Abstract
Rationale:
Granulocyte colony-stimulating factor (G-CSF) is most frequently used in healthy donors to mobilize progenitor cells into the peripheral blood for collection. While mild thrombocytopenia is common in allogeneic peripheral blood stem cell transplant donors after G-CSF mobilization, serious thrombocytopenia is rarely reported. Herein, we report a case of severe thrombocytopenia caused by G-CSF in a 14-year-old healthy donor and review the relevant literature. To our knowledge, this is the first reported case of severe thrombocytopenia caused by G-CSF in a healthy adolescent donor.
Patient concerns:
A 14-year-old sister of a girl with T lymphocyte leukemia was selected as a matched donor for transplantation. The donor was healthy with normal blood parameters.
Diagnoses:
The donor received 10 μg/kg/day G-CSF via subcutaneous injection. On day 4 of G-CSF administration, blood tests before stem cell collection indicated that platelets dropped to 51 g/L. Abdominal ultrasound showed that the spleen was mildly enlarged.
Interventions:
In order to prevent blood loss and other effects caused by a too low platelet count after collection, the donor's peripheral blood hematopoietic stem cells were collected after platelet transfusion.
Outcomes:
Checkups for 1 year after G-CSF administration showed normal blood parameters.
Lessons:
Due to the rare risk of severe thrombocytopenia in G-CSF mobilization, it is necessary to routinely monitor blood parameters during mobilization to ensure smooth progress of the transplantation process.
Keywords: granulocyte colony-stimulating factor, healthy donor, thrombocytopenia
1. Introduction
Granulocyte colony-stimulating factor (G-CSF) is most frequently used for mobilizing progenitor cells into the peripheral blood for collection in healthy donors.[1,2] Common side effects of G-CSF include bone and muscle pain, headache, fever, and inflammation at the injection site. Serious complications include thrombosis, allergic reactions, and spleen rupture.[3,4] While mild thrombocytopenia is common in allogeneic peripheral blood stem cell transplant donors after G-CSF mobilization, serious thrombocytopenia is rarely reported. Herein, we report a case of severe thrombocytopenia caused by G-CSF in a 14-year-old healthy donor and review the relevant literature. To the best of our knowledge, this is the first reported case of severe thrombocytopenia caused by G-CSF in a healthy adolescent donor.
2. Case report
This study was approved by the Ethics Committee of Tongji Medical college, Huazhong University of Science and Technology, Wuhan, China. The patient has given informed consent to publish these case details.
The 14-year-old sister of a girl with T lymphocyte leukemia was selected as a matched donor for transplantation. The donor was previously healthy and had no history of surgery, trauma, infectious diseases, or drug allergies. Clinical examination, chest computed tomography, electrocardiogram, abdominal ultrasound, blood chemistry, and urine analysis results were normal. Serology was negative for hepatitis B and C viruses, and IgG was negative for cytomegalovirus and Epstein–Barr virus.
The donor received 10 μg/kg/day G-CSF for 6 days via subcutaneous injection. Blood test results before donor mobilization were within the normal range (white blood cells (WBC), 8.35 G/L; hemoglobin, 147 g/L; platelets (PLT): 169 G/L). Routinely, blood parameters are not continuously monitored. On day 4 of G-CSF administration, blood tests before stem cell collection indicated that platelets dropped to 51 g/L (WBC, 42.47 G/L; hemoglobin, 137 g/L; PLT: 51 G/L). Clinical examination showed the following: temperature, 36.7°C; pulse, 79 bpm; breath, 18 bpm; heart rate, 80 bpm; and blood pressure, 115/76 mm Hg. No positive findings were found upon palpation. Abdominal ultrasound showed that the spleen was slightly enlarged, being 12.4 cm long and 3.6 cm thick (Fig. 1). Screening for autoimmune diseases was negative. Repeated blood tests showed that platelets were still significantly reduced (PLT, 47 G/L). In order to prevent blood loss and other effects caused by a too low platelet count after collection, the donor's peripheral blood hematopoietic stem cells were collected after a platelet transfusion. The collection process using a continuous flow blood cell separator (COBE Spectra, Lakewood, CO, USA) was smooth. The PLT count was 58 G/L afterwards. Since this was a haploid transplant, to ensure a smooth transplant process, peripheral blood hematopoietic stem cells were collected for the second time. The PLT count reduced to 42 G/L. On day 7 after collection, PLT count slightly increased to 66 G/L, while, on day 11, the PLT count rose to the normal range (185 G/L). WBC and PLT counts are shown in Figure 2. Ultrasonic measurement of spleen size indicated no change in size (length, 12.7 cm; Thickness, 3.6 cm). Checkups for 1 year after G-CSF administration showed normal blood parameters and spleen size. In addition, the transplant recipient's stem cells were well implanted and the recipient was healthy at the time.
Figure 1.
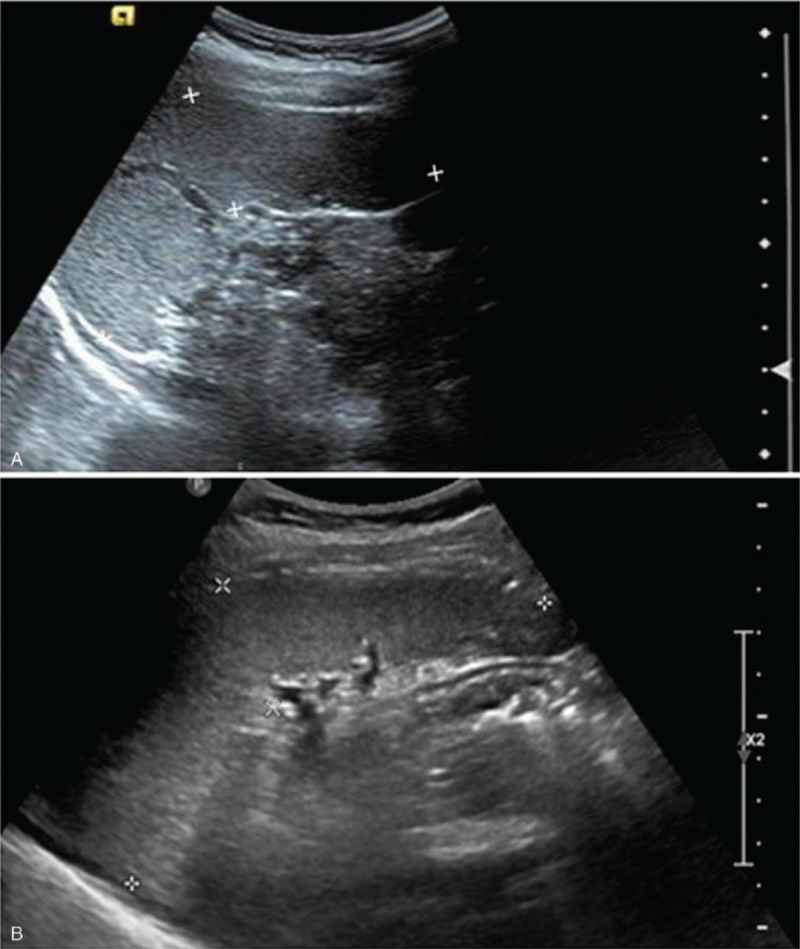
Ultrasonic image of spleen. A, before mobilization; B, after mobilization.
Figure 2.
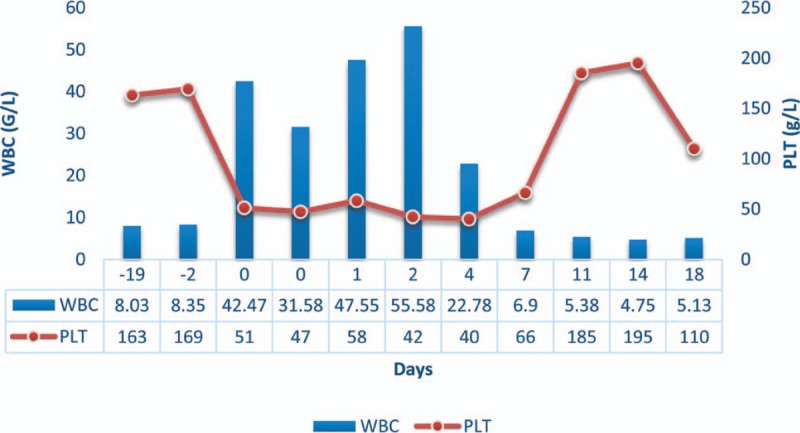
WBC and PLT counts. gr2PLT = platelets, WBC = white blood cells.
3. Discussion
Allogeneic hematopoietic stem cell transplantation is the preferred treatment for many malignant hematologic diseases. G-CSF is used as a mobilizing agent for healthy donors to rapidly increase peripheral blood stem cells (PBSCs) in a short period of time so as to collect the cells with a continuous flow blood cell separator. In the past 20 years, PBSCs mobilized by G-CSF have been widely used in stem cell transplantation instead of bone marrow-derived stem cells. After the clinical application of G-CSF, several studies have confirmed its safety and effectiveness in mobilizing hematopoietic stem cells for allogeneic transplantation.[5] Hölig et al [4] followed up 3928 healthy donors for 12 years and found that the adverse reactions of G-CSF used in healthy donors were slight, mainly in the form of bone pain (93.5%), and headache (34%). Another prospective study analyzed 2408 adverse events of healthy donors of peripheral blood stem cells from 1999 to 2004[3] and found that only 15 cases (0.6%) were accompanied by serious complications, among which the complications related to G-CSF medication were severe headache and nausea.
Several studies have shown that G-CSF-mobilized healthy donors can show a slight drop in PLT count (P < .05), while remaining within the normal range (125–350 G/L).[1,4] The exact cause of this drop is not clear. It is considered that this may be related to insufficient expression of proliferation-related genes in megakaryocytes, such as PF4 and PTFN4.[6] However, cases of serious thrombocytopenia directly caused by G-CSF are rarely reported. In 1993, Wun et al[7] reported a case of a significant decrease in PLT count after G-CSF mobilization. On the first day of medication, the PLT count dropped to 50 G/L, but gradually recovered to the normal range within 65 days after G-CSF application. Notably, the patient suffered from Felty Syndrome, a triad of rheumatoid arthritis, splenomegaly, and neutropenia. Ultrasonic examination showed that the spleen was enlarged, being 17.3 cm in length; on the 60th day, ultrasonic examination showed that the length of the spleen reduced to 13.9 cm. The author considered that a significant decrease in PLT count after G-CSF mobilization was closely related to the Felty syndrome. In 2007, Kovacic et al[8] reported a case of profound thrombocytopenia in a 57-year-old male with refractory cardiac ischemia, who received G-CSF during an angiogenesis trial. The platelet count was 71 G/L at the beginning of the treatment and gradually decreased to the lowest point of 5 G/L, without splenomegaly. They found that the anti-PLT antibodies were weakly positive, possibly because G-CSF aggravates immune-mediated thrombocytopenia. The mechanism may consist of G-CSF leading to hypersplenism and extensive activation of the reticuloendothelial system, which in turn increases PLT consumption.
Healthy donors without autoimmune diseases also experienced thrombocytopenia after G-CSF mobilization. In 2009, Minelli et al[9] reported a 51-year-old healthy male donor. The PLT count decreased on day 1 after G-CSF mobilization and was 78 G/L on day 5, decreasing to 30 G/L after collection. On day 7, since the PLT count was 27 G/L, the collection procedure was suspended, and the PLT count was monitored. On day 9 day after terminating G-CSF treatment, the PLT count returned to the normal range. The author believed that the significant decrease in the PLT count during G-CSF administration was directly related to the treatment because the decrease had been observed before the start of the collection, there was no splenomegaly during the whole process, and all antibodies related to autoimmune diseases were negative; the only positive finding was the donor's history of allergies to certain drugs, which the author considered as a possible cause.
Our case is a 14-year-old healthy donor, whose examination prior to transplantation showed that the spleen was normal in size and that there was no autoimmune disease. Moreover, the donor had no previous history of disease or drug allergy. After analyzing this case with respect to existing literature, we recognized that there is a risk of serious PLT decline after G-CSF mobilization among hematopoietic stem cell transplant donors. Before this case occurred, the center did not strictly monitor the blood test of healthy transplant donors, only the blood indexes before mobilization and collection. This case has prompted us to exert more caution when choosing donors. Spleen enlargement, allergic history of drugs, or history of immune system diseases all appear to increase the risk of severe thrombocytopenia. Before mobilization, it is necessary to explain the possible complications in more detail to the donors and their families, and it is necessary to monitor the donor's blood parameters closely during the mobilization process. Once an obvious drop in donor PLT is detected, early clinical intervention is needed to ensure the safety of the donor.
Notably, when thrombocytopenia occurred in the donor in our case, the recipient had already completed the pretreatment. Had the hematopoietic stem cell infusion not been able to be completed, the recipient would have been exposed to extremely high risk. Therefore, when the donor has serious thrombocytopenia and chooses the treatment plan and timing, the current situation of both the donor and the recipient should be fully considered.
4. Conclusion
Although PLT count reduction caused by G-CSF is rare, when it does occur, it may put the donor as well as the recipient to extremely high risk. Therefore, clinicians ought to monitor the PLT count of the donor during G-CSF mobilization to ensure smooth progress of the transplantation process.
Author contributions
Funding acquisition: Xuan Lu, Yu Wu.
Writing – original draft: Xuan Lu, Yu Wu.
Writing – review & editing: Huafang Wang, Linghui Xia.
Footnotes
Abbreviations: G-CSF = granulocyte colony-stimulating factor, PBSCs = peripheral blood stem cells.
Xuan Lu and Yu Wu contributed equally to this work.
This study was supported by National Natural Science Foundation of China (Grants: 81500109, 81601540). The authors declare they have no conflicts of interest to declare with respect to this manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].de la Rubia J, de Arriba F, Arbona C, et al. Follow-up of healthy donors receiving granulocyte colony-stimulating factor for peripheral blood progenitor cell mobilization and collection. Results of the Spanish Donor Registry. Haematologica 2008;93:735–40. [DOI] [PubMed] [Google Scholar]
- [2].Ozkan MC, Sahin F, Saydam G. Peripheral blood stem cell mobilization from healthy donors. Transfus Apher Sci 2015;53:13–6. [DOI] [PubMed] [Google Scholar]
- [3].Pulsipher MA, Chitphakdithai P, Miller JP, et al. Adverse events among 2408 unrelated donors of peripheral blood stem cells: results of a prospective trial from the National Marrow Donor Program. Blood 2009;113:3604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hölig K, Kramer M, Kroschinsky F, et al. Safety and efficacy of hematopoietic stem cell collection from mobilized peripheral blood in unrelated volunteers: 12 years of single-center experience in 3928 donors. Blood 2009;114:3757–63. [DOI] [PubMed] [Google Scholar]
- [5].Tvedt THA, Melve GK, Tsykunova G, et al. Immunological heterogeneity of healthy peripheral blood stem cell donors-effects of granulocyte colony-stimulating factor on inflammatory responses. Int J Mol Sci 2018;19:E2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hernández JM, Castilla C, Gutiérrez NC, et al. Mobilisation with G-CSF in healthy donors promotes a high but temporal deregulation of genes. Leukemia 2005;19:1088–91. [DOI] [PubMed] [Google Scholar]
- [7].Wun T. The Felty syndrome and G-CSF-associated thrombocytopenia and severe anemia. Ann Intern Med 1993;118:318–9. [DOI] [PubMed] [Google Scholar]
- [8].Kovacic JC, Macdonald P, Freund J, et al. Profound thrombocytopenia related to G-CSF. Am J Hematol 2007;82:229–30. [DOI] [PubMed] [Google Scholar]
- [9].Minelli O, Falzetti F, Di Ianni M, et al. G-CSF-induced thrombocytopenia in a healthy donor. Bone Marrow Transplant 2009;43:263–4. [DOI] [PubMed] [Google Scholar]


