflammatory cytokine produced by activated Tregs,[13] may modulate NKG2D ligand expression on melanoma cells and NK cell cytotoxicity in vitro.[14] Surface molecules on Tregs, such as TGF-β, directly bind to corresponding receptors on the target cell, and studies have shown that CD4+CD25+ Tregs inhibit the cytotoxic activity of NK lymphocytes via direct cell-to-cell interactions in vitro [15]; However, the exact mechanism by which CD4+CD25+Foxp3+Tregs inhibit NKG2D on NK cells (i.e., via cell-to-cell interaction or upregulated TGF-β and IL-10 production) remains unclear.
In the present study, we explored the effect of CD4+CD25+Foxp3+ Tregs on NKG2D expression on NK cells and the potential underlying mechanism. We found that CD4+CD25+Foxp3+Tregs inhibited NK cell cytotoxicity by downregulating NKG2D expression on NK cells, which was mediated by a cell contact-dependent mechanism and increased TGF-β and IL-10 production.
2. Methods
2.1. Antibodies
Mouse anti-human NKG2D monoclonal antibodies (mAbs) were purchased from eBioscience (No.16–5880–85, San Diego, CA). Rabbit anti-human TGF-β polyclonal antibodies, Mouse anti-human IL-10 mAbs and normal human IgG control antibodies were purchased from R&D Systems (No. AB-100NA, MAB217R-100 and 1–001-A, Minneapolis, MN, USA). Mouse anti-human-CD3 mAbs and mouse anti-human CD28 mAbs were purchased from Biolegend (San Diego, CA) and eBioscience, respectively. We performed flow cytometry analyses to detect the expression of CD107a and NKG2D using mouse anti-human CD107a-FITC mAb, purchased from BD Pharmingen (No. 560949, San Jose, CA), and mouse anti-human NKG2D Alexa Fluor 488-conjugated mAbs, purchased from R&D Systems (No. FAB139G-100). Mouse PE anti-human-CD3 mAbs and mouse APC anti-human-CD56 were obtained from BD Biosciences (No. 300310 and 318310 San Jose, CA).
2.2. Isolation of NK and Tregs
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation (No. 1114544, Axis-Shield Density Gradient Media, Oslo, Norway) using blood obtained from healthy volunteers. NK cells were isolated via negative selection using a human NK cell isolation kit (No. 130–092–657, Miltenyi Biotec, Bergish Gladbach, Germany) according to the manufacturer's instructions, and Tregs were isolated using a human CD4+CD25+ regulatory T cell isolation kit (No. 130–091–301, Miltenyi Biotec). The purity of the NK cells and CD4+CD25+Foxp3+ regulatory T cell was greater than 90%, as detected by fluorochrome-coupled monoclonal antibodies.
2.3. Cell culture and neutralization
Cells were cocultured in a 96-well sterile cell culture plate containing RPMI 1640 medium, 10% fetal bovine serum, 100 U/ml streptomycin and 100 U/ml penicillin. The NKG2D-blocking antibody (10 μg/ml) and IL-2 (200 U/ml; No. AF-200–02–10, PeproTech, Rocky Hill, NJ) were added to the NK cell cultures for 24 hours. An anti-TGF-β (10 μg/ml), anti-IL-10 (10 μg/ml) or normal human IgG control (10 μg/ml) mAbs was added to NK and CD4+CD25+Foxp3+ Treg cell cocultures with concurrent activation by anti-CD3 (5 μg/ml) and anti-CD28 (4 μg/ml) plus IL-2 for 24 hours. All cells were incubated at 37°C and 5% CO2.
2.4. Transwell assay
Two groups were established depending on whether freshly isolated CD4+CD25+Foxp3+ Tregs were placed in the upper or lower chamber of the Transwell insert. In the treatment group, CD4+CD25+Foxp3+ Tregs (1.5 × 105 cells/well) were placed in the upper chamber, and NK cells (1.5 × 105 cells/well) were placed in the lower chamber. In the control group, CD4+CD25+Foxp3+ Tregs and NK cells were both placed in the lower chamber. The cells were incubated for 24 hours.
2.5. NK cell cytotoxicity
NK cell cytotoxicity was assessed using a Non-Radioactive Cytotoxicity Assay kit (No. G1780, Promega, Madison, WI) that quantitatively measured the production of lactose dehydrogenase (LDH). Coculture units of neutralization were detected using this kit, whereby NK cells (5 × 104 cells/well) acted as effector cells, and human colon carcinoma HT29 cells (5 × 103 cells/well) or human erythroleukemia K562 cells (5 × 103 cells/well) acted as target cells. The percentage of cytotoxicity was calculated using the following formula:
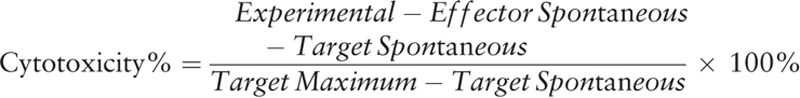 |
2.6. Enzyme-linked immunosorbent assay (ELISA)
IFN-γ is released by NK cells to promote antitumor efficacy. In this study, IFN-γ production in supernatant was measured using a Human IFN-gamma Uncoated ELISA kit (No. 88–7316–86, Thermo Fisher Scientific, Carlsbad, CA) according to the manufacturer's instructions. TGF-β and IL-10 production in supernatant were detected by human TGF-beta 1 ELISA kit (EH010–96, Shanghai ExCell Biology, Shanghai, China) and human IL-10 ELISA kit (EH006–96, Shanghai ExCell Biology, Shanghai, China).
2.7. Flow cytometry
To assess CD107a degranulation, collected cells were incubated with anti-CD107a-FITC (20 μl/106 cells) for 1 hour; Monensin (2 μmol/L; No. 00–4505–51, eBioscience) was then added, and the cells were incubated for 4 hours. Anti-CD3-PE (10 μl/106 cells) and anti-CD56-APC (10 μl/106 cells) antibodies were added for an additional 20 minutes. NKG2D expression on the NK cell surface was evaluated using a human NKG2D Alexa Fluor 488-conjugated antibody (10 μl/106 cells). At the same time, the cells were incubated with anti-CD3-PE (10 μl/106 cells) and anti-CD56-APC (10 μl/106 cells) antibodies for 30 minutes. Stained cells were counted using a FACSCalibur flow cytometer (BD LSRFortessa); at least 10,000 cells were counted.
2.8. Statistical analysis
Data analysis were conducted using the statistical software GraphPad Prism 5, and the results were presented as the mean ± standard deviation (SD). Unpaired t tests were applied to examine significant differences; according to the α = 0.05 standard, P < .05 indicated a statistically significant difference.
3. Results
3.1. Decreased NKG2D expression downregulates NK cell activity
We first verified the function of NKG2D by neutralization with anti-NKG2D mAbs. Compared to cells treated with the control antibody, NK cell cytotoxicity and IFN-γ production were decreased when the anti-NKG2D mAbs was added to the NK-HT29 coculture, as shown in Figure 1A and B. Additionally, blocking NKG2D using anti-NKG2D mAbs decreased the expression of CD107a on NK cells compared with that in control cells (32.9% vs 21.6%; P < .05; Fig. 1C). As expected, flow cytometry analysis showed that compared with control cells, NKG2D expression in NK cells was decreased in the presence of anti-NKG2D mAbs (58.4% vs 24.2%; P < .01; Fig. 1D). And the similar results in K562 cells were shown in supplementary Figure 1.
Figure 1.
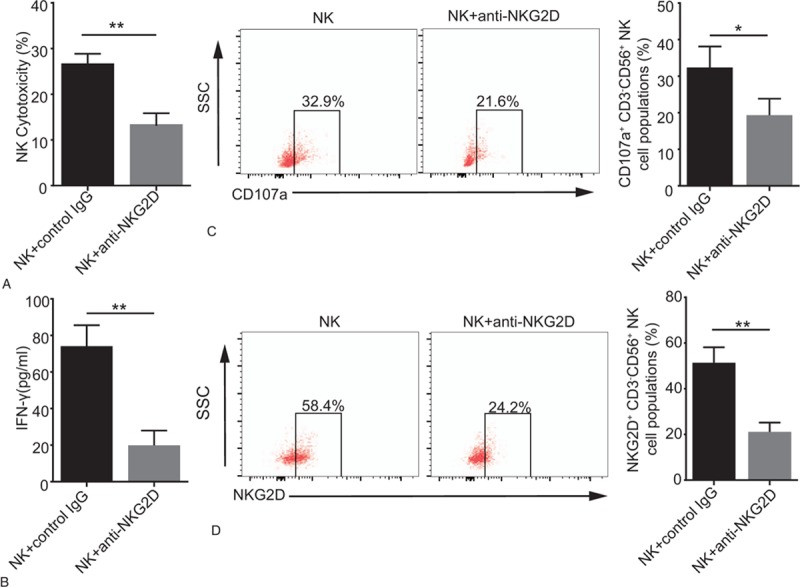
Decreased NKG2D expression upregulates NK cell cytotoxicity. An anti-NKG2D (10 μg/ml) mAbs or control IgG (10 μg/ml) was added to the NK-HT29 coculture (10:1 cell ratio). (A) Histogram represents NK cytotoxicity detected by a Non-Radioactive Cytotoxicity Assay following blockades with different antibodies. (B) Histogram represents IFN-γ production detected by ELISA following blockades with different antibodies. (C) Graphs represent CD107a expression on gated CD3-CD56+ NK cells as determined by flow cytometry following blockades with different antibodies. (D) Graphs represent NKG2D expression on NK cells following blockades with different antibodies. Numerical data are shown as the mean ± SD of 3 independent experiments (∗P < .05 and ∗∗P < .01).
3.2. Blocking TGF-β increases NKG2D expression on NK cells downregulated by CD4+CD25+Foxp3+ Tregs
To analyze whether CD4+CD25+Foxp3+ Tregs suppress NKG2D expression and the exact mechanism underlying this phenomenon, an anti-TGF-β mAbs that blocks the expression of TGF-β was added to a coculture of NK cells, CD4+CD25+Foxp3+ Tregs, and HT29 cells. TGF-β production in the presence of the anti-TGF-β mAbs was decreased which indicated the anti-TGF-β mAbs worked (Fig. 2A). As shown in Figure 2C, the NK cell cytotoxicity in the presence of the anti-TGF-β mAbs was higher than that in the controls (18.02% vs 8.45%; P < .05). Consistently, IFN-γ level in the supernatant was increased in the presence of the anti-TGF-β mAbs (Fig. 2D). Moreover, an elevated percentage of CD107a+CD3- CD56+NK cells was observed in the presence of the anti-TGF-β mAbs compared with that in the control (12.8% vs 5.12%; P < .05; Fig. 3A). We also assessed the impact of CD4+CD25+ Foxp3+ Tregs on NKG2D on NK cells and found that compared with the control treatment, blocking TGF-β obviously enhanced the percentage of NKG2D+CD3-CD56+ NK cells (20.4% vs 15.1%; P < .05; Fig. 3B). And the similar results in K562 cells were shown in supplementary Figure 2 and 3.
Figure 2.
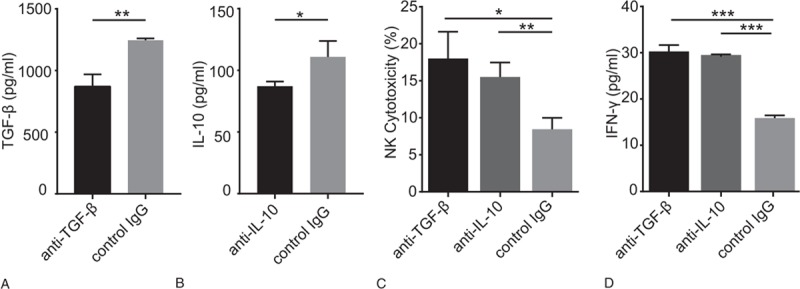
Blocking TGF-β and IL-10 enhances NK cytotoxicity. An anti-TGF-β (10 μg/ml) mAbs, anti-IL-10 (10 μg/ml) mAb or control IgG (10 μg/ml) was added to the NK-CD4+CD25+Foxp3+ Tregs- HT29 coculture (10:10:1 ratio). (A) Histogram represents the concentration of TGF-β after the neutralization; (B) Histogram represents the concentration of IL-10 after the neutralization; (C) Cytotoxic effects of NK cells following blockades with different antibodies in each coculture; (D) Representative histogram of IFN-γ production following blockades with different antibodies (∗P < .05, ∗∗P < .01 and ∗∗∗P < .001).
Figure 3.
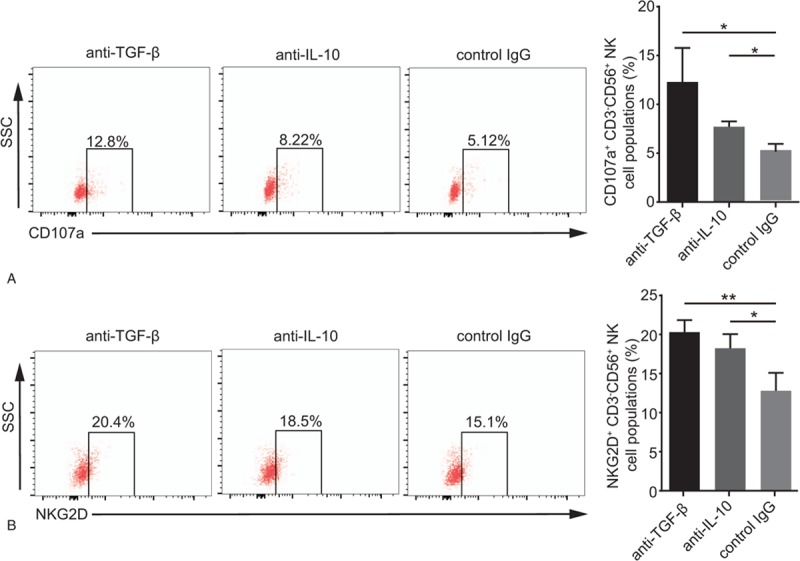
Neutralization of TGF-β and IL-10 elevates CD107a and NKG2D expression on NK cells. An anti-TGF-β (10 μg/ml) mAbs, anti-IL-10 (10 μg/ml) mAbs or control IgG (10 μg/ml) was added to the NK-CD4+CD25+Foxp3+ Tregs- HT29 coculture (10:10:1 ratio). (A) Histograms of CD107a expression on gated CD3-CD56+NK cells following blockades with different antibodies. (B) The percentage of NKG2D+CD3-CD56+NK cells following blockades with different antibodies is shown in graphs. Numerical data are shown as the mean ± SD of 3 independent experiments (∗P < .05 and ∗∗∗P < .001).
3.3. Blocking IL-10 enhances NKG2D expression on NK cells downregulated by CD4+CD25+Foxp3+ Tregs
We further investigated the function of IL-10 when CD4+CD25+Foxp3+ Tregs inhibited NKG2D expression on NK cells by adding an anti-IL-10 mAbs to the NK- CD4+CD25+Foxp3+ Tregs-HT29 coculture. IL-10 production in the presence of the anti-IL-10 mAbs was decreased which indicated the anti-IL-10 mAbs worked (Fig. 2B). As shown in Figure 2C, NK cytotoxicity was upregulated by the presence of the anti-IL-10 mAbs, and NK cells exhibited increased IFN-γ production (Fig. 2D). Moreover, an increased percentage of CD107a+ CD3-CD56+NK cells was also prominent in the presence of the anti-IL-10 mAbs compared with that in the presence of the control antibody (8.22% vs 5.96%; P < .05; Fig. 3A). Abundant NKG2D on NK cells was further confirmed by 3-color immunofluorescence staining (18.5% vs 10.5%; P < .05; Fig. 3B). And the similar results in K562 cells were shown in supplementary Figure 2 and 3.
3.4. CD4+CD25+Foxp3+Tregs inhibit NKG2D expression on NK cells via a cell contact-dependent mechanism
To further test the hypothesis that CD4+CD25+Foxp3+Treg function is mediated by a cell contact-dependent mechanism, we applied Transwell inserts to segregate CD4+CD25+Foxp3+ Tregs and NK cells. As expected, coincubation in Transwell inserts resulted in significantly elevated IFN-γ production, as shown in Figure 4A. Consistently, flow cytometric analysis revealed that CD107a expression on NK cells was downregulated in the absence of Transwell inserts (7.15% vs 5.37%; P < .05; Fig. 4B). We also investigated whether CD4+CD25+Foxp3+Tregs suppress NKG2D expression of NK cells via a cell contact-dependent mechanism. As shown in Figure 4C, approximately 17.9% of CD3-CD56+NK cells expressed NKG2D in the presence of Transwell inserts, compared to approximately 9.59% in the control group. And the similar results in K562 cells were shown in supplementary Figure 4.
Figure 4.
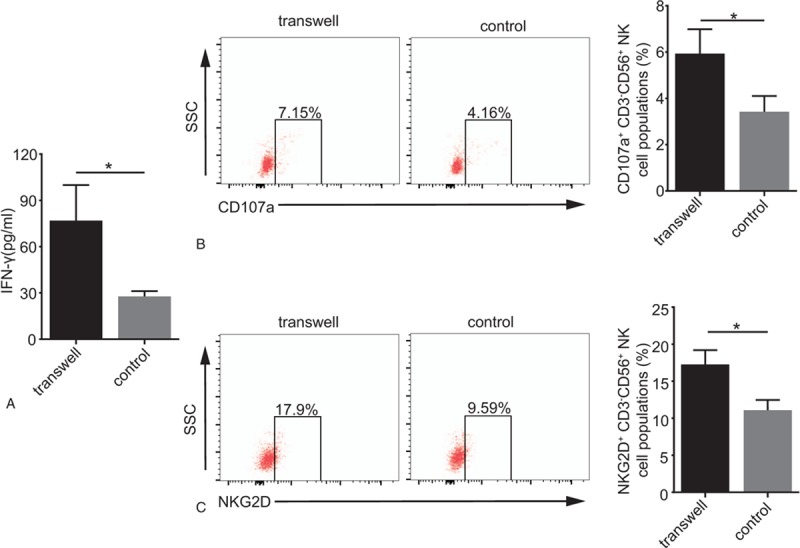
CD4+CD25+Foxp3+Tregs suppress NKG2D expression on NK cells via a cell contact-dependent mechanism. Transwell inserts contained CD4+CD25+Foxp3+Tregs in the upper chamber, NK and HT29 cells in the lower chamber; in the control, CD4+CD25+ Foxp3+Tregs, NK cells and HT29 cells were mixed together. The ratio of NK: CD4+CD25+Foxp3+Treg: HT29 was 10:10:1. (A) Histogram of IFN-γ production after incubation. (B) Representative graphs of CD107a expression on gated CD3-CD56+NK cells after incubation. (C) Representative graphs of NKG2D expression on NK cells after incubation. Numerical data are shown as the mean ± SD of 3 independent experiments (∗P < .05).
4. Discussion
In the present study, blocking NKG2D expression on NK cells resulted in decreased NK cell cytotoxicity, demonstrating that antitumor immunity induced by NK cells partly depends on the induction of NKG2D expression in peripheral blood. The results verified evidence from a recent study showing that melanoma cell lines were effectively lysed by activated NK cells that exhibited high NKG2D expression and were obtained from PBMCs in vitro.[16,17] Moreover, we herein showed that blocking TGF-β and IL-10, which are expressed by CD4+CD25+Foxp3+ Tregs, partly and overtly enhanced NK cell cytotoxicity, demonstrating that TGF-β and IL-10 play essential roles in the CD4+CD25+Foxp3+ Treg cell-mediated inhibition on NKG2D expression in NK cells. Interestingly, blocking TGF-β and IL-10 markedly enhanced NKG2D expression on NK cells, which is indirect compelling evidence that CD4+CD25+Foxp3+Tregs inhibit NK cell cytotoxicity via decreasing NKG2D expression. This finding is consistent with those of a series of other studies showing that Tregs suppress the antitumor activity of NK cells via downregulating NKG2D expression and that suppression on NK cells in the melanoma microenvironment is mediated by a unique subset of Tregs that produce IL-10 and TGF-β1.[6,12,14] In addition, our results showed that Transwell inserts reduced IFN-γ production and CD107a and NKG2D expression, indicating that CD4+CD25+Foxp3+ Tregs might suppress NKG2D expression on NK cells via a cell contact-dependent mechanism.
However, the current results are somewhat inconsistent with our previous results showing significantly decreased NKG2D expression but no significant correlation between the upregulation of Tregs and downregulation of NKG2D expression on NK cells in the peripheral blood of patients with colorectal cancer.[8,9] Our present study was conducted in vitro using purified CD4+CD25+Foxp3+ Tregs and NK cells from the peripheral blood of healthy individuals and HT29 cells or K562 cells, whereas the previous study was conducted using Spearman correlation analysis, which has certain limitations. CD4+CD25+Foxp3+Tregs are well known to produce anti-inflammatory cytokines, such as TGF-β and IL-10 [18], yet our results indicated that the alleviation caused by IL-10-blocking antibodies was lower than that caused by TGF-β-blocking antibodies in vitro. According to the literature, this phenomenon occurred because TGF-β primarily guides the induction of Foxp3 in conventional T cells, whereas IL-10 mainly directs the conversion of conventional T cells to Tregs.[19] Nonetheless, a contrary result is that Tregs inhibit NKG2D-mediated cytolysis largely via a TGF-β-dependent mechanism rather than via IL-10-dependent signaling in a melanoma mouse model.[6] The difference in experimental subjects and human tumor cell lines may have led to these diverse results. In addition, based on comparative analysis of the previous study, we propose that further studies are needed to explore the accurate mechanism of IL-10-mediated suppression by CD4+CD25+Foxp3+Tregs on NK cell NKG2D expression. In the presence of Transwell inserts, NK cell cytotoxicity and NKG2D expression were increased by approximately 1.5- to 2.7-fold compared with that in the absence of the inserts. CD4+CD25+Foxp3+Tregs display latent TGF-β1 on the cell surface, which would not contact NK cells in Transwell inserts and likely leads to non-suppression.[20] This finding was in accordance with the result that cell-to-cell contact plays a relatively important role in Treg cell suppression.[21]
Despite numerous attempts to develop therapeutic approaches for cancers based on the CD4+CD25+Foxp3+ Treg cell number or function, limited success has been achieved thus far, as the suppression mechanisms remain largely unknown.[16] In recent years, an increasing number of studies have been performed to explore the inhibitory abilities of the cytokines TGF and IL-10. A TGF-β receptor kinase inhibitor strongly activates NK cell cytotoxicity, though it does result in exposure to pathological levels of TGF-β in mice with colon cancer metastasis.[22] An inhibitory monoclonal antibody against the IL-10 receptor alleviates melanoma cell MICA expression such that it enhances the recognition of effective immune cells.[14] The availability and beneficial effects of anti-IL-10 or anti-TGF-β mAbs that play important roles in peripheral blood provide new avenues for developing bio-immune targeted cancer therapy. However, such improvements have not been sufficient to completely reverse suppression, which might be explained by the capacity of other NK cell receptors and cytokines to mediate NK cell cytotoxicity. To address this issue, advanced investigations need to be performed to seek efficient immunotherapy for cancers. Moreover, interaction between CD4+CD25+Foxp3+ Tregs and NKG2D may be a focal point for efforts to reverse this inhibition, potentially by blocking the delivery of signals between them.
In conclusion, our results demonstrate that CD4+CD25+Foxp3+ Tregs inhibit NKG2D expression on NK cells to further suppress NK cell cytotoxicity in peripheral blood, which occurs via a cell contact-dependent mechanism and increased TGF-β and IL-10 production. Furthermore, we emphasize the therapeutic value of anti-TGF-β and anti-IL-10 mAbs in enhancing NK cell immunity. These results lay an experimental foundation for further investigations of CD4+CD25+Foxp3+ Treg cell function and antitumor immunotherapy using NK cells.
Acknowledgments
We thank all the participants in this study for their involvement.
Author contributions
Investigation: Xu Geng, Ming Li, Peng Zhang.
Methodology: Bin Cui, Chao Lu, Xiaowen Liu, Bin Liu, Chunyan Ma.
Project administration: Yajuan Shen, Zhiming Lu.
xu geng orcid: 0000-0003-3201-0652.
Supplementary Material
Footnotes
Abbreviations: CD107a = cluster of differentiation 107a, ELISA = enzyme-linked immunosorbent assay, IFN-γ = interferon-γ, IL-10 = interleukin 10, NK cells = natural killer cells, NKG2D = natural killer group 2 member D, TGF-β = transforming growth factor beta, Treg cells = regulatory T cells.
This work was supported by the Shandong Natural Science Foundation [No. ZR2013HM057 and No. ZR2017MH088].
The authors who have participated in this study declare that they have no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Osada T, Hsu D, Hammond S, et al. Metastatic colorectal cancer cells from patients previously treated with chemotherapy are sensitive to T cell killing mediated by CEA/CD3-bispecifc T cell-engaging BiTE antibody. Br J Cancer 2010;102:124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hall BM. T cells: soldiers and spies-the surveillance and control of effector T cells by regulatory T cells. Clin J Am Soc Nephrol 2015;10:2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Martín-Antonio B, Suñe G, Perez-Amill L, et al. Natural killer cells: angels and devils for immunotherapy. Int J Mol Sci 2017;18:1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Molfetta R, Quatrini L, Santoni A, et al. Regulation of NKG2D-dependent NK cell functions: the yin and the yang of receptor endocytosis. Int J Mol Sci 2017;18:1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mirjačić KMM, Vuletić AM, Lj NB, et al. Attenuated in vitro effects of IFN-α, IL-2 and IL-12 on functional and receptor characteristics of peripheral blood lymphocytes in metastatic melanoma patients. Cytokine 2017;96:30–40. [DOI] [PubMed] [Google Scholar]
- [6].Smyth MJ, Teng MW, Swann J, et al. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol 2006;176:1582–7. [DOI] [PubMed] [Google Scholar]
- [7].Bergmann C, Wild CA, Narwan M, et al. Human tumor-induced and naturally occurring Tregs differentially affect NK cells activated by either IL-2 or target cells. Eur J Immunol 2011;41:3564–73. [DOI] [PubMed] [Google Scholar]
- [8].Shen Y, Wang Q, Qi Y, et al. Peripheral Foxp3+ regulatory T cells and natural killer group 2, member D expression levels in natural killer cells of patients with colorectal cancer. Mol Med Rep 2014;10:977–82. [DOI] [PubMed] [Google Scholar]
- [9].Shen Y, Lu C, Tian W, et al. Possible association of decreased NKG2D expression levels and suppression of the activity of natural killer cells in patients with colorectal cancer. Int J Oncol 2012;40:1285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhao H, Liao X, Kang Y. Tregs: where we are and what comes next? Front Immunol 2017;8:1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen W, Konkel JE. Development of thymic Foxp3 (+) regulatory T cells: TGF- βmatters. Eur J Immunol 2015;45:958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chang WC, Li CH, Chu LH, et al. Regulatory T cells suppress natural killer cell immunity in patients with human cervical carcinoma. Int J Gynecol Cancer 2016;26:156–62. [DOI] [PubMed] [Google Scholar]
- [13].Schmetterer KG, Pickl WF. The IL-10/STAT3 axis: Contributions to immune tolerance by thymus and peripherally derived regulatory T cells. Eur J Immunol 2017;47(8.):1256–65. [DOI] [PubMed] [Google Scholar]
- [14].Serrano AE, Menares-Castillo E, Garrido-Tapia M, et al. Interleukin 10 decreases MICA expression on melanoma cell surface. Immunol Cell Biol 2011;89:447–57. [DOI] [PubMed] [Google Scholar]
- [15].Trzonkowski P, Szmit E, Myśliwska J, et al. CD4+CD25+T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol 2004;112:258–67. [DOI] [PubMed] [Google Scholar]
- [16].Parkhurst MR, Riley JP, Dudley ME, et al. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res 2011;17:6287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Huergo-Zapico L, Acebes-Huerta A, Gonzalez-Rodriguez AP. Expansion of NK cells and reduction of NKG2D expression in chronic lymphocytic leukemia. Correlation with progressive disease. PLoS One 2014;9:e108326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen X, Du Y, Lin XQ, et al. CD4+CD25+ regulatory T cells in tumor immunity. Int Immunopharmacol 2016;34:244–9. [DOI] [PubMed] [Google Scholar]
- [19].Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010;10:490. [DOI] [PubMed] [Google Scholar]
- [20].Stockis J, Dedobbeleer O, Lucas S. Role of GARP in the activation of latent TGF- β1. Mol Biosyst 2017;13:1925–35. [DOI] [PubMed] [Google Scholar]
- [21].Strauss L, Bergmann C, Szczepanski M, et al. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-(1 mediates suppression in the tumor microenvironment. Clin Cancer Res 2007;13:4345–54. [DOI] [PubMed] [Google Scholar]
- [22].Otegbeye F, Ojo E, Moreton S, et al. Inhibiting TGF-beta signaling preserves the function of highly activated, in vitro expanded natural killer cells in AML and colon cancer models. PloS One 2018;13:e0191358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


