Abstract
Background:
Prognostic nutritional index (PNI) is an easily obtained index inflecting both one's nutritional and inflammatory status. Its clinical role in esophageal squamous cell carcinoma (ESCC) remains still in debate. The aim of this meta-analysis was to assess the prognostic value and the clinical-pathological features of pre-treatment PNI in ESCC patients.
Methods:
A comprehensive search of online databases (PubMed, Embase, Web of Science) was performed. Studies explored the relationship between pre-treatment PNI and long-term survival of ESCC patients were regarded eligible for this meta-analysis. Outcomes were extracted and synthesized. Hazard ratio (HR) and relative ratio (RR) with 95% confidence interval (CI) were used to evaluate the prognostic value of PNI on long-term survival and association with clinical-pathological features, respectively. The heterogeneity levels and publication bias between studies were also estimated.
Results:
Nine observational studies involving 2276 patients were considered eligible for this meta-analysis. The pooled results showed that low PNI score was significantly correlated with poorer overall survival (OS) of esophageal cancer (pooled HR = 1.418 95%CI: 1.200–1.676, P = .000), poorer recurrence free survival (HR = 1.880 95%CI: 1.207–2.929, P = .005) but not cancer specific survival (CSS) (HR = 1.948 95%CI: 0.544–6.977, P = .306). The PNI value was not related with patient age, sex, depth of tumor invasion, nodular metastasis, and differential grade but the TNM stage (III/IV vs 0/I/II, RR = 1.276, 95% CI 1.146–1.420).
Conclusions:
Low pre-treatment PNI was significantly related with OS and recurrence free survival but not CSS for ESCC. PNI was a reliable prognostic factor of ESCC, and higher stage ESCC have higher incidence of low PNI.
Keywords: esophageal squamous cell carcinoma, prognosis, prognostic nutritional index
1. Introduction
Esophageal cancer is a main cause of cancer-related death worldwide. About four hundred seventy-seven thousand patients were newly diagnosed of esophageal cancer and three hundred seventy-five thousand patients died of esophageal cancer each year in China.[1] Even though multimodal treatments have gained great advances in treating esophagus cancer, the long-term survivals remain relative low. Esophageal squamous cell carcinoma (ESCC) accounts for a majority of patients in esophageal cancer in Eastern countries. We wonder whether certain traditional biomarkers could be useful for predicting prognoses in ESCC before treatment begins.
Inflammatory response plays important roles in carcinogenesis and progression.[2] The prognosis roles of inflammatory indices or scores calculated from peripheral blood measurements such as C reactive protein, Glasgow prognostic score, neutrophil-to-lymphocyte ratio, and prognostic nutritional index (PNI) have been further studied in various kinds of solid malignancies.[3] PNI was first reported by Buzby and colleagues,[4] regarded as a simply obtained nutritional and immunological variable calculated with serum albumin level and peripheral lymphocyte count. Then PNI was simplified by Onodera et al, who confirmed that PNI score was correlated with prognosis after gastrointestinal surgery.[5] After that, PNI was further studied for its prognostic role in various types of malignancies.
In recent years, the prognostic role of PNI in esophageal cancer has been investigated in several studies. However, whether PNI could predict clinical outcome of patients with ESCC is still in debate. Therefore, we reviewed the published studies and conducted this meta-analysis to evaluate the prognostic role of PNI in ESCC.
2. Materials and methods
This meta-analysis was performed according to the statement of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).[6] An additional checklist is available as supplementary material PRISMA checklist. This study has been approved by the Sichuan University, West China Hospital's clinical trials and biomedical ethics committee.
2.1. Search strategy
A comprehensive search of PubMed, Embase (via OvidSP), and Web of Science was implemented by two independent researchers for studies that investigated the prognosis with PNI in ESCC published before April 30, 2018. The search terms were:PNI and esophageal cancer. The titles and abstracts of each identified studies were browsed to screen relevant publications. Full texts of all potentially eligible studies were retrieved, and their references were also browsed to find other relevant studies. Disagreements between the investigators were settled by team discussion.
2.2. Inclusion and exclusion criteria
Studies eligible for this meta-analysis should meet the following criteria:
-
1.
blood tests used to calculate PNI were performed before any treatment;
-
2.
evaluated the long-term prognostic value (overall survival [OS], cancer specific survival [CSS], recurrence-free survival [RFS]) of PNI in ESCC;
-
3.
reported a cut-off value for PNI score and patients was divided into high and low PNI grade groups;
-
4.
literatures published with full text in English.
Exclusion criteria including:
-
1.
studies published as reviews, conference abstracts, comments, case or serious reports;
-
2.
studies with insufficient survival data for which hazard ratio (HR) and 95% confidence interval (95% CI) could be determined;
-
3.
studies performed based on the same cohort patients or data duplicated.
2.3. Data extraction and quality assessment
Useful information from eligible studies was extracted by two investigators independently (PFL and KZ). Information including: first author, publication year, country, number of patients, follow-up period; tumor stage, TNM edition, treatment strategy, cut-off value, source of HR, survival analysis mode was extracted. When the HR and with 95% CI was available from the original articles, it would be used directly. Otherwise we extracted data from the Kaplan–Meier survival curves. Results based on multivariate analysis were preferred when both univariate and multivariate analysis data were reported. PNI was calculated on the basis of laboratory data using the reported formula: 10 ∗ albumin value (g/dL) + 0.005 ∗ total lymphocyte count in the peripheral blood. The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of each individual study.[7] The NOS consists of three parameters of quality: selection (three terms), comparability (one term), and outcome assessment (three terms). The NOS score ranged from 0 to 9, and studies with NOS scores ≥ 6 were considered of high qualities.
2.4. Statistical analysis
HR with 95% CI was used to estimate the prognostic value of PNI on the long-term survival of ESCC.[8] Cochrane's Q test (Chi-squared test) and I2 metric were used to calculate the statistical heterogeneity of the pooled HR with 95% CI.[9] For I2 statistics, I2 < 25% indicates a low heterogeneity, I2 = 25% to 50% indicates a moderate heterogeneity and I2 > 50% indicates a significant heterogeneity. The fixed-effect model was adopted when no statistically significant heterogeneity was observed (P ≥ .1 or I2 < 50%), otherwise the random-effect model would be preferred. When HR > 1, a poor prognosis for esophageal cancer patients was indicated by low PNI level. Sensitivity analysis was performed to omit each individual study sequentially to explore the influence of each study on the pooled estimate for OS. Publication bias was evaluated by funnel plot (qualitative) and Egger's test (quantitative). When publication bias existed, the nonparametric trim and fill method would be applied to re-estimate a corrected effect size after adjustment for publication bias.[10] All P values were based on two-sided test, and a P value <.05 was considered to be statistically significant. STATA 12.1 software (Stata Corporation, College Station, TX) was used to conduct all statistical analysis.
3. Results
3.1. Characteristics of eligible studies
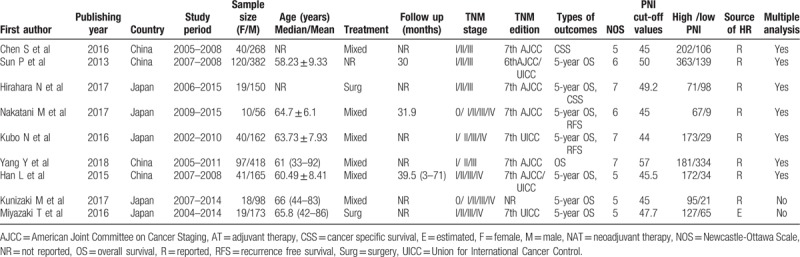
A total of 407 records were identified through initial database searching. 119 duplicated records were excluded. After browsing the titles and abstracts, 30 studies were screened out to read the full texts. Finally, 9 eligible studies, all from Asian countries,[11–19] including 2276 patients were selected for quantitative analysis (Fig. 1). Detailed characteristics of these studies were summarized in Table 1. Their sample sizes ranged from 66 to 515, of which eight provided available HR with 95% CI directly, and it was calculated from Kaplan–Meier survival curve in the remaining one. All of the studies were considered to have moderate or high qualities (NOS: 5–7). Details of all included studies were depicted in Table 1.
Figure 1.
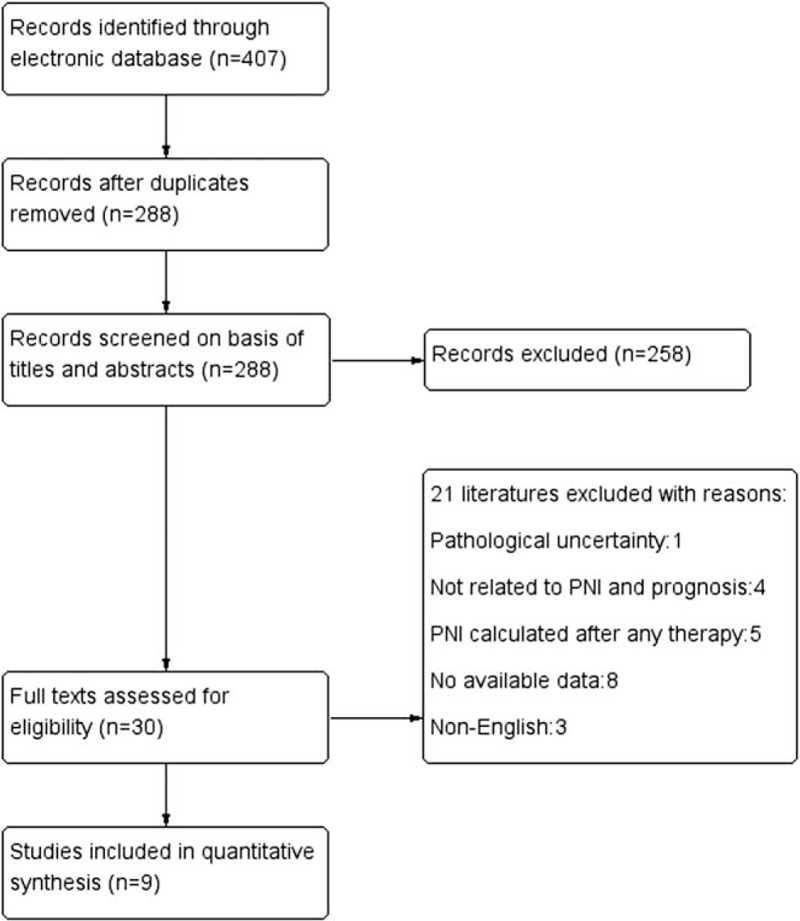
Flow diagram of literature review.
Table 1.
Basic characteristics of all studies included.
3.2. Prognostic impact of PNI on OS
Eight enrolled studies[12–19] provided data about PNI in OS. Pooled HR from eligible studies was 1.418 (95%CI 1.200–1.676, P = .000), which indicated that the low-PNI group had adverse OS, with moderate heterogeneity (I2 = 32.2%, P = .171) (Fig. 2).
Figure 2.
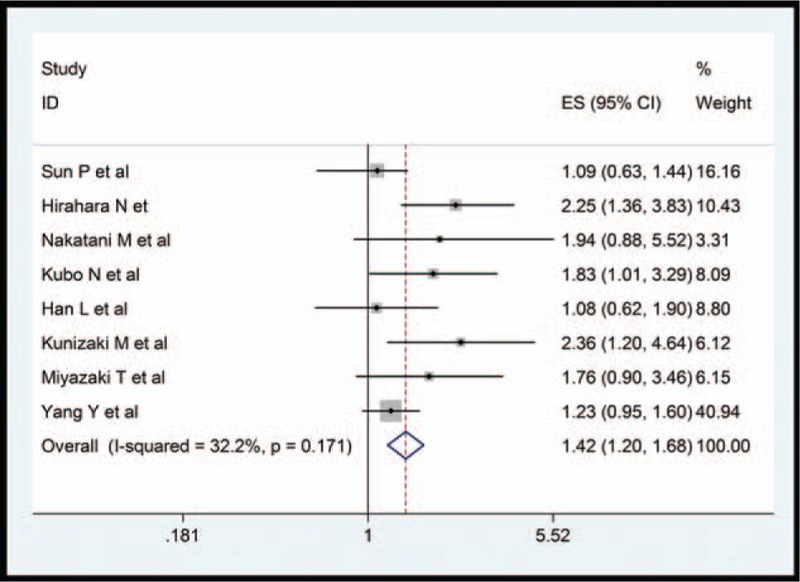
Forest plot for the association between PNI and overall survival.
Subgroup analysis was used to explore the potential source of heterogeneity for OS among several related clinical features, demonstrating that low-PNI score predicted worse OS in patient with ESCC regardless of the sample size (≥200 or <200), cut-off value (>45 and ≤45), publication country (China or Japan). On the other hand, we observed no heterogeneity in the subgroup of sample size and country, which could partly explain the observed heterogeneity (Table 2).
Table 2.
Results of subgroup analysis for relationship between PNI and overall survival.
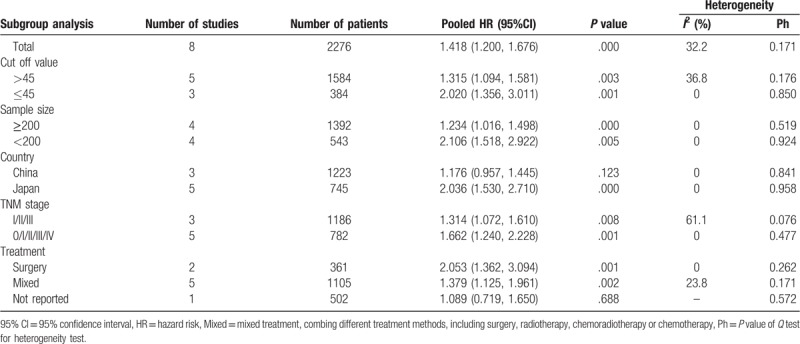
Subgroups stratified by TNM stage suggested that a low PNI predicted negative OS in ESCC without stage IV (HR = 1.314, 95% CI 1.072–1.610, P = .008, I2 = 61.1%), or at all stages (HR = 1.662, 95% CI 1.240–2.228, P = .001, I2 = 0%). In the subgroups divided by treatment strategy, the same outcome was also observed in groups treated by surgery alone (HR = 1.662, 95% CI 1.240–2.228, P = .001, I2 = 0%), or with mixed therapies (HR = 1.379, 95% CI 1.125–1.961, P = .002, I2 = 23.8%). Details of subgroup analysis were presented in Table 2.
3.3. Sensitivity analysis and publication bias
Sensitivity analysis was performed to estimate the influence by omitting one study at a time and calculate the combined HR. The result indicated that no study had significant influence on the observed effect size for OS (Fig. 3).
Figure 3.
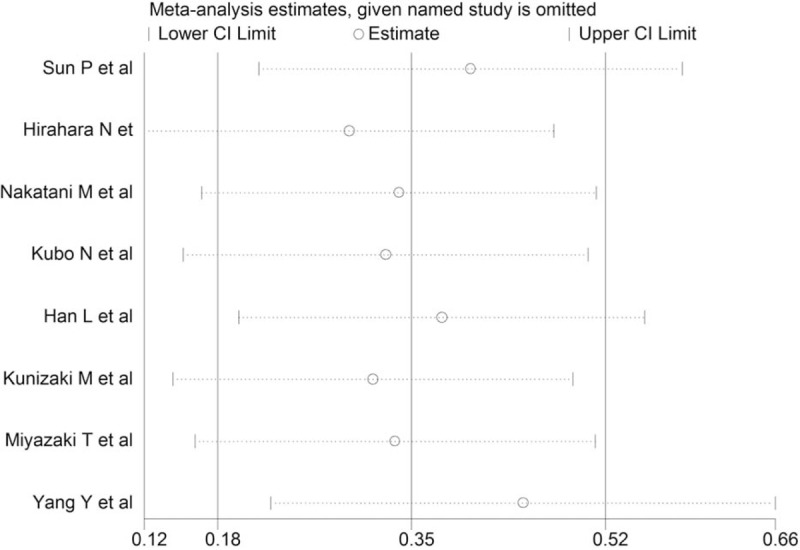
Sensitivity analysis for the relationship between PNI and overall survival.
Funnel plot and Egger's tests with pseudo 95% CI were performed to estimate the publication bias of the eight studies regarding OS. There was apparent asymmetry with the funnel plot but no significant publication bias observed with the Egger's test (P = .095). To validate the result, the nonparametric trim and fill method was further conducted to evaluate the impact of this bias and the pooled HR for OS. The results indicated no publication bias was observed in the trim and fill method after three potential studies were filled and the pooled HR remained significant (Fig. 4).
Figure 4.

(A) Funnel plots of publication bias on the correlation between PNI and overall survival; (B) Egger's tests of publication bias; (C) Trim and fill method to test the publication bias.
3.4. Prognostic impact of PNI on CSS
Two studies[11,13] provided the CSS data about PNI in ESCC. The pooled HR was 1.352 (95%CI 0.991–1.845) which showed that low-PNI did not show any prognostic significance on CSS (P = .057) with significant heterogeneity (I2 = 90.4%, P = .001) (Fig. 5A).
Figure 5.

Forest plot for the association between PNI and (A) cancer specific survival; (B) progression free survival.
3.5. Prognostic impact of PNI on RFS
Two studies[14,15] provided the RFS data about PNI in esophageal cancer. The pooled HR was 1.880 (95% CI 1.207–2.929). The result indicated that low-PNI score was statistically significant correlated with early relapse of ESCC (P = .005) with no heterogeneity (I2 = 0.0%, P = .938) (Fig. 5B).
3.6. Relationship between PNI and clinic-pathological features
3.6.1. PNI and age
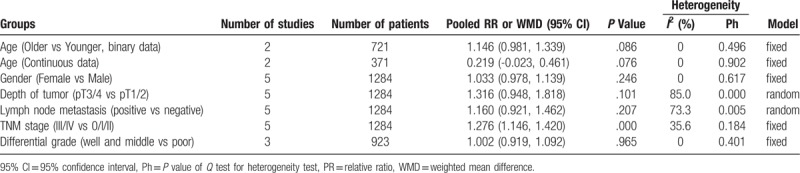
Four studies assessed the association between PNI and age. Two studies provided continuous data, and another two provided binary data. The pooled results from neither model indicated that the age have association with PNI (weighted mean difference = 0.210, 95% CI −0.023 to 0.461, P = .076), (RR = 1.146, 95% CI 0.981–1.339, P = .086) (Table 3).
Table 3.
Result of meta-analysis for relationship between PNI level and clinicopathologic features in ESCC.
3.6.2. PNI and gender
Five studies assessed the association between PNI and gender. The pooled result showed gender have no association with PNI (RR = 1.033, 95% CI 0.978–1.139, P = .246) (Table 3).
3.6.3. PNI and tumor stage and differential grade
Five studies provided data on PNI and tumor stage. No significance was discovered between in pT3 and pT4 groups compared to the pT1 and pT2 groups (RR = 1.316, 95% CI 0.948–1.818, P = .101, I2 = 85.0%), in the lymph node positive groups compared to the lymph node negative groups (RR = 1.160, 95% CI 0.921–1.462, P = .207, I2 = 73.3%), and in the well and moderate differential grade group compared with the poor differential grade group (RR = 1.002, 95% CI 0.919–1.092, P = .965, I2 = 0%). However, incidence of low PNI was significantly higher in the stage III and IV group than in the earlier stage group (RR = 1.276, 95% CI 1.146–1.420, P = .000, I2 = 35.6%) (Table 3).
4. Discussion
The PNI can be easily obtained from peripheral blood test. It is deemed to reflect nutritional status as well as inflammatory condition of the cancer patients. Since reported in 1980, several studies have investigated the prognostic role of PNI in ESCC, however, no consensus reached. To the best of our knowledge, our meta-analysis is the first to pool the results of the prognostic role of PNI in ESCC. We come to the results that low-PNI score was significantly associated with poorer OS and RFS, but not CSS, and higher stage ESCC patients tended to have higher rate of low PNI.
In the subgroup analysis, the same outcomes were observed regardless of the cut-off value, sample size, publication country, whether including stage IV patients and the treatment strategies. We observed moderate heterogeneity which might come from sample size or publication country. In the subgroup analysis stratified by TNM stage, we were only able to classify the studies to two groups of whether including stage IV or not. For that insufficient data were available to stratify the studies to more detailed groups even though that cancer stage plays significant roles in prognosis. Despite this, we compared the PNI in stage III/IV compared to stage 0/I/II (Table 3), which showed that the PNI was relevant to TNM stages. For ESCC staged at II–III, therapy strategy changed in different medical centers, and PNI could be changed during the neoadjuvant therapy period,[14] so we only included studies in which blood samples were collected before treatments began to exclude the clinical heterogeneity. Considering these, we believe that our result was robust and credible.
Of the included eight studies that investigated the relationship between PNI and OS, three demonstrated favorable results while the other five studies indicated that PNI was not associated with OS in ESCC. As to our knowledge, several studies have also explored the relationship between PNI score and long-term survival in other types of solid malignant tumors. Yang and his colleagues conducted a meta-analysis of eleven studies demonstrated that low PNI was significantly associated with poorer OS and CSS in colorectal cancer.[20] Sun et al revealed in another meta-analysis that low PNI was related to poorer OS despite cancer types, but it had no relationship with CSS.[21] Regarding our results, low-PNI score has strong correlation with poorer OS and RFS. Among the nine studies included in our research, only two provided RFS, which limited the prognostic role of PNI in predicting RFS. However, no such significance was detected on CSS in esophageal cancer in our research, which almost reached statistical significance (95%CI 0.991–1.845). The reason for this difference compared to previous studies may be attributed to the limited number of studies that reported relevant data. So, more studies are warranted to further confirm its prognostic value in CSS.
Even though the underlying mechanism about the association between the PNI and prognosis in ESCC is uncertain, some standpoints might potentially explain this intriguing phenomenon. PNI reflects the nutritional and immunological aspects of patients simultaneously because it is based on the peripheral lymphocyte count and serum albumin levels.[22] Studies have proven that lymphocytes and serum albumin are both independent prognostic risk factors of cancer patients.[23,24] Hypoalbuminemia or lymphocytopenia was the reason of low PNI. For esophageal cancer patients, hypoalbuminemia is not only contributes by impaired digestive function due to esophagectomy, but also associated with inflammatory response from tumor or host reaction. As the consequences of malnutrition and inflammatory response, cancer tends to relapse.[25] On the other hand, lymphocytes play an important role in immunologic reaction to suppress carcinogenesis. Of note, the peripheral lymphocytes and the lymphocytes of tumor microenvironment may have different influence on tumor recurrence, invasion, and metastasis. Besides, different types of lymphocytes have distinct roles in tumorigenesis.[26] Thus, more studies are required to investigate the underlying mechanism of interaction between peripheral lymphocytes, tumor microenvironment, and tumor itself.
Some limitations in this meta-analysis should not be ignored. First, both the number and the simple size of included studies were relative small, and all of the studies were retrospective observational studies, which leads to the decline in evidence level. Secondly, not all of the HRs with 95% CIs were directly extracted from the studies, and the reconstructed HR calculated via Kaplan–Meier survival curves unavoidably brought some unexpected errors, and not all of the survival analyses of the eligible studies were conducted by the multivariate analysis, some confounding factors might exist. Thirdly, all of the included studies were conducted in Asian countries, therefore ethnic bias may exist even though we did not setup region restriction when searching literature. Fourthly, the PNI cut-off values of the studies in our meta-analysis varied from 43.2 to 57, because there was no consensus about the cut-off value of the PNI, which could obviously increase the clinical heterogeneity. Moreover, survival rates varied from each study the pooling of their results which inevitably cause clinical heterogeneity. Last but not least, a relative large discrepancy in survival rates existed among the studies. The integration of these studies with large variation survival rates were of great clinical heterogeneity which could not be eliminated by subgroup analysis or other approaches.
In conclusion, low PNI was significantly related with OS and recurrence free survival but not CSS in ESCC. PNI was a reliable prognostic factor of ESCC, and higher stage ESCC patients have higher incidence of low PNI.
Author contributions
PFL, XW, and GWC conceived and drafted the study; XW, KZ, and YTL conducted the literature research and collected all data; YXT is a statistician who conducted statistical analysis. All authors commented on drafts of the paper and approved the final manuscript.
Conceptualization: Xin Wang, Pengfei Li, Guowei Che.
Data curation: Yutian Lai, Kun Zhou, Yuxin Tang.
Formal analysis: Yuxin Tang.
Investigation: Yutian Lai, Yuxin Tang.
Methodology: Xin Wang, Yutian Lai, Kun Zhou, Yuxin Tang.
Writing – original draft: Xin Wang, Pengfei Li, Guowei Che.
Guowei Che orcid: 0000-0003-0500-6180.
Footnotes
Abbreviations: CI = confidence interval, CSS = cancer specific survival, ESCC = esophageal squamous cell carcinoma, HR = hazard ratio, OS = overall survival, PNI = prognostic nutritional index, RFS = recurrence-free survival, RR = relative ratio.
XW and PL contributed equally to this work and should be regarded as co-first authors.
Compliance with ethical standards.
The authors declare that they have no conflict of interest.
References
- [1].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;2:115–32. [DOI] [PubMed] [Google Scholar]
- [2].Di Caro G, Marchesi F, Laghi L, et al. Immune cells: plastic players along colorectal cancer progression. J Cell Mol Med 2013;17:1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sylman JL, Mitrugno A, Atallah M, et al. The predictive value of inflammation-related peripheral blood measurements in cancer staging and prognosis. Front Oncol 2018;8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Buzby GP, Mullen JL, Matthews DC, et al. Prognostic nutritional index in gastrointestinal surgery. Am J Surg 1980;139:160–7. [DOI] [PubMed] [Google Scholar]
- [5].Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984;85:1001–5. [PubMed] [Google Scholar]
- [6].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [8].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [10].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [11].Chen S, Yang X, Feng JF. A novel inflammation-based prognostic score for patients with esophageal squamous cell carcinoma: the c-reactive protein/prognostic nutritional index ratio. Oncotarget 2016;7:62123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sun P, Zhang F, Chen C, et al. Comparison of the prognostic values of various nutritional parameters in patients with esophageal squamous cell carcinoma from Southern China. J Thorac Dis 2013;5:484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hirahara N, Tajima Y, Fujii Y, et al. Preoperative prognostic nutritional index predicts long-term surgical outcomes in patients with esophageal squamous cell carcinoma. World J Surg 2018;42:2199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nakatani M, Migita K, Matsumoto S, et al. Prognostic significance of the prognostic nutritional index in esophageal cancer patients undergoing neoadjuvant chemotherapy. Dis Esophagus 2017;30:1–7. [DOI] [PubMed] [Google Scholar]
- [15].Naoshi Kubo, Masaichi Ohira, Tatsuro Tamura, et al. Prognostic significance of baseline nutritional index for patients with esophageal squamous cell carcinoma after radical esophagectomy. Esophagus 2017;84:1612–9067. [Google Scholar]
- [16].Han L, Song Q, Jia Y, et al. The clinical significance of systemic inflammation score in esophageal squamous cell carcinoma. Tumour Biol 2016;37:3081–90. [DOI] [PubMed] [Google Scholar]
- [17].Yang Y, Xu H, Zhou L, et al. Platelet to lymphocyte ratio is a predictive marker of prognosis and therapeutic effect of postoperative chemotherapy in non-metastatic esophageal squamous cell carcinoma. Clin Chim Acta 2018;479:160–5. [DOI] [PubMed] [Google Scholar]
- [18].Kunizaki M, Tominaga T, Wakata K, et al. Clinical significance of the C-reactive protein-to-albumin ratio for the prognosis of patients with esophageal squamous cell carcinoma. Mol Clin Oncol 2018;8:370–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Miyazaki T, Sakai M, Sohda M, et al. Prognostic significance of inflammatory and nutritional parameters in patients with esophageal cancer. Anticancer Res 2016;36:6557–62. [DOI] [PubMed] [Google Scholar]
- [20].Yang Y, Gao P, Chen X, et al. Prognostic significance of preoperative prognostic nutritional index in colorectal cancer: results from a retrospective cohort study and a meta-analysis. Oncotarget 2016;7:58543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sun K, Chen S, Xu J, et al. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol 2014;140:1537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lucijanic M, Veletic I, Rahelic D, et al. Assessing serum albumin concentration, lymphocyte count and prognostic nutritional index might improve prognostication in patients with myelofibrosis. Wien Klin Wochenschr 2018;130:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang L, Su Y, Chen Z, et al. The prognostic value of preoperative inflammation-based prognostic scores and nutritional status for overall survival in resected patients with nonmetastatic Siewert type II/III adenocarcinoma of esophagogastric junction. Medicine (Baltimore) 2017;96:e7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang H, Zhao J, Zhang M, et al. The combination of plasma fibrinogen and neutrophil lymphocyte ratio (F-NLR) is a predictive factor in patients with resectable non small cell lung cancer. J Cell Physiol 2018;233:4216–24. [DOI] [PubMed] [Google Scholar]
- [25].Rey-Ferro M, Castaño R, Orozco O, et al. Nutritional and immunologic evaluation of patients with gastric cancer before and after surgery. Nutrition 1997;13:878–81. [DOI] [PubMed] [Google Scholar]
- [26].Lin EW, Karakasheva TA, Hicks PD, et al. The tumor microenvironment in esophageal cancer. Oncogene 2016;35:5. [DOI] [PMC free article] [PubMed] [Google Scholar]


