Abstract
Several studies have investigated the relationship between Manganese (Mn) levels and hepatocellular carcinoma (HCC), but the results were inconsistent. Thus, we conducted a systematic review and meta-analysis to evaluate the association between Mn levels and HCC. Nine studies focusing on hair Mn levels, 6 studies on serum Mn levels and 6 studies on tissue Mn levels were identified in a systematic search of PubMed, CNKI, Wanfang and SinoMed databases. Standard mean differences (SMD) with the corresponding 95% confidence intervals (CI) were pooled to compare the Mn levels between HCC and controls. In serum, the Mn levels in HCC were significantly lower than in healthy controls (SMD (95% CI): −0.941 (−1.559, −0.323)). In hair, the Mn levels in HCC were slightly lower than in healthy controls, but not significant (SMD (95% CI): −0.168 (−0.766, 0.430)). In tissue, the Mn levels in tumors were significantly lower than in adjacent normal tissues (SMD (95% CI): −4.867 (−7.143, −2.592)). Subgroup analysis showed consistent results. In conclusion, this meta-analysis suggested an inverse association between Mn levels and HCC.
Keywords: hepatocellular carcinoma, Manganese, meta-analysis
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers around the world, and it is the second leading cause of cancer-related death among males.[1] Multiple factors were reported to be related with the pathogenesis of HCC, like chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV), obesity and heavy alcohol consumption.[2,3] Trace elements also played a role in the development of HCC. In the meta-analysis of Zhang et al, HCC patients had lower selenium levels (serum/blood/toenail measurement) than healthy controls (SMD (95% CI): −1.08 (−0.136, −0.08)).[4] In the systematic review of Gurusamy, Iron, Zinc, and Copper content were lower in HCC than surrounding tissues.[5] Manganese (Mn) is an essential element that is required for many biological processes including bone health, macronutrient metabolism, and defense against reactive oxygen species (ROS).[6] The beneficial effects of Mn are due to the incorporation of the metal into metalloproteins, such as arginase (rate-limiting enzyme in urea synthesis), acetyl-CoA carboxylase (critical catalyst for endogenous fatty acid synthesis), phosphoenolpyruvate decarboxylase, pyruvate carboxylase (gluconeogenesis), Mn superoxide dismutase (mitochondrial antioxidant), glutamine synthetase (critical for brain ammonia metabolism), and glycosyltransferases (bone health).[7] Furthermore, several Mn metalloproteins had been reported in association with HCC.[8–10] However, no meta-analyses have focused on the Mn levels in HCC, although several studies reached inconsistent results.[11–29] Hence, we conducted a systematic review and meta-analysis to clarify these conflicting results and compare the Mn levels between HCC and controls.
2. Methods
2.1. Literature search
This meta-analysis was restricted to the studies which investigated the difference in hair, serum, and tissue Mn levels between HCC and controls. Two independent reviewers searched PubMed, China Knowledge Resource Integrated Database (CNKI), China Wanfang Database and China SinoMed Database from inception to September 2018, using the key words including: (“Manganese concentration” OR “Manganese content” OR “Manganese level” OR “Manganese” OR “trace element”) AND (“hepatocellular” OR “hepatic” OR “liver”) AND (“cancer” OR “tumor” OR “carcinoma”). Moreover, we also reviewed the references of related studies and reviews for undetected studies. The study was approved by the ethnic committee of The First People's Hospital of Qinzhou.
2.2. Study selection and exclusion
The studies were included if meeting the following criteria:
-
1.
all patients were diagnosed by pathology or other methods;
-
2.
In the samples of hair and serum, the controls were from healthy individuals;
-
3.
In tissue samples, the controls were adjacent normal tissues in HCC (obtained as far from the tumor as possible);
-
4.
assessed the Mn levels in HCC and controls. The exclusion criteria were as follows: animal studies, reviews, or case reports.
2.3. Data extraction and quality assessment
Two authors extracted the data by a standardized collection form. All differences were resolved by discussion. In each study, the following information was extracted: first author, publication year, area, Mn measurement, type of samples, number of cases and controls, and Mn levels. The Newcastle–Ottawa Scale (NOS) was used to assess the methodological quality of included studies.
2.4. Statistical analysis
Standard mean differences (SMD) and the corresponding 95% confidence intervals (CI) were pooled to compare hair, serum and tissue Mn levels between HCC and controls respectively. The heterogeneity among studies was estimated by Q test and I2 statistic.[30]I2 > 50% represented substantial heterogeneity, and the summary estimate was analyzed by a random-effects model. Otherwise, a fixed-effects model was applied. Sensitivity analysis was conducted to estimate the stability of the meta-analysis by omitting one study at a time during repeated analyses. Publication bias was assessed by using funnel plots and Egger test. All statistical analyses were performed using software STATA version 12.0 (StataCorp LP, College Station, TX).
3. Results
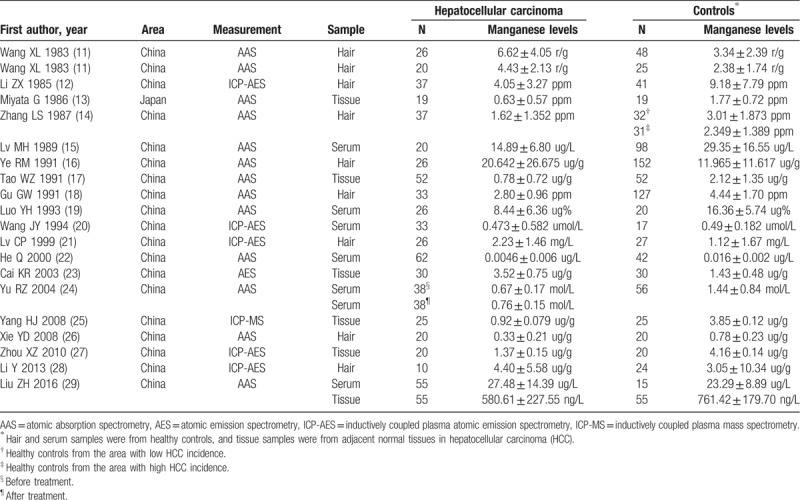
3.1. Characteristics of the included studies
The search strategy identified 1984 records: 1238 from PubMed, 325 from CNKI, 238 from Wanfang, and 183 from SinoMed (Fig. 1). After excluding duplicated and irrelevant records, 19 records (20 studies) were included into this meta-analysis (Table 1).[11–29] The research by Wang et al was based on 2 cohorts, which were regarded as 2 individual studies. Nineteen studies were from China, and one was from Japan. Nine studies investigated the hair Mn levels in 235 HCC cases and healthy controls. Six studies investigated the serum Mn levels in 272 HCC cases and 248 healthy controls. Six studies investigated the tissue Mn levels in the tumors and adjacent normal tissues of 201 HCC cases. Most studies used the method of atomic absorption spectrometry (AAS) to measure Mn levels, followed by inductively coupled plasma atomic emission spectrometry (ICP-AES). In quality assessment, all included studies had an NOS score of 6.
Figure 1.
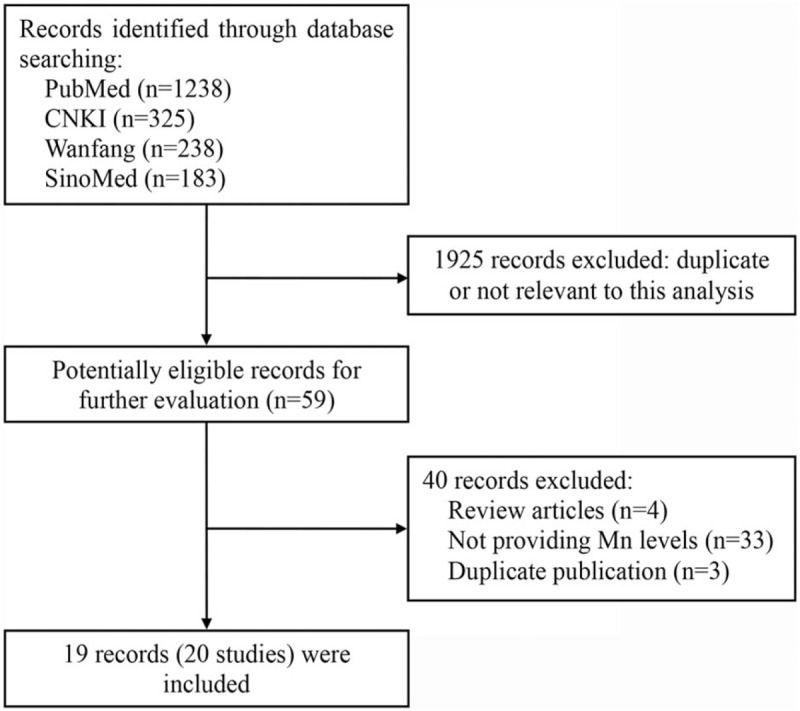
Flowchart of literature search.
Table 1.
Characteristics of included studies.
3.2. Hair Mn levels and HCC
Nine studies investigated the difference of hair Mn levels between HCC and healthy controls. The research by Zhang et al included 2 groups of healthy controls from the areas with low and high incidence of HCC. It was found that hair Mn levels in HCC were lower than in controls, but not significant (SMD (95% CI): −0.168 (−0.766, 0.430); I2 = 92.4%, Pheterogeneity < .001) (Fig. 2). Sensitivity analysis showed the result was robust. Egger test detected no significant publication bias (P = .850).
Figure 2.
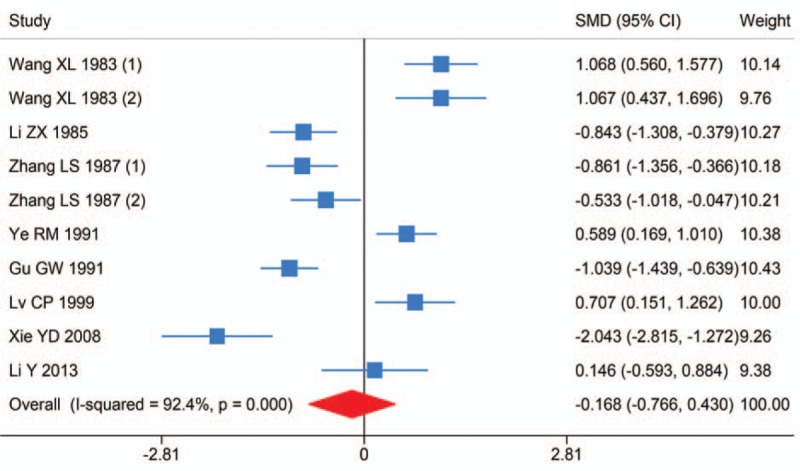
Forest plot of meta-analysis on hair Manganese levels and hepatocellular carcinoma.
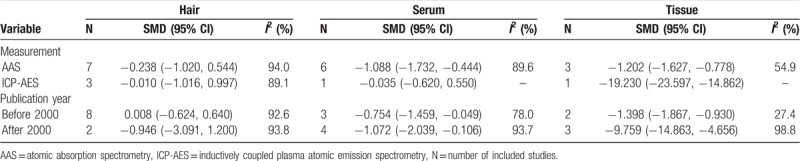
Subgroup analysis was conducted on measurement and publication year. No substantial changes of the primary result were found between subgroups (Table 2).
Table 2.
Subgroup analysis of Manganese levels and hepatocellular carcinoma.
3.3. Serum Mn levels and HCC
Six studies investigated the difference of serum Mn levels between HCC and healthy controls. The study by Yu et al also investigated the serum Mn levels of HCC cases before and after treatment. It was found that serum Mn levels in HCC were significantly lower than in controls (SMD (95% CI): −0.941 (-1.559, −0.323); I2 = 90.0%, Pheterogeneity < .001) (Fig. 3). Sensitivity analysis showed the result was robust. Egger test detected no significant publication bias (P = .483).
Figure 3.
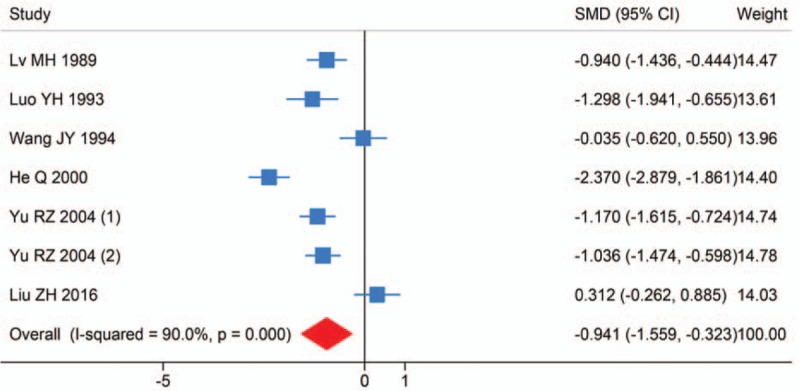
Forest plot of meta-analysis on serum Manganese levels and hepatocellular carcinoma.
Subgroup analysis was conducted on measurement and publication year. No substantial changes of the primary result were found between subgroups (Table 2).
3.4. Tissue Mn levels and HCC
Six studies investigated the difference of tissue Mn levels between the tumors and adjacent normal tissues in HCC. It was found that tissue Mn levels in tumors were significantly lower than in adjacent normal tissues (SMD (95% CI): −4.867 (−7.143, −2.592); I2 = 98.2%, Pheterogeneity < .001) (Fig. 4). Sensitivity analysis showed the result was robust. Egger test detected no significant publication bias (P = .268).
Figure 4.
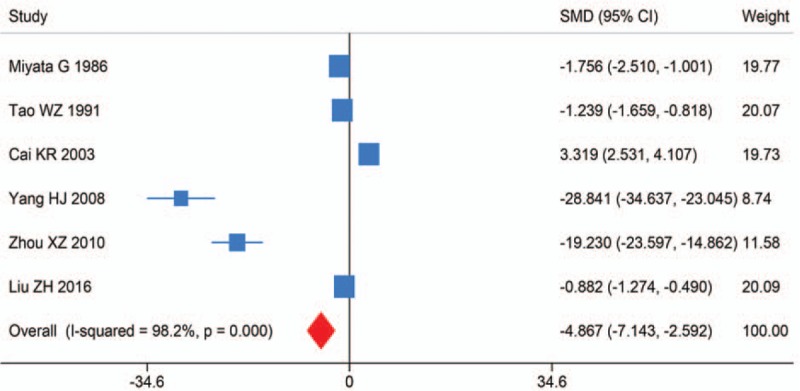
Forest plot of meta-analysis on tissue Manganese levels and hepatocellular carcinoma.
Subgroup analysis was conducted on measurement and publication year. No substantial changes of the primary result were found between subgroups (Table 2).
4. Discussion
As an essential trace element, Mn played an important role in a wide range of biological processes. It is a cofactor of some important enzymes, like Mn superoxide dismutase (MnSOD). MnSOD is a mitochondrial antioxidant enzyme which is down-regulated in a majority of cancers and suggested as a tumor suppressor due to potent antioxidant activity.[31] Thus, Mn was thought to be associated with multiple diseases. In the meta-analysis of Du et al, patients with Alzheimer's disease and mild cognition impairment had significantly reduced serum Mn levels compared to healthy controls (SMD (95% CI): −0.39 (−0.71, −0.08); −0.37 (−0.60, −0.13)).[32] In the meta-analysis of Shen et al, a significant association was found between deficient Mn levels and breast cancer (SMD (95% CI): −1.51 (−2.47, −0.56)), and different sample specimens showed consistent results (serum: −1.24 (−2.31, −0.16); hair: −1.99 (−3.91, −0.06)).[33] Furthermore, in the recent study by Ma et al, a population-based epidemiological research was conducted to investigate the relationship between dietary trace element intake and liver cancer risk.[34] It was found that dietary intake of Mn was inversely associated with liver cancer risk (highest vs lowest quintile, hazard ratio (95% CI): 0.51 (0.35–0.73), Ptrend < .001). However, no meta-analyses have focused on the Mn levels in HCC, although several studies reached inconsistent results.
In this meta-analysis, we also suggested a significant association between Mn levels and HCC. In serum, the Mn levels in HCC were significantly lower than in healthy controls. In tissue, the Mn levels in tumors were significantly lower than in adjacent normal tissues. Furthermore, in the study by Yu et al, the serum Mn levels in HCC increased significantly after treatment (SMD (95% CI): −0.561 (−1.020, −0.103)), which also indicated an inverse association between Mn levels and HCC. In hair, the Mn levels in HCC were slightly lower than in healthy controls, but not significant, which might contribute to tissue specificity. Similarly, breast cancer patients had a higher nickel (Ni) level in serum (SMD: 1.76, 95% CI: 0.82–2.70), but it was not significant in hair (SMD: 0.16, 95% CI: −1.08 to 1.40).[35] Breast cancer patients had a lower Zinc (Zn) level in hair (SMD: −1.99, 95% CI: −3.46 to −0.52), but it was not significant in serum (SMD: −0.65, 95% CI: −1.42 to 0.13).[36] Nevertheless, Zinc supplementation could significantly increase natural killer T (NKT) cells, which were involved in the direct killing of target cells and thus provided anti-tumor protection.[37]
This meta-analysis had several strengths. First, to the best of our knowledge, this is the first meta-analysis to evaluate the association between Mn levels and HCC. Second, the conditions in hair, serum, and tissue were considered respectively. Third, sensitivity analysis and Egger test were conducted to estimate the stability of pooled results and potential publication bias. However, several limitations in this study should be considered. First, the number of cases and controls in each study was relatively small. Second, the obvious heterogeneity between studies was observed. For this, we conducted a sensitivity analysis to evaluate the stability of pooled results.
In conclusion, this meta-analysis suggested an inverse association between Mn levels and HCC.
Author contributions
Conceptualization: Xiubing Chen, Yuehui Wei, Youbao Zou.
Data curation: Xiubing Chen, Yuehui Wei, Youbao Zou.
Formal analysis: Xiubing Chen, Yuehui Wei, Xiu-Ke Chen.
Investigation: Xiubing Chen.
Methodology: Xiubing Chen, Yuehui Wei, Xiu-Ke Chen, Jia-Yan Nie.
Software: Xiubing Chen, Jian Zhong.
Supervision: Jian Zhong, Jia-Yan Nie.
Validation: Jian Zhong.
Visualization: Xiubing Chen.
Writing – original draft: Xiubing Chen, Youbao Zou.
Writing – review & editing: Xiu-Ke Chen, Youbao Zou, Jia-Yan Nie.
Footnotes
Abbreviations: AAS = atomic absorption spectrometry, CI = confidence interval, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, ICP-AES = inductively coupled plasma atomic emission spectrometry, Mn = Manganese, MnSOD = Manganese superoxide dismutase, NOS = Newcastle-Ottawa Scale, SMD = standard mean difference.
XBC, YHW, and XKC contributed equally to this study.
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends-an update. Cancer Epidemiol Biomarkers Prev 2016;25:16–27. [DOI] [PubMed] [Google Scholar]
- [2].El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118–27. [DOI] [PubMed] [Google Scholar]
- [3].Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 2013;47:S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang Z, Bi M, Liu Q, et al. Meta-analysis of the correlation between selenium and incidence of hepatocellular carcinoma. Oncotarget 2016;7:77110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gurusamy K. Trace element concentration in primary liver cancers--a systematic review. Biol Trace Elem Res 2007;118:191–206. [DOI] [PubMed] [Google Scholar]
- [6].Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Crit Rev Toxicol 2005;35:1–32. [DOI] [PubMed] [Google Scholar]
- [7].Erikson KM, Aschner M. Manganese: its role in disease and health. Met Ions Life Sci 2019;doi: 10.1515/9783110527872-016. [DOI] [PubMed] [Google Scholar]
- [8].Clark I, Shah SS, Moreira R, et al. A subset of well-differentiated hepatocellular carcinomas are Arginase-1 negative. Hum Pathol 2017;69:90–5. [DOI] [PubMed] [Google Scholar]
- [9].Wang MD, Wu H, Fu GB, et al. Acetyl-coenzyme A carboxylase alpha promotion of glucose-mediated fatty acid synthesis enhances survival of hepatocellular carcinoma in mice and patients. Hepatology 2016;63:1272–86. [DOI] [PubMed] [Google Scholar]
- [10].Jeon JY, Lee H, Park J, et al. The regulation of glucose-6-phosphatase and phosphoenolpyruvate carboxykinase by autophagy in low-glycolytic hepatocellular carcinoma cells. Biochem Biophys Res Commun 2015;463:440–6. [DOI] [PubMed] [Google Scholar]
- [11].Wang XL, Pu WX, Lu XJ, et al. The study on the role of manganese in the etiology of hepatocellular carcinoma. J Youjiang Med Coll Natl 1983;2:1–5. [Google Scholar]
- [12].Li ZX, Liang YC, Sheng SY, et al. Determination of hair trace elements in hepatocellular carcinoma patients and healthy controls in Shunde area of Guangdong Province. Guangzhou Med J 1985;1:42–7. [Google Scholar]
- [13].Miyata G. The comparison of trace element concentration in tumor and normal tissues of hepatocellular carcinoma. Jpn J Digest Dis 1986;83:2091. [Google Scholar]
- [14].Zhang LS, Huang ZS, Liang RX, et al. The analyses of trace elements in the hair of liver cancer patients, high liver cancer incidence family members and healthy clusters. Chin J Cancer 1987;8:32–4. [Google Scholar]
- [15].Lv MH, Jiang HM, Xu BQ, et al. Viral hepatitis, cirrhosis and liver cancer and trace elements in serum. Shandong Med Univ 1989;27:20–5. [Google Scholar]
- [16].Ye RM, Cao GH, Qian ZY, et al. Preliminary study on the association between trace elements and hepatocellular carcinoma. Chin J Clin Hepatol 1991;7:46–7. [Google Scholar]
- [17].Tao WZ, Peng Q, Wu CZ, et al. The study on the association between the concentration of manganese, copper, zinc, iron and cadmium and the etiology of hepatocellular carcinoma. Chin J Clin Exp Pathol 1991;7:19–22. [Google Scholar]
- [18].Gu GW. A preliminary study on the relationship between primary liver cancer and trace elements in Qidong Region. Chin J Clin Oncol 1991;18:20–2. [Google Scholar]
- [19].Luo YH, Yuan AD. The change in the concentration of serum copper, zinc and manganese in primary liver cancer and cirrhosis. J Third Milit Med Univ 1993;15:71–2. [Google Scholar]
- [20].Wang JY, Yin SC, Chen YQ, et al. Determination of element contents, caeruloplasmin, and T lymphocites in the serum of patients with carcinoma of liver and their clinical significance. Guangdong Trace Elem Sci 1994;1:34–7. [Google Scholar]
- [21].Lv CP, Li J, Lv DM. Study on the content of inorganic elements in the hair of patients with cancers. Guangdong Trace Elem Sci 1999;6:1–4. [Google Scholar]
- [22].He Q, Ma RH, Li YB, et al. Determination of trace elements Cu, Zn, Mg, Cr, Mn in serum of people with hepatoma, cirrhosis, hepatapstema disease. Chin J Spectrosc Spectr Analy 2000;20:540–1. [PubMed] [Google Scholar]
- [23].Cai KR, Cai C, Jie XM, et al. Studies of trace elements in the primary liver cancer and side of cancer. Guangdong Trace Elem Sci 2003;10:28–30. [Google Scholar]
- [24].Yu RZ, Dai DM, Du JJ. The influence of chemotherapy on the concentration of trace elements in hepatocellular carcinoma. J Mod Lab Med 2004;19:38–9. [Google Scholar]
- [25].Yang HJ, Hu CH, Li LB. Mass spectrometry in determination of trace elements in the tissues of liver cancer. Contemp Med 2008;14:11–3. [Google Scholar]
- [26].Xie YD. Study on relationship of trace elements level of hair to the hepatitis B, cirrhosis and liver carcinoma. J Hainan Med Coll 2008;14:140–4. [Google Scholar]
- [27].Zhou XZ, Nie XD. Study on simultaneous determination of trace elements in carcinoma of liver by ICP-AES. Guangdong Trace Elem Sci 2010;17:27–30. [Google Scholar]
- [28].Li Y, Zhao ZZ, Zhou YZ. Identification of heavy metals sources in scalp hair and associated influence factors in liver cancer's high incidence area in Pearl River delta. China Guangdong Trace Elem Sci 2013;20:1–0. [Google Scholar]
- [29].Liu ZH, Yang WP, Wei CY, et al. The study on the concentration and correlation between tissue and serum zinc, copper, manganese and selenium in primary liver cancers. J Guangxi Med Univ 2016;33:66–70. [Google Scholar]
- [30].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analysis. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Case AJ, Domann FE. Absence of manganese superoxide dismutase delays p53-induced tumor formation. Redox Biol 2014;2:220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Du K, Liu M, Pan Y, et al. Association of serum manganese levels with Alzheimer's disease and mild cognitive impairment: a systematic review and meta-analysis. Nutrients 2017;9. [Google Scholar]
- [33].Shen F, Cai WS, Li JL, et al. The association between deficient manganese levels and breast cancer: a meta-analysis. Int J Clin Exp Med 2015;8:3671–80. [PMC free article] [PubMed] [Google Scholar]
- [34].Ma X, Yang Y, Li HL, et al. Dietary trace element intake and liver cancer risk: Results from two population-based cohorts in China. Int J Cancer 2017;140:1050–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yu M, Zhang J. Serum and hair Nickel levels and breast cancer: systematic review and meta-analysis. Biol Trace Elem Res 2017;179:32–7. [DOI] [PubMed] [Google Scholar]
- [36].Wu X, Tang J, Xie M. Serum and hair zinc levels in breast cancer: a meta-analysis. Sci Rep 2015;5:12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Baltaci SB, Mogulkoc R, Baltaci AK, et al. The effect of zinc and melatonin supplementation on immunity parameters in breast cancer induced by DMBA in rats. Arch Physiol Biochem 2018;124:247–52. [DOI] [PubMed] [Google Scholar]


