Supplemental Digital Content is available in the text
Keywords: fenestration, Fontan procedure, meta-analysis, outcome
Abstract
Background:
The benefits of fenestration for patients undergoing Fontan procedure seem controversial at early and late postoperative stages.
Objective:
We aimed to compare the outcomes between the fenestrated and non-fenestrated Fontan procedures.
Methods:
Studies comparing the fenestrated and non-fenestrated Fontan procedures were identified by searching the PubMed, EMBASE, and Cochrane Library databases until July 2018. The assessed variables included postoperative oxygen saturation (SaO2), pulmonary artery pressure, mortality, cardiopulmonary bypass (CPB) time, ventilation time, intensive care unit stay, hospital stay, chest tube duration, protein-losing enteropathy, arrhythmia, and other follow-up outcomes including reintervention, stroke/thrombosis, and peak oxygen consumption. A random-effect/fixed-effect model was used to summarize the estimates of the mean difference (MD)/odds ratio (OR) with 95% confidence interval (CI). Subgroup analysis stratified by early and late outcomes was performed.
Results:
A total of 1929 Fontan patients from 14 studies were included. The early postoperative SaO2 was lower with fenestration than without fenestration (MD −2.52, 95% CI −4.16 to −0.87, P <.05); however, the late postoperative SaO2 showed no difference between the 2 approaches. The CPB time was shorter without fenestration than with fenestration (MD 10.72, 95% CI 2.54–18.9, P <.05); however, the incidence of arrhythmia was lower with fenestration than without fenestration (OR 0.43, 95% CI 0.25–0.75, P <.05). Other variables showed no significant differences between the 2 approaches in Fontan patients.
Conclusion:
Fenestration appears to result in a lower incidence of arrhythmia but with a longer CPB time and lower early SaO2. Other outcomes are comparable between the 2 approaches.
1. Introduction
The Fontan procedure was introduced for a functional single ventricle more than 40 years ago,[1,2] and it has undergone several major technical modifications.[3] However, in patients with risk factors such as pulmonary artery pressure (PAP) >18 mmHg, end-diastolic pressure >12 mmHg, valvar regurgitation, pulmonary artery distortion, pulmonary vascular resistance >2 Woods’ units, ventricular outflow obstruction, and complex anatomy, the postoperative mortality rate with this procedure remained high because of elevated systemic venous pressure and decreased cardiac output.[4,5] Therefore, Bridges et al[4] initially reported baffle fenestration in the intra-lateral tunnel (ILT) Fontan procedure, which allowed right-to-left shunt, maintained cardiac output, and limited right atrial pressure. Such fenestration provided a smoother early postoperative course.[3,5]
The introduction of extracardiac conduit (ECC) Fontan procedure was, however, associated with a technical challenge of maintaining fenestration patency.[6] Many centers chose to perform the non-fenestrated extracardiac Fontan procedure,[7–9] and early outcomes involving “hospitalization duration” and “pleural drainage” appeared not to be poor without fenestration.[8] Meanwhile, some centers insisted that routine elective fenestration is justified in all Fontan patients, with decreased Fontan failure rates and decreased occurrence of significant postoperative pleural effusion.[10] Consequently, the adoption of fenestration in the Fontan procedure remains controversial.
Therefore, we aimed to compare the outcomes between the fenestrated and non-fenestrated Fontan procedures and evaluate the effects of both the methods to provide evidence for establishing an appropriate clinical strategy.
2. Methods
Ethical approval was not necessary, because this work is a meta-analysis.
2.1. Search strategy
This study was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses guidelines.[11] A literature search of computerized medical literature was performed using the PubMed, EMBASE, and Cochrane Library databases. The keywords searched were “Fontan” or “total cavopulmonary connection” and “fenestration” or “fenestrated” (Supplementary File 1). The search was conducted for published papers from inception until July 2018, and there were no language restrictions. To ensure that the search was complete, reference lists of all retrieved articles were manually searched to identify additional relevant studies by DL and ML.
2.2. Inclusion and exclusion criteria
All included studies were required to report baseline characteristics of patients, and original data for dichotomous and continuous variables were required to be provided or were required to be assessable from the data source. Studies were selected using the following inclusion criteria:
-
1)
reported comparisons of outcomes between fenestration and no fenestration in patients who underwent the Fontan procedure;
-
2)
described at least 1 of the following variables:
-
1.
postoperative oxygen saturation (SaO2), PAP, or peak oxygen consumption (VO2);
-
2.
in-hospital data, including cardiopulmonary bypass (CPB) time, ventilation time, intensive care unit (ICU) stay, hospital stay, and chest tube duration; and
-
3.
complications, including arrhythmia, protein-losing enteropathy (PLE), reintervention, stroke/thrombosis, and death.
Simple abstracts without complete information were excluded because quality was difficult to assess. Additionally, letters, editorials, animal trails, case reports, and literature reviews were excluded.
2.3. Study quality and level of evidence
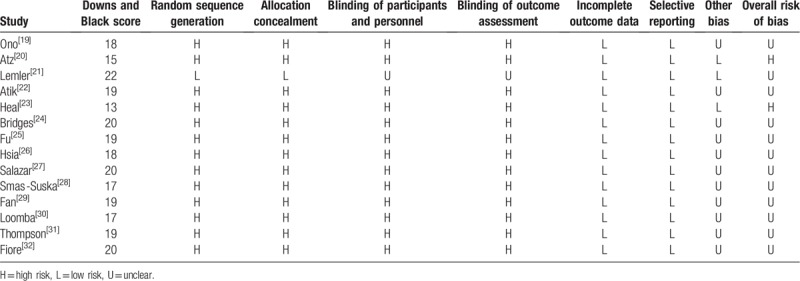
The level of evidence of the included studies was categorized according to the criteria of the Center for Evidence-Based Medicine in Oxford, United Kingdom.[12] Studies with a score of ≥3b were considered to be of high quality. The methodological quality of the included studies was assessed by DL and ML. The quality of individual studies was evaluated using the Downs and Black quality assessment method, in which a list of 27 criteria are used to evaluate both randomized controlled trials (RCTs) and non-RCTs.[13] The Downs and Black scores were grouped into the following 4 quality levels: excellent (26–28), good (20–25), fair (15–19), and poor (<14). The risk of bias was assessed independently by 2 reviewers using the Cochrane Collaboration tool.[14] Every domain was scored as high risk of bias, low risk of bias, or unclear. The overall assessment of each study was graded as “low risk” (if all domains were assessed as low risk of bias), “unclear” (if at least 1 domain was assessed as unclear), or “high risk” (otherwise).
2.4. Data extraction and outcomes of interest
The full text of the included studies was reviewed by DL and ML. According to a prespecified protocol, all data were extracted independently by the 2 authors. The following data were extracted from each eligible study by using a standardized data-collection form: first author's name, study design, publication year, country where the study was conducted, sex, sample size, age, weight, main diagnosis, Fontan procedure type, fenestration size, preoperative SaO2, PAP, and follow-up interval. Values of comparative data were also collected. The parameters were as follows: primary outcomes including postoperative SaO2 (%), PAP (mmHg), and mortality; secondary outcomes including CPB time (min), ventilation time (min), ICU stay (days), hospital stay (days), chest tube duration (days), PLE, and arrhythmia; and other follow-up outcomes including reintervention, stroke/thrombosis, and peak VO2.
2.5. Statistical analysis
The measure of the effect of interest was the odds ratio (OR)/mean difference (MD) with 95% confidence interval (CI). We imputed the missing standard deviation (SD) of the difference between before and after the Fontan procedure using the following formula recommended in the Cochrane Handbook: 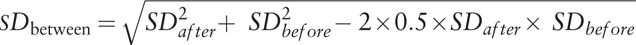 .[15] We used the Cochrane chi-square test (Q test) and the I2 test to evaluate potential heterogeneity between studies. When significant heterogeneity (P <.05 or I2 >50%) was detected, we pooled data using a random-effect model.[16] Otherwise, a fixed-effect model was used.[17] The funnel plots were visually inspected to identify any potential publication bias. In addition, Egger test was used to examine the degree of publication bias (P <.05 was considered indicative of statistically significant publication bias).[18] All statistical analyses were performed using Review Manager software (version 5.3; Cochrane Collaboration, Oxford, UK) and Stata software (version 12.0; Stata Corp., College Station, TX).
.[15] We used the Cochrane chi-square test (Q test) and the I2 test to evaluate potential heterogeneity between studies. When significant heterogeneity (P <.05 or I2 >50%) was detected, we pooled data using a random-effect model.[16] Otherwise, a fixed-effect model was used.[17] The funnel plots were visually inspected to identify any potential publication bias. In addition, Egger test was used to examine the degree of publication bias (P <.05 was considered indicative of statistically significant publication bias).[18] All statistical analyses were performed using Review Manager software (version 5.3; Cochrane Collaboration, Oxford, UK) and Stata software (version 12.0; Stata Corp., College Station, TX).
3. Results
3.1. Search results and characteristics of the included studies
According to the inclusion and exclusion criteria, 14 studies (from 1992 to 2017, involving 1929 patients) were included in the analysis.[19–32] The flow diagram shows the detailed literature search steps (Fig. 1). The characteristics and quality of the individual studies are presented in Table 1. Of the 14 studies, 9 were conducted in America (8 in the United States and 1 in Brazil), 4 in Europe (2 in Germany, 1 in the UK, and 1 in Poland), and 1 in Asia (China). Patients in 5 studies underwent the ECC procedure, and those in 1 study underwent the ILT procedure. The other studies reported more than 1 kind of Fontan technique. The fenestration size in most studies ranged from 4 to 6 mm. The study by Smaś-Suska et al had the longest follow-up record.[28] Eleven studies were retrospective cohort studies, 2 were cross-sectional studies, and 1 was an RCT. The results of the methodological quality and bias risk of the included studies are shown in Table 2.
Figure 1.
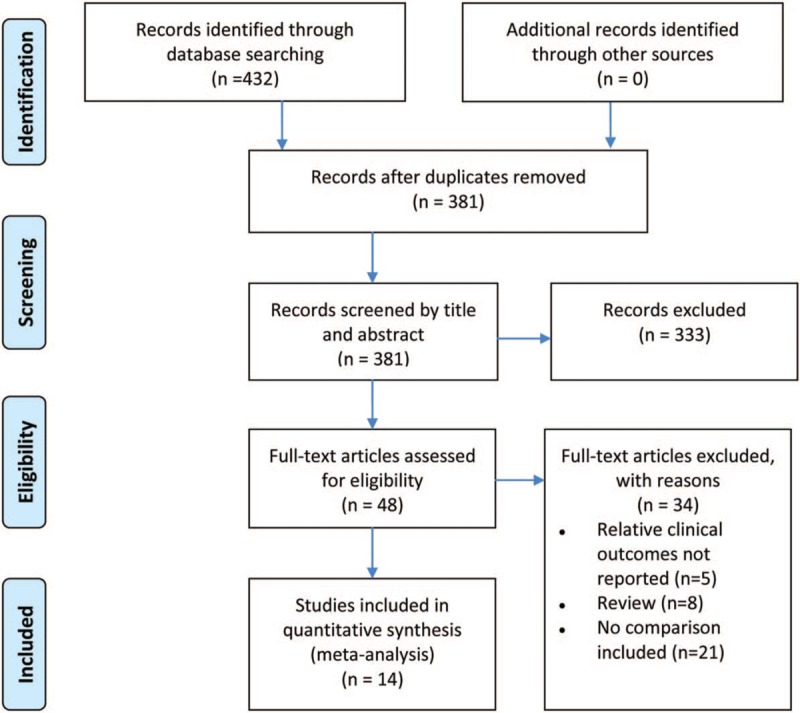
Flow diagram of literature search and study selection.
Table 1.
Characteristics of included studies.
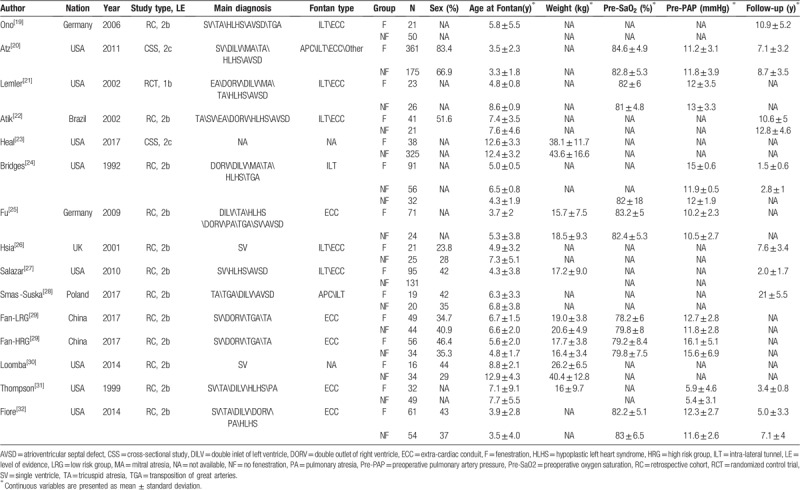
Table 2.
Methodological quality (Downs and Black scale) and risk of bias (Cochrane collection) of included studies.
3.2. Primary outcomes
Eleven studies compared postoperative SaO2 between patients treated with fenestration and those treated without fenestration (Fig. 2A). In subgroup analysis, the pooled MD of early postoperative SaO2 favored the no fenestration group (−2.52, 95% CI −4.26 to −0.87, P <.05), whereas the MD of late postoperative SaO2 did not indicate a difference between the 2 groups (−2.51, 95% CI −9.37 to 4.35, P = .47). The pooled estimates of the SaO2 change before and after surgery in the 2 groups are shown in Fig. 2B. The pooled MD of early SaO2 change favored the no fenestration group (−1.86, 95% CI −3.25 to −0.47, P <.05), whereas the MD of late SaO2 change did not indicate a difference between the 2 groups (1.80, 95% CI −0.14 to 3.74, P = .07). Additionally, no differences were found in postoperative PAP (MD −0.39, 95% CI −1.63 to 0.86, P = .54, Fig. 3A) and PAP change (MD −1.67, 95% CI −4.25 to 0.92, P = .21, Fig. 3B) before and after surgery between the 2 groups. Moreover, no differences were found in early mortality (OR 0.65, 95% CI 0.32–1.31, P = .23, Fig. 4) and late mortality (OR 1.00, 95% CI 0.34–2.94, P = .88) between the 2 groups.
Figure 2.
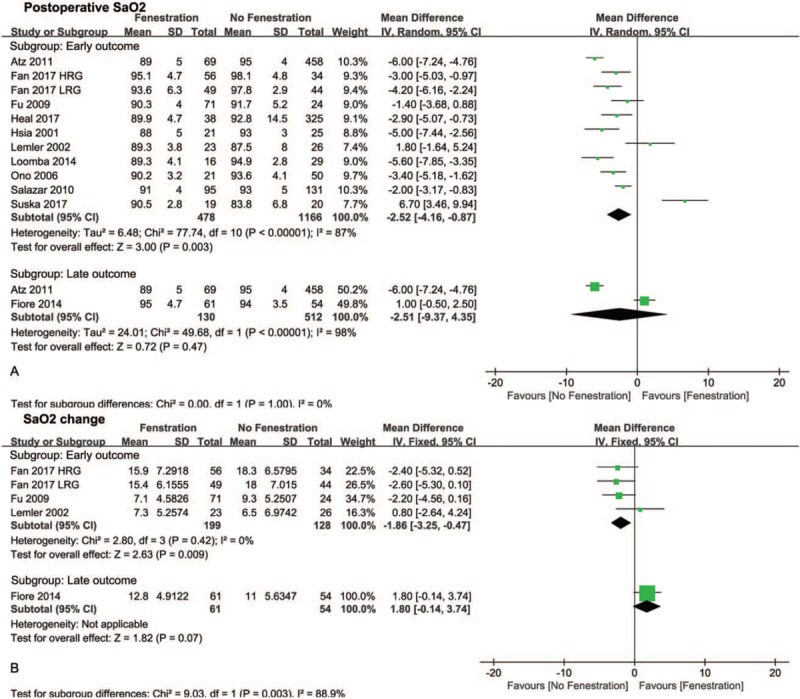
Forest plots of postoperative SaO2 (A) and SaO2 change before and after Fontan procedure (B). HRG = high risk group, LRG = low risk group, SaO2 = oxygen saturation.
Figure 3.
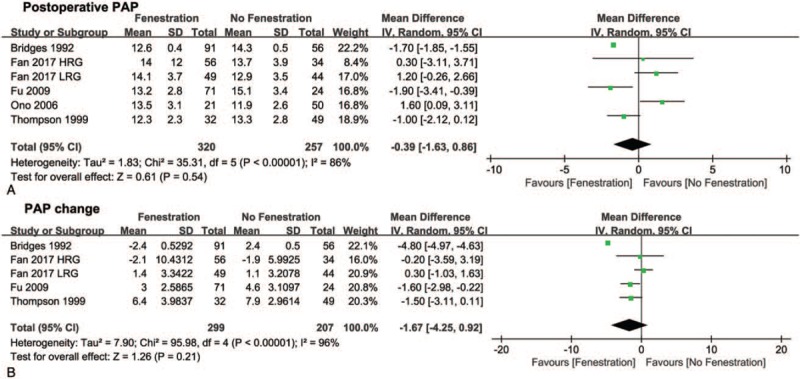
Forest plots of postoperative PAP (A) and PAP change before and after Fontan procedure (B). PAP = pulmonary artery pressure. HRG = high risk group, LRG = low risk group.
Figure 4.
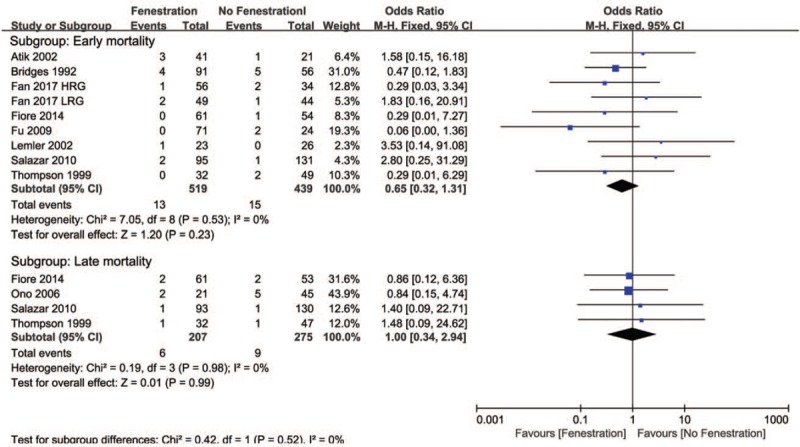
Forest plots and subgroup analysis of mortality. HRG = high risk group, LRG = low risk group.
3.3. Secondary outcomes
The pooled MD of the CPB time favored the no fenestration group (10.72, 95% CI 2.54–18.9, P <.05, Fig. 5A), whereas the pooled ORs of PLE (0.24, 95% CI 0.06–096, P <.05, Fig. 5B) and arrhythmia (0.38, 95% CI 0.23–0.62, P <.05, Fig. 5C) favored the fenestration group. In addition, no significant differences were found in ventilation time, ICU stay, hospital stay, and chest tube duration between the 2 groups (Fig. 6A–D).
Figure 5.
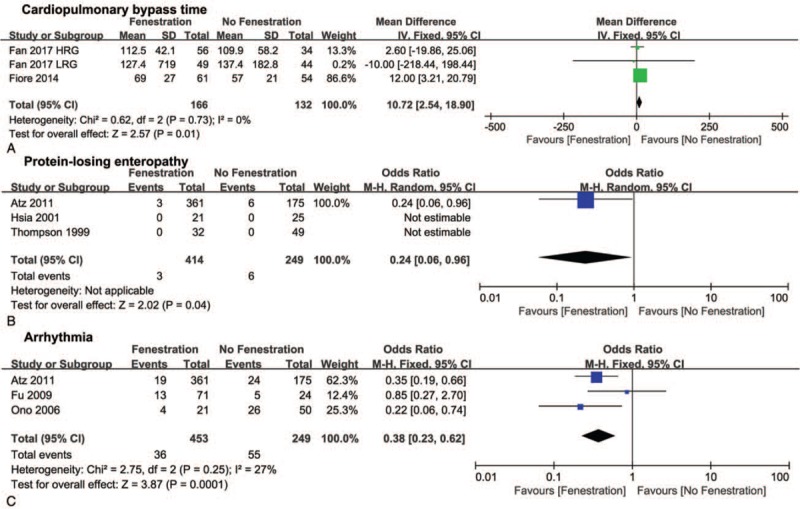
Forest plots of cardiopulmonary bypass time (A), protein-losing enteropathy (B), and arrhythmia (C). HRG = high risk group, LRG = low risk group.
Figure 6.
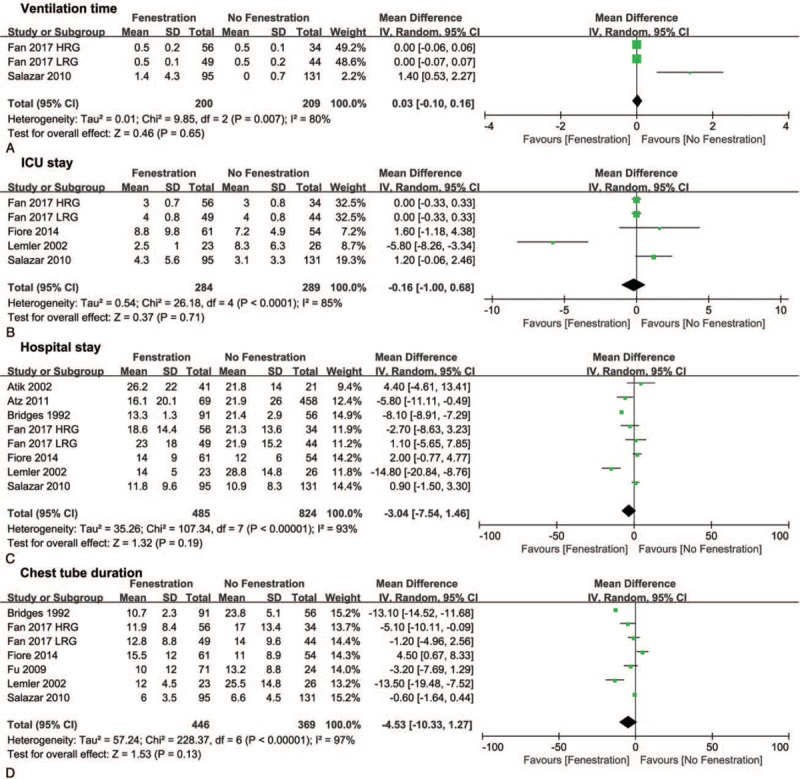
Forest plots of ventilation time (A), ICU stay (B), hospital stay (C), and chest tube duration (D). HRG = high risk group, LRG = low risk group.
3.4. Follow-up outcomes
During the follow-up, the pooled OR of stroke favored the fenestration group (0.17, 95% CI 0.05–0.61, P <.05, Fig. 7A), although there was no difference in thrombosis between the 2 groups (Fig. 7B). The pooled ORs of reintervention involving readmission, reoperation, and Fontan take-down did not indicate differences between the 2 groups (Fig. 8A–C). Additionally, no difference was found in peak VO2, which is an index for cardiopulmonary function, between the 2 groups (Fig. 8D).
Figure 7.
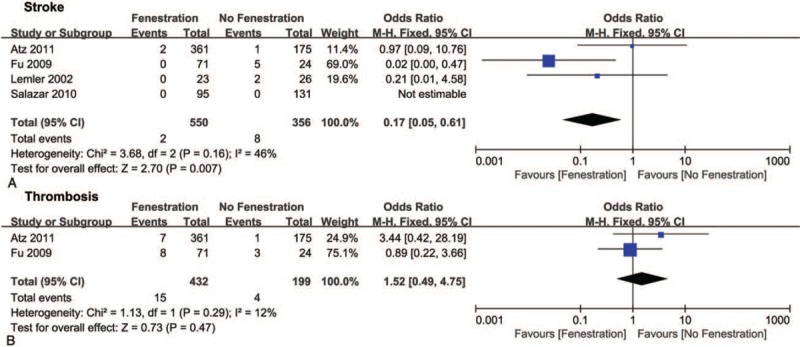
Forest plots of stroke (A) and thrombosis (B).
Figure 8.
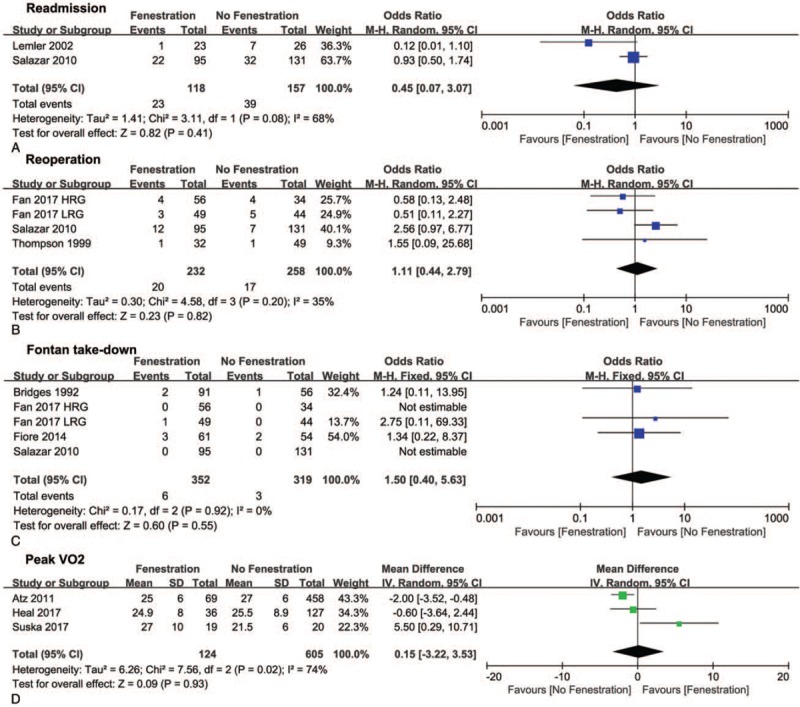
Forest plots of readmission (A), reoperation (B), Fontan take-down (C), and peak VO2 (D). HRG = high risk group, LRG = low risk group, VO2 = oxygen consumption.
3.5. Sensitivity analysis and publication bias
We conducted sensitivity analyses to ascertain the primary origin of heterogeneity. The outcomes of studies by Smaś-Suska et al[28] and Lemler et al[21] showed marked effects on the pooled MD of early postoperative SaO2 (Supplemental Fig 1). After elimination of these 2 studies, which might have been related to the heterogeneity, the pooled MD of early postoperative SaO2 favored the no fenestration group. The funnel plots of studies reporting postoperative SaO2 showed symmetrical distributions, suggesting that there was no evidence of publication bias in the meta-analysis (Supplemental Fig 2).
4. Discussion
To our knowledge, this is the first meta-analysis to compare the fenestrated with non-fenestrated Fontan procedures. In this meta-analysis, we found that early postoperative SaO2 and CPB time favored the no fenestration group, whereas the incidence of postoperative arrhythmia favored the fenestration group. Other variables showed no significant differences between the fenestration and no fenestration groups.
Most studies have reported early desaturation, whereas few studies have mentioned increased SaO2 after fenestration.[21,28] Atz et al reported that long-term SaO2 was lower with fenestration than without fenestration, whereas Fiore et al. reported that there was no significant difference in long-term saturation between the 2 approaches.[20,32] This might be because of the increase in saturation with the fenestration spontaneously closing during long-term follow-up. The SaO2 change before and after surgery was greater with fenestration than without fenestration.[21,25,29,32]
Although our pooled estimate suggested that the effect of fenestration on PAP change was not significantly different from that of no fenestration, Bridge et al and Hu et al indicated that fenestration was more beneficial for reducing systemic venous pressure.[24,25] And they concluded that there was a decrease in PAP with fenestration after surgery,[24,25] whereas Fan et al concluded that there was no significant difference in the decrease in PAP between window fenestration and no fenestration.[29] Additionally, Ono et al reported that postoperative PAP was higher with fenestration than without fenestration.[19] In terms of early and late mortalities, there were no significant differences between the 2 approaches in many centers.[22,29,31,32]
Although the operation time would theoretically be longer with fenestration than without fenestration, the CPB time was reported not to be significantly different between fenestration and no fenestration by Fiore et al.[32] Fan et al reported that the ventilation time did not differ significantly between the 2 approaches, but Salazar et al mentioned that the ventilation time was longer with fenestration than without fenestration.[27] With regard to ICU stay, Lemler et al reported that the stay was shorter with fenestration, Salazar et al reported that the stay was shorter without fenestration, and Fiore et al reported that the stay did not differ significantly between the 2 approaches.[21,27,32] Atz et al and Lemler et al reported that hospital stay was shorter with fenestration than without fenestration, whereas Atik et al and Salazar et al reported that hospital stay did not differ significantly between the 2 approaches.[20–22,27] As systemic venous pressure decreases after fenestration, the time of pleural effusion should be shorter with fenestration than without fenestration after surgery. However, both Fan et al and Fiore et al reported that there was no significant difference between the 2 approaches.[29,32]
With regard to postoperative complications, Atz et al reported that the incidence of PLE was lower with fenestration than without fenestration.[20] However, the absence of PLE was reported by both Hsia et al and Thompson et al.[26,31] Atz et al reported that the incidence of early arrhythmia was lower with fenestration than without fenestration, whereas Fu et al reported that there was no significant difference between the 2 approaches.[20,25] Additionally, Ono et al reported that the incidence of late arrhythmia was lower with fenestration than without fenestration.[19]
Although the incidence of stroke was higher without fenestration than with fenestration, there was no significant difference in the overall incidence of thrombotic events between the 2 approaches.[25] Additionally, Atz et al and Lemler et al reported that the incidence of stroke did not differ significantly between the 2 approaches.[20,21]
Reintervention included readmission and reoperation. Lemler et al and Salazar et al reported that readmission did not differ significantly between the 2 approaches.[21,27] Salazar et al reported that the reoperation rate was higher with fenestration than without fenestration.[27] Furthermore, Bridges et al, Fan et al, and Fiore et al found no significant difference in Fontan take-down between the 2 approaches.[24,29,32]
During the follow-up, peak VO2 is an important parameter of exercise tolerance. Atz et al reported that peak VO2 was higher with fenestration than without fenestration, whereas Smaś-Suska et al reported that peak VO2 was higher without fenestration than with fenestration.[20,28] On the other hand, Heal et al reported that peak VO2 did not differ significantly between the 2 approaches.[23]
5. Limitations
This study has several limitations. First, there was heterogeneity in some results of our pooled estimates. The heterogeneity might be associated with different kinds of anomalies, different risk levels, and different types of Fontan procedures. We were unable to perform subgroup analyses stratified by these factors. Second, only 1 of the 14 studies was a prospective RCT, and the remaining studies were observational studies. Owing to the biases, such as design bias, selection bias, and treatment bias, inherent in observational studies, the results of this study might be restricted to some extent. Third, it is well known that all surgical outcomes mentioned earlier might be influenced by the surgeon's learning curve. Additionally, the Fontan procedure has undergone several significant modifications with regard to technique and material. Fourth, whether the fenestration was open was not confirmed using a catheter in all patients. Thus, our analysis is an intention-to-threat analysis. Fifth, some time-related parameters, such as mortality, are best analyzed with hazard ratios; however, as our evaluations were limited to the original research data, only ORs could be used. Considering these limitations, RCTs with patients stratified by the risk level and Fontan type are needed to confirm our findings.
6. Conclusion
Fenestration appears to result in a lower incidence of arrhythmia but with a longer CPB time and lower early SaO2. However, other outcomes are comparable between the fenestrated and non-fenestrated Fontan procedures.
Author contributions
Data curation: Dongxu Li, Mengsi Li.
Formal analysis: Dongxu Li, Mengsi Li.
Methodology: Dongxu Li, Xu Zhou.
Project administration: Qi An.
Supervision: Qi An.
Writing – original draft: Dongxu Li.
Writing – review & editing: Dongxu Li, Mengsi Li, Qi An.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CPB = cardiopulmonary bypass, ECC = extracardiac conduit, ICU = intensive unit care, ILT= intra lateral tunnel, MD = mean difference, OR = odds ratio, PAP = pulmonary artery pressure, PLE = protein-losing enteropathy, RCT = randomized control trial, SaO2 = oxygen saturation, SD = standard deviation, VO2 = oxygen consumption.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax 1971;26:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kreutzer GO, Vargas FJ, Schlichter AJ, et al. Atriopulmonary anastomosis. J Thorac Cardiovasc Surg 1982;83:427–36. [PubMed] [Google Scholar]
- [3].Jonas RA. The intra/extracardiac conduit fenestrated fontan. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2011;14:11–8. [DOI] [PubMed] [Google Scholar]
- [4].Bridges ND, Lock JE, Castaneda AR. Baffle fenestration with subsequent transcatheter closure. Modification of the Fontan operation for patients at increased risk. Circulation 1990;82:1681–9. [DOI] [PubMed] [Google Scholar]
- [5].Laks H. The partial Fontan procedure. A new concept and its clinical application. Circulation 1990;82:1866–7. [DOI] [PubMed] [Google Scholar]
- [6].Marcelletti C, Corno A, Giannico S, et al. Inferior vena cava-pulmonary artery extracardiac conduit. A new form of right heart bypass. J Thorac Cardiovasc Surg 1990;100:228–32. [PubMed] [Google Scholar]
- [7].Schreiber C, Kostolny M, Hörer J, et al. Can we do without routine fenestration in extracardiac total cavopulmonary connections? Report on 84 consecutive patients. Cardiol Young 2006;16:54–60. [DOI] [PubMed] [Google Scholar]
- [8].Schreiber C, Hörer J, Vogt M, et al. Nonfenestrated extracardiac total cavopulmonary connection in 132 consecutive patients. Ann Thorac Surg 2007;84:894–9. [DOI] [PubMed] [Google Scholar]
- [9].Harada Y, Uchita S, Sakamoto T, et al. Do we need fenestration when performing two-staged total cavopulmonary connection using an extracardiac conduit. Interact Cardiovasc Thorac Surg 2009;9:50–4. [DOI] [PubMed] [Google Scholar]
- [10].Airan B, Sharma R, Choudhary SK, et al. Univentricular repair: is routine fenestration justified. Ann Thorac Surg 2000;69:1900–6. [DOI] [PubMed] [Google Scholar]
- [11].Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349:g76471–25. [DOI] [PubMed] [Google Scholar]
- [12].Howick J. Oxford centre for evidence-based medicine: levels of evidence. Centre for Evidence Based Medicine. March 2009. Available from: Available at: http://www.cebm.net/index.aspx?o=1025 [access date July 20, 2018]. [Google Scholar]
- [13].Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d59281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. March 2011. Available at: http://handbook-5-1.cochrane.org/ [access date July 20, 2018]. [Google Scholar]
- [16].DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007;28:105–14. [DOI] [PubMed] [Google Scholar]
- [17].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Egger M, Davey Smith G, Schneider M, et al. Bias in metaanalysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ono M, Boethig D, Goerler H, et al. Clinical outcome of patients 20 years after Fontan operation--effect of fenestration on late morbidity. Eur J Cardiothorac Surg 2006;30:923–9. [DOI] [PubMed] [Google Scholar]
- [20].Atz AM, Travison TG, McCrindle BW, et al. Late status of Fontan patients with persistent surgical fenestration. J Am Coll Cardiol 2011;57:2437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lemler MS, Scott WA, Leonard SR, et al. Fenestration improves clinical outcome of the fontan procedure: a prospective, randomized study. Circulation 2002;105:207–12. [DOI] [PubMed] [Google Scholar]
- [22].Atik E, Ikari NM, Martins TC, et al. Fontan operation and the cavopulmonary technique: immediate and late results according to the presence of atrial fenestration. Arq Bras Cardiol 2002;78:162–6. [DOI] [PubMed] [Google Scholar]
- [23].Heal ME, Jackson LB, Atz AM, et al. Effects of persistent Fontan fenestration patency on cardiopulmonary exercise testing variables. Congenit Heart Dis 2017;12:399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bridges ND, Mayer JE, Jr, Lock JE, et al. Effect of baffle fenestration on outcome of the modified Fontan operation. Circulation 1992;86:1762–9. [DOI] [PubMed] [Google Scholar]
- [25].Fu S, Valeske K, Akinturk H, et al. Fontan extracardiac tunnel connection: fenestration or not. Chin Med J (Engl) 2009;122:2335–8. [PubMed] [Google Scholar]
- [26].Hsia TY, Khambadkone S, Redington AN, et al. Effect of fenestration on the sub-diaphragmatic venous hemodynamics in the total-cavopulmonary connection. Eur J Cardiothorac Surg 2001;19:785–92. [DOI] [PubMed] [Google Scholar]
- [27].Salazar JD, Zafar F, Siddiqui K, et al. Fenestration during Fontan palliation: now the exception instead of the rule. J Thorac Cardiovasc Surg 2010;140:129–36. [DOI] [PubMed] [Google Scholar]
- [28].Smaś-Suska M, Róg B, Weryński P, et al. Long-term effects of percutaneous fenestration following the fontan procedure in adult patients with congenital univentricular heart. Med Sci Monit 2018;24:3506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fan F, Liu Z, Li S, et al. Effect of fenestration on early postoperative outcome in extracardiac fontan patients with different risk levels. Pediatr Cardiol 2017;38:643–9. [DOI] [PubMed] [Google Scholar]
- [30].Loomba RS, Danduran ME, Dixon JE, et al. Effect of Fontan fenestration on regional venous oxygen saturation during exercise: further insights into Fontan fenestration closure. Pediatr Cardiol 2014;35:514–20. [DOI] [PubMed] [Google Scholar]
- [31].Thompson LD, Petrossian E, McElhinney DB, et al. Is it necessary to routinely fenestrate an extracardiac fontan. J Am Coll Cardiol 1999;34:539–44. [DOI] [PubMed] [Google Scholar]
- [32].Fiore AC, Tan C, Armbrecht E, et al. Comparison of fenestrated and nonfenestrated patients undergoing extracardiac Fontan. Ann Thorac Surg 2014;97:924–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


