Abstract
This study retrospectively evaluated the effect of lutein supplement (LS) on patients with non-proliferative diabetic retinopathy (NPDR).
A total of 72 patients with NPDR were included in this study. All patients received Zeaxanthin during the study period. In addition, 36 patients also received LS and were assigned to the treatment group, while the other 36 patients did not receive LS and were assigned to the control group. All patients were treated for a total of 4 months. The endpoints included visual acuity (VA), contrast sensitivity (CS), and glare sensitivity (GS). In addition, any adverse events were also assessed. All endpoints were measured before and after 4-month treatment.
Before treatment, there were no significant differences in VA (P = .75), CS (P = .71), and GS (P = .73) between two groups. After 4-month treatment, there were still no significant differences in all endpoints of VA (P = .66), CS (P = .58), and GS (P = .61) between two groups. No adverse events were recorded in either group.
The results of this retrospective study showed that LS may not benefit for patients with NPDR after 4-month treatment. More high quality randomized controlled trials should still be needed to warrant the results of this study.
Keywords: effect, lutein supplement, non-proliferative diabetic retinopathy
1. Introduction
Diabetic retinopathy (DR) is a chronic metabolic retinal damage condition,[1–2] which is characterized by hyperglycemia due to the complications of diabetic mellitus (DM).[3–5] This condition is also a frequent and leading cause that accounts for the adult vision impairment and blindness, especially among the population between 20 and 60 years of age.[6] Previous studies have reported that DR accounted for 4.8% (1.8 million) cases for the whole 37 million blind people around the world in 2002.[6] Other studies also reported that the global prevalence of DR among different ethnic groups ranges widely from 20.8% in Asians to 46.7% in Caucasians.[7] Although a variety of managements are reported to treat this disorder, no permanent cure is still available.[8–15] Thus, effective therapies are needed to help in preventing and treating patients with DR to delay and to prevent its progression for visual function impairment.
Several previous studies have found that oxidative stress is involved in the pathogenesis of DM and its complications, especially for cataract and non-proliferative diabetic retinopathy (NPDR).[16–19] Zeaxanthin and lutein supplement (LS) are both reported to contain rich antioxidant properties.[20–23] Furthermore, several previous studies also reported to utilize LS for prevention and treatment of NPDR, and also achieved satisfied outcomes.[24–25] However, present evidence is still insufficient and more evidence is still needed to support this intervention. Thus, it is still necessary to investigate the effect of LS for patients with NPDR. In this retrospective study, we assessed the effect of LS for patients with NPDR.
2. Methods and patients
2.1. Ethic approval
This retrospective study was approved by the Ethical Committee of First Affiliated Hospital of Jiamusi University. The written informed consent was waived because this study just analyzed all endpoints data from completed medical records.
2.2. Design
This retrospective study was conducted from January 2016 to December 2017 at First Affiliated Hospital of Jiamusi University. We included 72 patients with NPDR in this study. Of these, 36 patients received Zeaxanthin and LS and were assigned to the treatment group, while the other 36 patients received Zeaxanthin alone and were assigned to the control group according to the different treatments they received. Patients in both groups were treated for a total of 4 months. All efficacy endpoints were measured before and after 4-month treatment in this study. In this study, all researchers, patients, and outcome assessors were not blinded, except the data analyst.
2.3. Inclusion and exclusion criteria
This study included patients aged from 40 to 75 years old. All of them received complete ophthalmologic examination and were clinically diagnosed of type 2 DM with NPDR in mild or moderate phrase. However, patients were excluded if they had proliferated DR or other conditions except the NPDR. These conditions included type 1 DM, macular degeneration, diabetic macular edema, retinal detachment, glaucoma; or history of eye trauma, pregnancy, or breast feeding; or received other treatments for NPDP 1 month before this study; or receiving other therapies for NPDP during the period of study.
2.4. Treatment schedule
All 72 participants in both groups received Zeaxanthin 0.5 mg daily for a total of 4 months. In addition, 36 patients in the treatment group also received LS 6 mg daily for a total of 4 months, while the other 36 patients in the control group did not receive LS.
2.5. Endpoints measurement
The efficacy endpoints consisted of visual acuity (VA),[26] contrast sensitivity (CS),[27] and glare sensitivity (GS).[28] All these endpoints were measured before and after 4-month treatment. Additionally, any expected and unexpected adverse events were also assessed in this study.
2.6. Statistical analysis
All endpoints data were analyzed by a blinded statistician using SPSS Statistics 15.0 (IBM Corp., Armonk, NY, USA). Mann–Whitney test or Student's t-test were used to analyze the continuous endpoints data. Fisher's exact test was used to analyze the categorical endpoints data. The value of P < .05 is considered as having statistical significance.
3. Results
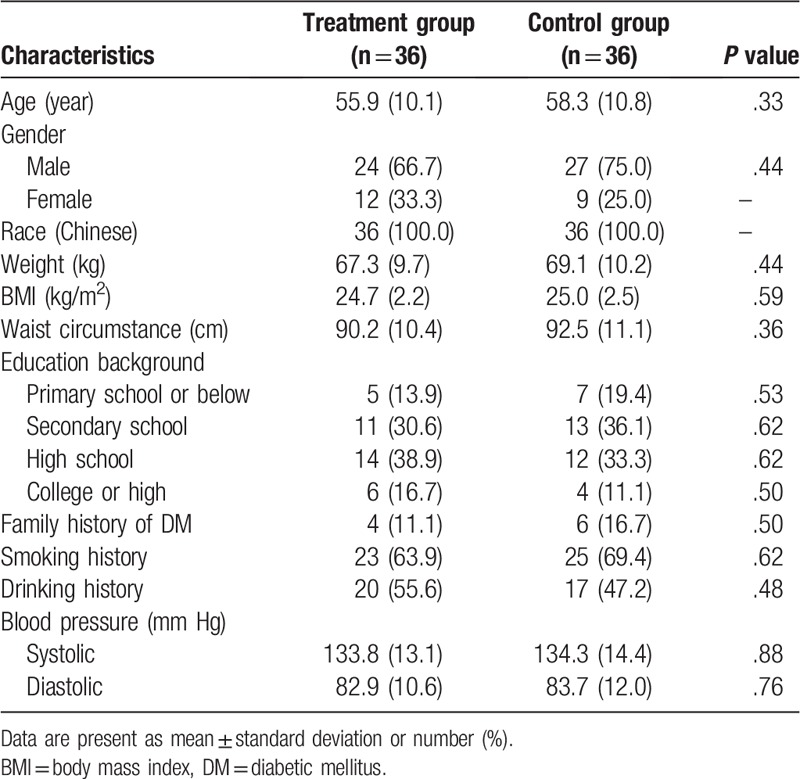
The comparison of all characteristics between two groups before this study is showed in Table 1. There were not significant differences regarding all characteristic values between two groups prior to the treatment in this study (Table 1).
Table 1.
Characteristics comparison between two groups before the study.
Before the treatment, no significant differences in all effect endpoints of VA (P = .75, Table 2), CS (P = .71, Table 3), and GS (P = .73, Table 4) were found between two groups.
Table 2.
Comparison of visual acuity between two groups.
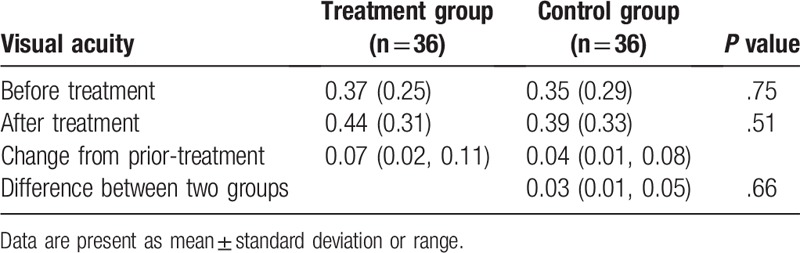
Table 3.
Comparison of contrast sensitivity between two groups.
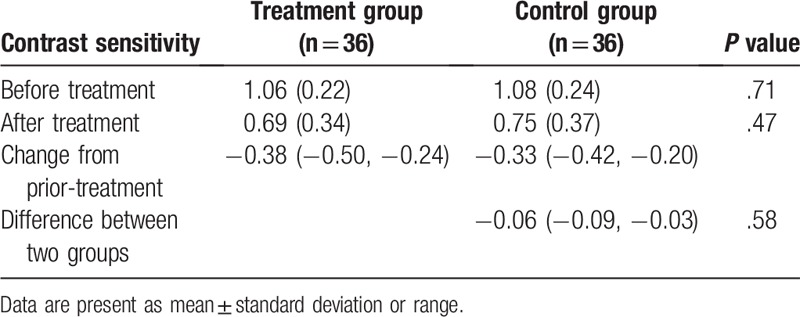
Table 4.
Comparison of glare sensitivity between two groups.
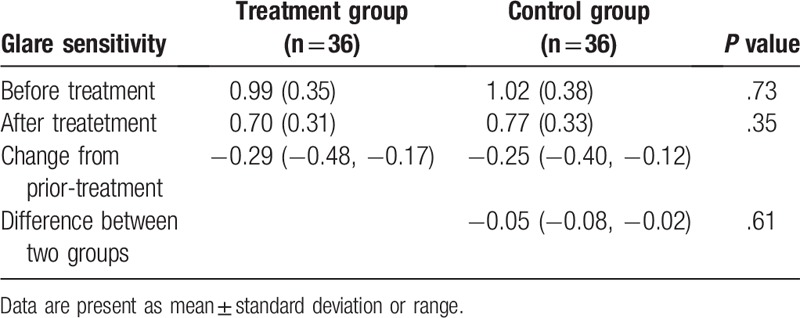
After 4-month treatment, there were still no significant differences in VA (P = .66, Table 2), CS (P = .58, Table 3), and GS (P = .61, Table 4) between two groups.
After 4-month treatment, no adverse events were reported in either group in this study. No death related to the treatments was reported in either group.
4. Discussion
Previous studies have reported that NPDR are highly associated with oxidative stress.[16–19] Meanwhile, LS is found to contain rich antioxidant properties.[20–23] In addition, two previous studies utilized it for preventing the mild or moderate NPDR, and also exerted a promising effect.[24–25] The results of the first study found that LS resulted in potential improvements in CS at low spatial frequency.[24] However, it only included 30 patients in this study. The other study compared the serum concentrations of Lutein and Zeaxanthin (L/Z) between patients with NPDR and normal participant. In addition, it also investigated the effect of L/Z supplementation on visual function in patients with NPDR.[25] Its results showed that L/Z can significantly lower the serum L/Z concentrations in NPDR patients, and also can enhance VA, CS, and macular edema.[25]
The present study compared the effect of Zeaxanthin and LS with Zeaxanthin alone for the prevention of patients with NPDR. Its results are not consistent with the previous studies.[24–25] The results of the present study demonstrated that after 4-month treatment, patients in the treatment group did not exert better effect endpoints in VA (P = .66), CS (P = .58), and GS (P = .61), than patients in the control group. The results indicated that LS may not benefit for patients with NPDR.
This study has several limitations. Firstly, although the sample size is much bigger than the previous study,[24] more large scale studies are still needed to warrant the results of this study. Second, this study did not apply procedures of randomization, masked to the patients and researchers, because all the data were collected from the completed medical records. Thus, it may cause higher risk of bias for patient selection. In addition, this study also did not apply blinding procedure to the outcome assessors, which may also bring higher risk of detection bias. Therefore, future studies should avoid those limitations.
5. Conclusion
The results of this study did not found that LS may be effective for patients with NPDR. More high quality studies with large scales are still needed to warrant the results of this study.
Author contributions
Conceptualization: Yong-bo Ren, Yan-xiu Qi, Qi Sun.
Data curation: Yong-bo Ren, Yan-xiu Qi, Xing-jie Su, He-qun Luan, Qi Sun.
Formal analysis: Yong-bo Ren.
Investigation: Yan-xiu Qi.
Methodology: Yong-bo Ren, Qi Sun.
Project administration: Yan-xiu Qi.
Resources: Yong-bo Ren, Yan-xiu Qi, Xing-jie Su, He-qun Luan, Qi Sun.
Software: Yong-bo Ren.
Supervision: Yan-xiu Qi, Xing-jie Su.
Validation: Yong-bo Ren, Xing-jie Su, He-qun Luan.
Visualization: Yong-bo Ren, Yan-xiu Qi, Xing-jie Su, He-qun Luan.
Writing – original draft: Yong-bo Ren, Yan-xiu Qi, Xing-jie Su, He-qun Luan, Qi Sun.
Writing – review & editing: Yong-bo Ren, Yan-xiu Qi, Xing-jie Su, He-qun Luan, Qi Sun.
Footnotes
Abbreviations: CS = contrast sensitivity, DM = diabetic mellitus, DR = Diabetic retinopathy, GS = glare sensitivity, L/Z = Lutein and Zeaxanthin, LS = lutein supplement, NPDR = non-proliferative diabetic retinopathy, VA = visual acuity.
Y-bR and Y-xQ contributed equally to this work.
The authors report no conflicts of interest.
References
- [1].Al-Kharashi AS. Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J Ophthalmol 2018;32:318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Song P, Yu J, Chan KY, et al. Prevalence, risk factors and burden of diabetic retinopathy in China: a systematic review and meta-analysis. J Glob Health 2018;8:010803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shah AR, Gardner TW. Diabetic retinopathy: research to clinical practice. Clin Diabetes Endocrinol 2017;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fenner BJ, Wong RLM, Lam WC, et al. Advances in retinal imaging and applications in diabetic retinopathy screening: a review. Ophthalmol Ther 2018;7:333–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017;128:40–50. [DOI] [PubMed] [Google Scholar]
- [6].Al-Turki YA. Blood sugar control, ophthalmology referral and creatinine level among adult diabetic patients in primary health care, Riyadh, Saudi Arabia. Saudi Med J 2002;23:1332–4. [PubMed] [Google Scholar]
- [7].Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35:556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang HW, Zhang H, Grant SJ, et al. Single herbal medicine for diabetic retinopathy. Cochrane Database Syst Rev 2018;12:CD007939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Julius A, Hopper W. A non-invasive, multi-target approach to treat diabetic retinopathy. Biomed Pharmacother 2019;109:708–15. [DOI] [PubMed] [Google Scholar]
- [10].Khadamy J, Abri Aghdam K, Falavarjani KG. An update on optical coherence tomography angiography in diabetic retinopathy. J Ophthalmic Vis Res 2018;13:487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stewart S, Lois N. Fenofibrate for diabetic retinopathy. Asia Pac J Ophthalmol (Phila) 2018;7:422–6. [DOI] [PubMed] [Google Scholar]
- [12].Rani PK, Nangia V, Murthy KR, et al. Community care for diabetic retinopathy and glaucoma in India: a panel discussion. Indian J Ophthalmol 2018;66:916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Moutray T, Evans JR, Lois N, et al. Different lasers and techniques for proliferative diabetic retinopathy. Cochrane Database Syst Rev 2018;3:CD012314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kowluru RA, Mishra M. Therapeutic targets for altering mitochondrial dysfunction associated with diabetic retinopathy. Expert Opin Ther Targets 2018;22:233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fiori A, Terlizzi V, Kremer H, et al. Mesenchymal stromal/stem cells as potential therapy in diabetic retinopathy. Immunobiology 2018;223:729–43. [DOI] [PubMed] [Google Scholar]
- [16].Rodríguez-Carrizalez AD, Castellanos-González JA, Martínez-Romero EC, et al. The effect of ubiquinone and combined antioxidant therapy on oxidative stress markers in non-proliferative diabetic retinopathy: a phase IIa, randomized, double-blind, and placebo-controlled study. Redox Rep 2016;21:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rodríguez-Carrizalez AD, Castellanos-González JA, Martínez-Romero EC, et al. The antioxidant effect of ubiquinone and combined therapy on mitochondrial function in blood cells in non-proliferative diabetic retinopathy: a randomized, double-blind, phase IIa, placebo-controlled study. Redox Rep 2016;21:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Alekseev IB, Vorob’eva IV, Mikhaleva LG, et al. Results of antioxidant therapy for non-proliferative diabetic retinopathy in patients with type 2 diabetes mellitus. Vestn Oftalmol 2013;129:66–71. [PubMed] [Google Scholar]
- [19].Rodríguez-Carrizalez AD, Castellanos-González JA, Martínez-Romero EC, et al. Oxidants, antioxidants and mitochondrial function in non-proliferative diabetic retinopathy. J Diabetes 2014;6:167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Firdous AP, Kuttan G, Kuttan R. Anti-inflammatory potential of carotenoid meso-zeaxanthin and its mode of action. Pharm Biol 2015;53:961–7. [DOI] [PubMed] [Google Scholar]
- [21].Bernstein PS, Li B, Vachali PP, et al. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res 2016;50:34–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Manikandan R, Thiagarajan R, Goutham G, et al. Zeaxanthin and ocular health, from bench to bedside. Fitoterapia 2016;109:58–66. [DOI] [PubMed] [Google Scholar]
- [23].Mares J. Lutein and Zeaxanthin isomers in eye health and disease. Annu Rev Nutr 2016;36:571–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang PC, Wu CR, Wang ZL, et al. Effect of lutein supplementation on visual function in nonproliferative diabetic retinopathy. Asia Pac J Clin Nutr 2017;26:406–11. [DOI] [PubMed] [Google Scholar]
- [25].Neelam K, Goenadi CJ, Lun K, et al. Putative protective role of lutein and zeaxanthin in diabetic retinopathy. Br J Ophthalmol 2017;101:551–8. [DOI] [PubMed] [Google Scholar]
- [26].Kaido M. Functional visual acuity. Invest Ophthalmol Vis Sci 2018;59:DES29–35. [DOI] [PubMed] [Google Scholar]
- [27].Maia OO, Jr, Takahashi WY, Sampaio MW, et al. Contrast sensitivity in diabetic retinopathy treated with argon laser panphotocoagulation. Arq Bras Oftalmol 2007;70:763–6. [DOI] [PubMed] [Google Scholar]
- [28].Babizhayev MA, Deyev AI, Yermakova VN, et al. Image analysis and glare sensitivity in human age-related cataracts. Clin Exp Optom 2003;86:157–72. [DOI] [PubMed] [Google Scholar]


