Supplemental Digital Content is available in the text
Keywords: dapagliflozin, meta-analysis, monotherapy, type 2 diabetes
Abstract
Background:
Dapagliflozin, a novel inhibitor of sodium-glucose cotransporter-2 (SGLT-2), lowers blood glucose level by specifically inhibiting the activity of SGLT-2. Previous studies showed efficacy and safety of dapagliflozin combined with other antihyperglycemic agents in type 2 diabetes (T2DM), however, there are few studies for dapagliflozin as monotherapy. The aim of this study was to assess the efficacy and safety of dapagliflozin as a monotherapy in T2DM and provide theoretical basis for clinical rational use of drugs.
Methods:
We did a systematic review and meta-analysis of randomized, placbo-controlled clinical studies in patients with type 2 diabetes. We searched PubMed, Embase, Cochrane Library, CNKI, Wanfang, and VIP database through October 2018, we also manually screened list of references to the previous meta-analysis of dapagliflozin in the treatment of type 2 diabetes. Data search and extraction were completed with a standardized data form and any discrepancies were resolved by consensus. A meta-analysis was conducted by using RevMan 5.3 software.
Results:
Six randomized controlled trials (RCTs) including 2033 patients were analyzed. Compared with placebo, dapagliflozin monotherapy was associated with a reduction in glycosylated hemoglobin A1c (HbA1c) (weighted mean difference [WMD]: –0.60%; 95% confidence interval [CI]: –0.67%, –0.52%; P < .00001), fasting plasam glucose (FPG) (WMD: –1.30 mmol/L; 95% CI: –1.52, –1.08; P < .00001), and body weight (WMD: –1.50 kg; 95% CI: –1.67, –1.32; P < .00001). Dapagliflozin was associated with an increased risk of urinary tract infections (relative risk [RR]: 1.74; 95% CI: 1.21, 2.49; P = .003) and genital tract infections (RR: 3.52; 95% CI: 2.06, 6.03; P < .00001).
Conclusions:
Dapagliflozin monotherapy was well tolerated and effective in reducing the level of HbA1c, FPG, and body weight in patients with T2DM without increasing hypoglycaemia, although it may increase the risk of urinary tract infections and genital tract infections. This meta-analysis provides an evidence for the treatment in patients with T2DM. However, more randomized clinical evidences are still needed to verify the results.
1. Introduction
As the global population ages, the incidence of diabetes is increasing year by year and people have paid more and more attention to the treatment of the disease. It is estimated that there are 451 million (age 18–99 years) people with diabetes in the world in 2017, this figure was expected to increase to 693 million by 2045.[1] Diabetes mellitus is a chronic metabolic disease characterized by insulin secretion defects, insulin resistance, and a progressive loss of β-cell function, which causes an increase in plasma glucose levels.[2] Hyperglycaemia is associated with microvascular and macrovascular complications in people with diabetes, the incidence and severity of these complications can be reduced by early and sustained glycaemic control.[3] Therefore, the aim of lowering blood glucose is to reduce the risk of long-term complications such as the damage to eyesight and kidneys of diabetes. At present, different kinds of antidiabetic agents including metformin, sulphonylureas, thiazolidinediones, alpha-glucosidase inhibitors, dipeptidyl peptidase-4 inhibitors, and glucagon-like peptide-1 utilize different mechanisms to lower blood glucose level in patients with type 2 diabetes (T2DM) inadequately controlled by diet and exercise. However, most of them are dependent on insulin secretion or function, it is usually insufficient to achieve or maintain glycaemic goals with the progress of diabetes and progressive loss of β-cell function. Treatment for T2DM requires new mechanisms of action and synergistic drugs.
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors have been proposed as a novel therapeutic strategy for diabetes. The SGLT-2 that specifically expressed in the proximal S1 segment of renal tubular mediates glucose reabsorption in the early proximal tubule and almost 90% of glucose reabsorption by the kidney. SGLT-2 inhibitors can reduce renal glucose reabsorption in the proximal convoluted tubule by specifically inhibiting the activity of SGLT-2, leading to increased urinary glucose excretion.[4–6] Many members of SGLT-2 inhibitors currently are in varying stages of clinical development, and the drugs already approved in Europe including canagliflozin, empagliflozin, and dapagliflozin. The recommended starting dose of canagliflozin is 100 mg once a day in Europe, the mean absolute oral bioavailability is approximately 65%, peak plasma concentrations of canagliflozin occurs within 1 to 2 hours post-dose, and the half-life was 10.6 hours. The recommended starting dose of empagliflozin is 10 mg once a day in Europe, after oral administration, peak plasma concentrations of empagliflozin were reached at 1.5 hours post-dose, and the half-life was 12.4 hours. The recommended dose of dapagliflozin is 10 mg once daily in Europe, while the recommended starting dose in the United States and China is 5 mg. It is rapidly absorbed after oral administration, achieving maximal plasma concentrations within 2 hours, the oral bioavailability following the administration of a 10 mg dose is 78%, and the mean half-life was 12.9 hours. Dapagliflozin, a highly selective SGLT-2 inhibitor, was approved as a monotherapy or add-on therapy for T2DM in the European Union in November 2012 and in the USA in January 2014, it was also approved by China's State Food and Drug Administration in 2017. Previous studies[7,8] showed that dapagliflozin is effective in reducing HbA1c, FPG, and body weight in models of T2DM and in clinical trials with less hypoglycemic events. We found dapagliflozin monotherapy is effective in glucose control and patients have good adherence due to easy way to use (5–10 mg qd).[9–11]
At present, there are many clinical studies and meta-analysis on dapagliflozin combined with metformin, sulfonylureas, insulin, and other hypoglycemic agents for the treatment of T2DM, but there are few meta-analysis on dapagliflozin as monotherapy for T2DM. In this review, we summarize the available data concerning the efficacy and safety of this novel antidiabetes therapeutic monotherapy.
2. Methods
2.1. Search strategy
We searched PubMed, Embase, Cochrane Library, CNKI, Wanfang, and VIP database (any date up to October 31, 2018, restricted to randomized clinical trials) with language restrictions in English and Chinese, search terms used were “sodium–glucose co-transporter 2 inhibitor,” “SGLT2 inhibitor,” “dapagliflozin,” “efficacy,” and “monotherapy.” No attempt was made at identifying and retrieving unpublished studies.
2.2. Study selection criteria
All participants were men and women aged 18 to 80 years who were diagnosed with T2DM based on the standards criteria of American Diabetes Association (ADA).[11] Patients were required to be drug naive (defined as never received the prescription medication or received it for <24 weeks since the original diagnosis) at the enrollment visit. Randomized controlled trials (RCTs) included in this study were taken to evaluate the efficacy and safety of dapagliflozin as monotherapy in patients with T2DM. The trials should last for >12 weeks and the outcomes should include the changes of HbA1c, FPG, body weight from baseline. Patients were excluded if they had serious renal, endocrine, vascular, hematological, or oncological disease. Articles with significant shortcomings in the study protocol or data analysis were excluded.
2.3. Data extraction
Two investigators independently extracted data using a standardized tool. On the basis of the inclusion and exclusion criteria, the reviewers carefully scrutinized the baseline characteristics of participants, study design, daily dose of dapagliflozin, efficacy, and safety outcomes.
2.4. Quality assessment
All of the included studies were evaluated according to the Cochrane Collaboration's tool for assessing risk of bias that includes random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias.[12]
2.5. Statistical analysis
According to the Cochrane System Evaluator's Handbook, the data from different studies were transformed and combined, and then the meta-analysis was performed using RevMan software 5.3 (the software was released by the Cochrane Collaboration, the Nordic Cochrane Centre, Copenhagen, Denmark). I2 statistic was used to analyze the heterogeneity between different studies, P ≥ .1 showed no statistical heterogeneity between studies, and a fixed effect model was used, P < .1 indicates statistical heterogeneity between studies, which needs to be heterogeneous. We reduced the heterogeneity by the subgroup analysis. For continuous data including mean changes from baseline in HbA1c, body weight and FPG, we used weighted mean difference (WMD) and 95% confidence interval (95%CI), for dichotomous data like adverse events, while the outcomes were expressed as the relative risk (RR) and 95%CI. The difference was statistically significant when P < .05.
3. Results
3.1. Description of the studies
There were 431 possibly related studies obtained through the literature search, and 6 RCT studies finally included. The process of literature screening is shown in Supplementary Appendix 1. The main characteristics of the included studies are summarized in Table 1. These RCTs were published between 2009 and 2014. A total of 2033 participants were included, 470 patients were in the placebo group, and 1563 in the dapagliflozin groups with different doses. The study durations lasted from 12 to 24 weeks, the clinical characteristics were well matched for age, sex distribution, body mass index (BMI), HbA1c, FPG, and weight in all groups at the beginning of each study. The dapagliflozin was administered once per day in the morning in all trials,[13–18] at a dose of 1.0 to 50.0 mg, whereas the patients received dapagliflozin once daily in the evening in one of them.[16] All of the included studies compared dapagliflozin with placebo. All of the included studies were evaluated according to the Cochrane Collaboration's tool, the result were shown in Fig. 1(A–B).
Table 1.
Baseline characteristics of the study population included in the meta-analysis.
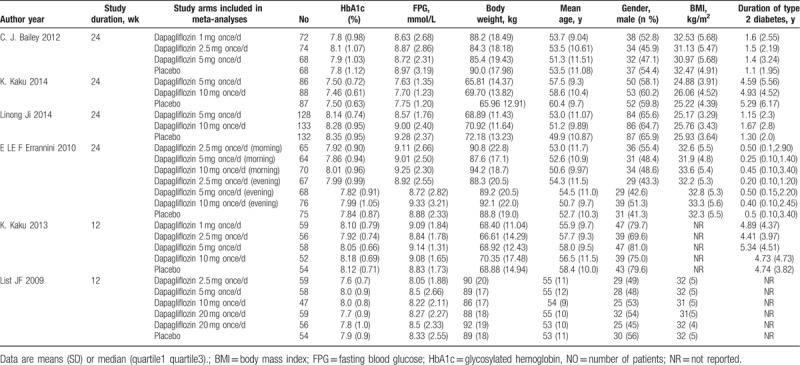
Figure 1.
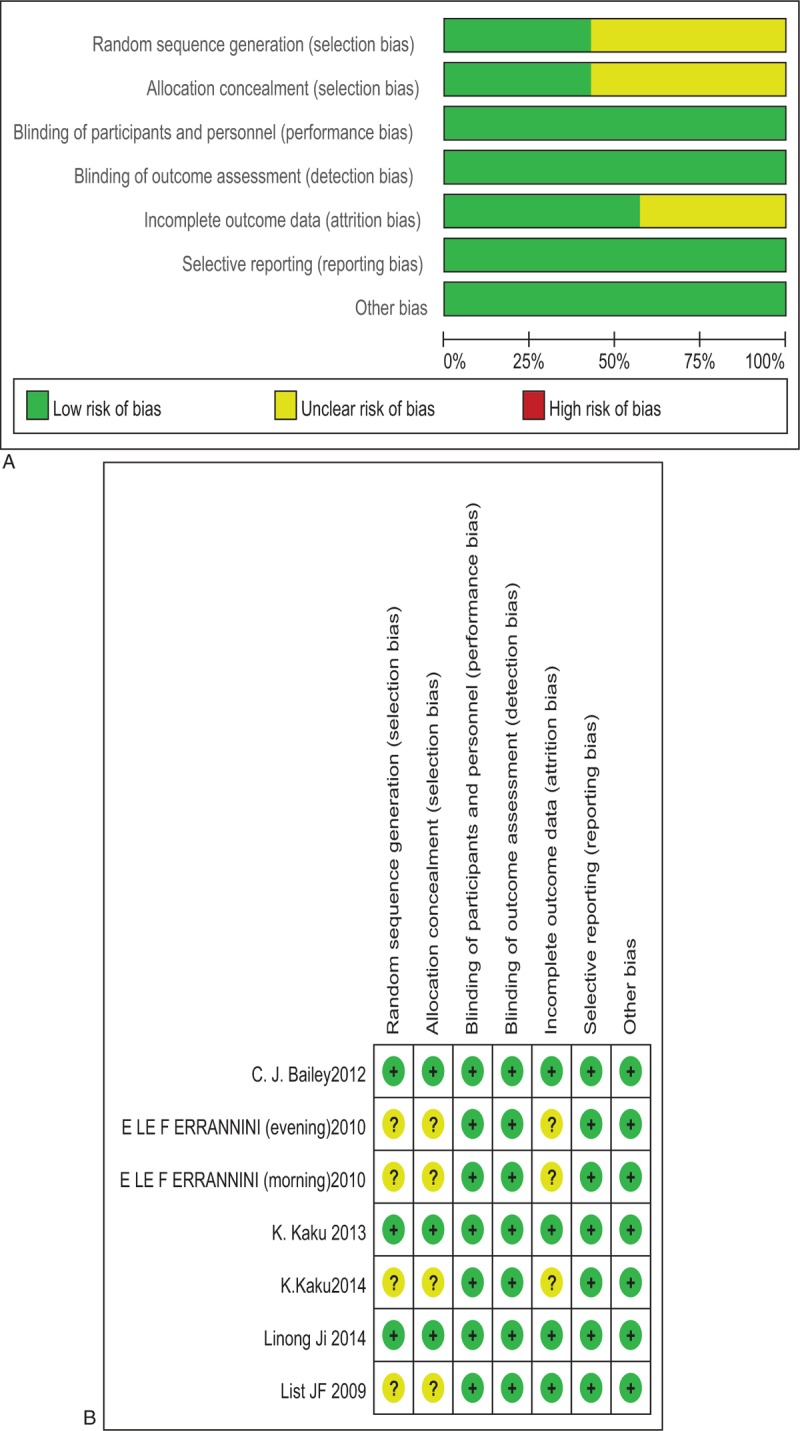
(A) Methodological quality graph. (B) Methodological quality summary.
3.2. Glycosylated hemoglobin A1c (HbA1c)
In all included studies,[13–18] dapagliflozin was compared with placebo, random effect models and the subgroup analysis were used to analyze this outcome because of the heterogeneity between the different groups. Six trials with total of 2033 patients provided data of HbA1c changed from baseline. The result shown that dapagliflozin produced greater mean reductions in HbA1c from baseline, compared with placebo (WMD: −0.60%; 95%CI: –0.67, –0.52; P < .00001, I2 = 51%). The subgroup analysis based on the dosage of dapagliflozin showed that, 1, 2.5, 5, and 10 mg/d dapagliflozin monotherapy lowered HbA1c compared with placebo (for 1 mg/d dapagliflozin: WMD: –0.55%; 95%CI: –0.74, –0.36; P < .00001; for 2.5 mg/d dapagliflozin: WMD: –0.52%; 95%CI: –0.63, –0.41; P < .00001; for 5 mg/d dapagliflozin: WMD: –0.61%; 95%CI: –0.75, –0.47; P < .00001; for 10 mg/d dapagliflozin: WMD: –0.65%; 95%CI: –0.81, –0.49; P < .00001) (Fig. 2).
Figure 2.
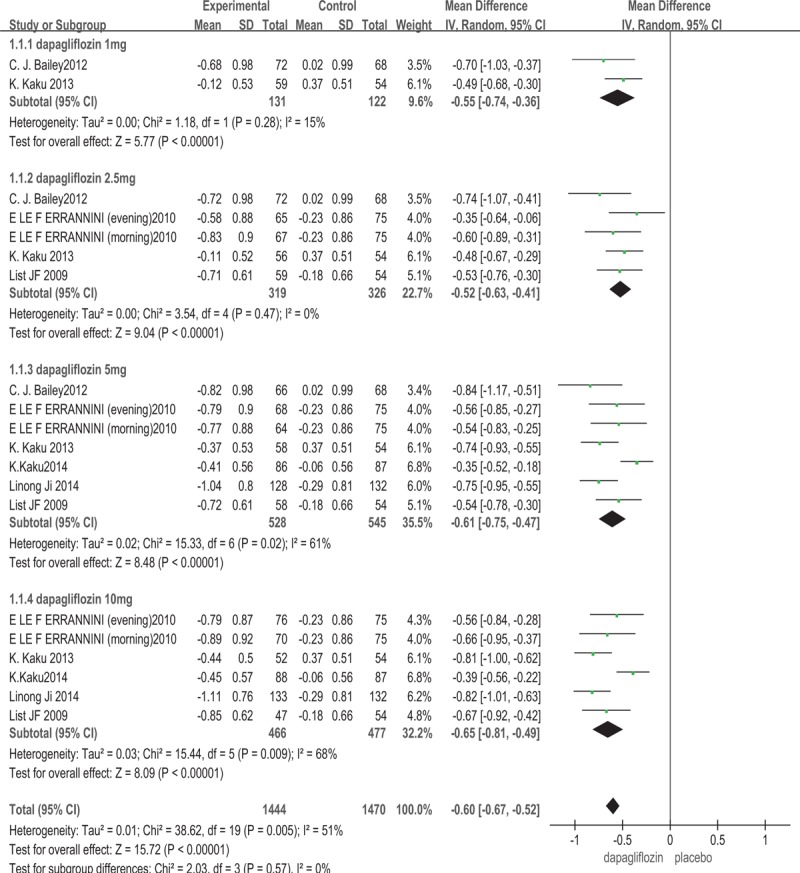
Glycosylated hemoglobin A1c (HbA1c): dapagliflozin versus placebo.
3.3. Fasting plasma glucose (FPG)
Six trials[13–18] measured the change from baseline of FPG. The results are shown in Fig. 3. Compared with placebo, dapagliflozin monotherapy was efficacy to control the level of FPG (WMD: –1.30 mmol/L; 95%CI: –1.52, –1.08; P < .00001, I2 = 75%). The subgroup analysis based on the dosage of dapagliflozin showed that, 1, 2.5, 5, and 10 mg/d dapagliflozin lowered FPG level compared with placebo (for 1 mg/d dapagliflozin: WMD: –1.19 mmol/L; 95%CI: –1.83, –0.56; P = .0002; for 2.5 mg/d dapagliflozin: WMD: –1.10 mmol/L; 95%CI: –1.57, –0.62; P < .00001; for 5 mg/d dapagliflozin: WMD: –1.29 mmol/L; 95%CI: –1.66, –0.93; P < .00001; for 10 mg/d dapagliflozin: WMD: –1.50 mmol/L; 95%CI: –1.95, –1.05; P < .00001).
Figure 3.
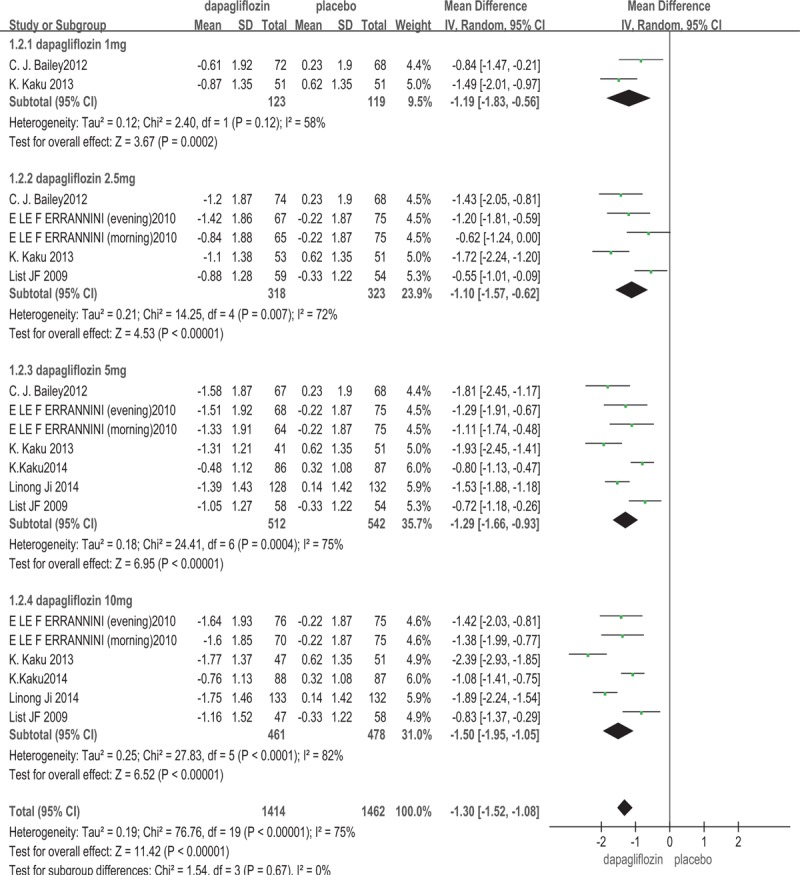
Fasting plasma glucose (FPG): dapagliflozin versus placebo.
3.4. Body weight
In this meta-analysis, all of the included studies[13–18] involving 2033 patients reported the change of body weight from baseline. The dapagliflozin was associated with a significant decrease in body weight compared with placebo (WMD: –1.50 kg; 95%CI: –1.67, –1.32; P < .00001, I2 = 0%). The subgroup analysis based on the dosage of dapagliflozin showed that, 1, 2.5, 5, and 10 mg/d dapagliflozin reduced body weight compared with placebo (for 1 mg/d dapagliflozin: WMD: –1.30 kg; 95%CI: –1.76, –0.84; P < .00001; for 2.5 mg/d dapagliflozin: WMD: –1.32 kg; 95%CI: –1.70, –0.94; P < .00001; for 5 mg/d dapagliflozin: WMD: –1.56 kg; 95%CI: –1.86, –1.26; P < .00001; for 10 mg/d dapagliflozin: WMD: –1.64 kg; 95% CI: –1.95, –1.33; P < .00001) (Fig. 4).
Figure 4.
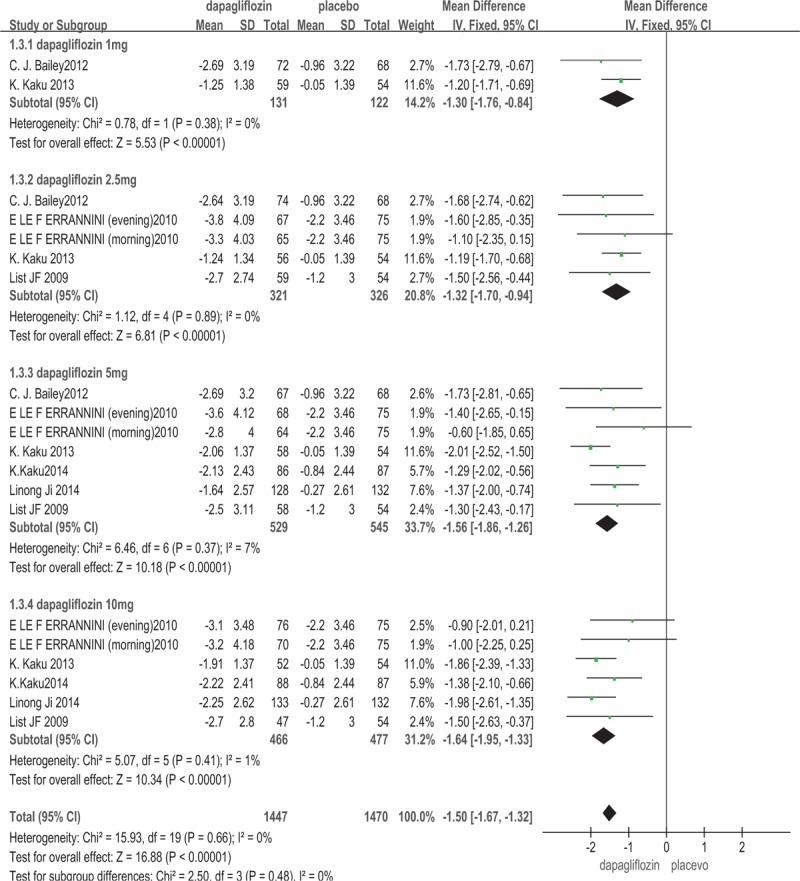
Body weight: dapagliflozin versus placebo.
3.5. Adverse events
3.5.1. Urinary tract infections (UTIs) and genital infections
All of the included studies[13–18] involving 2033 patients provided the data on the signs, symptoms, and events suggestive of urinary tract infections. Dapagliflozin increased the risk of UTIs (RR: 1.74; 95%CI: 1.21, 2.49; P = .003, I2 = 0%). Five trials[13,15–18] involving 1722 patients measured the signs, symptoms, and events that suggestive of genital infections like vulvovaginitis, balanitis, posthitis. The meta-analysis showed that dapagliflozin increased the risk of genital infections (RR: 3.52; 95%CI: 2.06, 6.03; P < .00001, I2 = 0%) (The outcome was shown in Figs. 5 and 6). Dapagliflozin increased the risk of UTIs and genital infections may be related to the drug's promotion of urine glucose excretion which can provide favorable conditions for bacterial and fungal reproduction of the genitourinary system.
Figure 5.
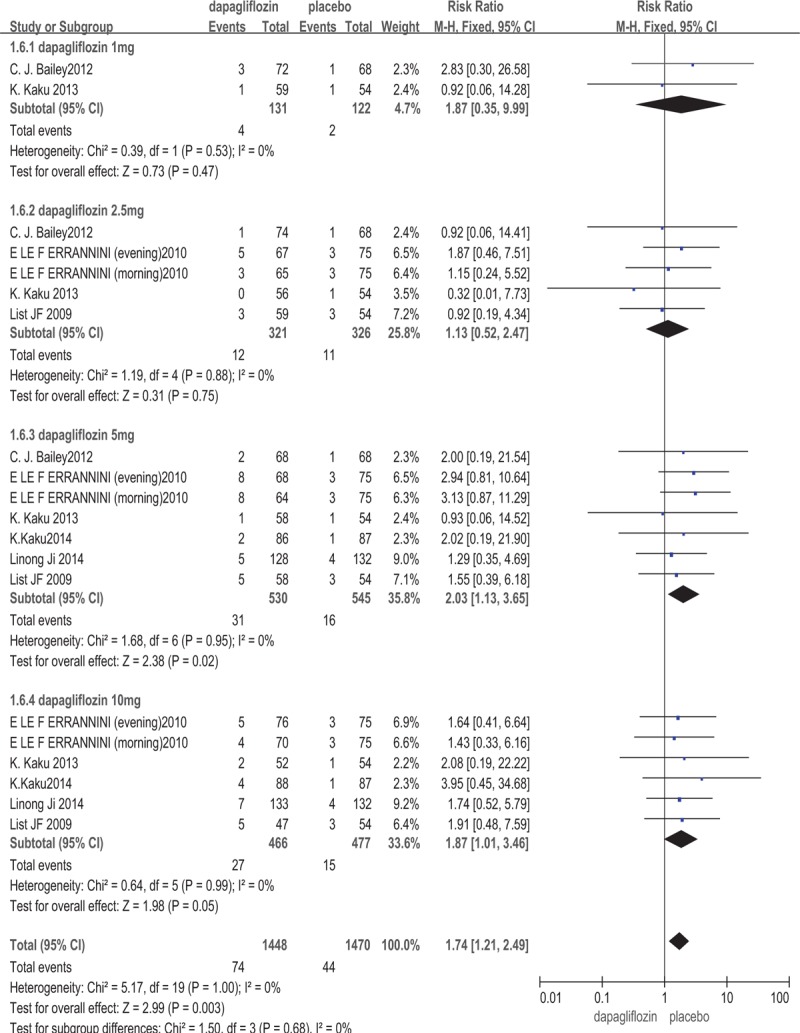
Urinary tract infections: dapagliflozin versus placebo.
Figure 6.
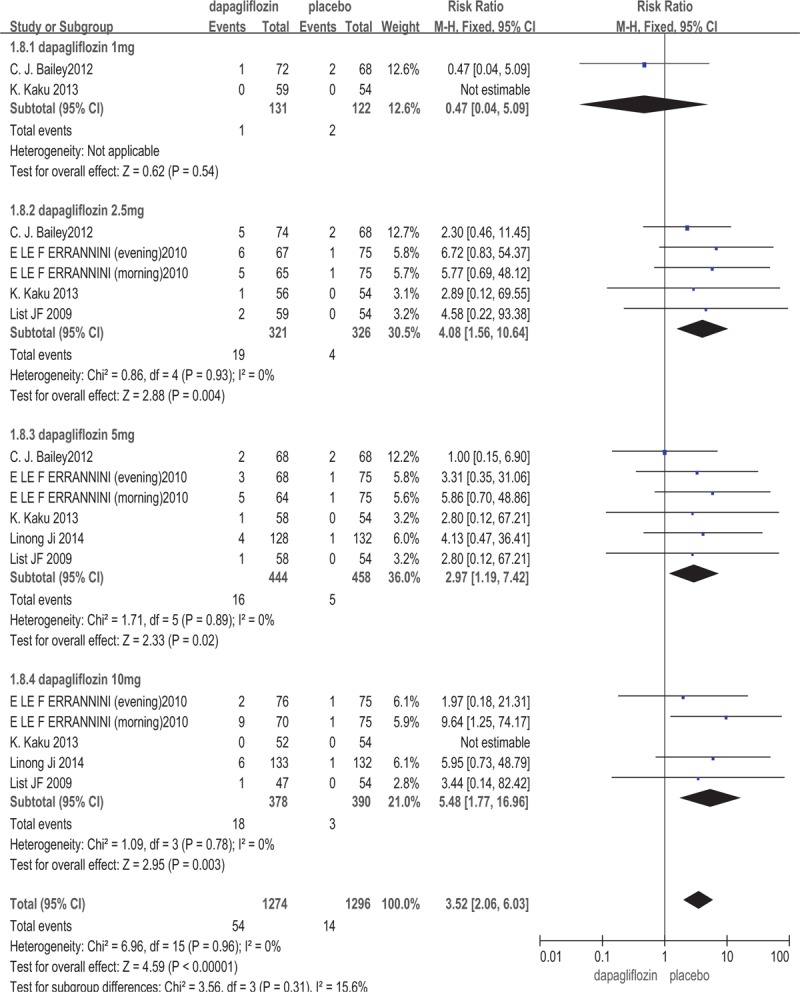
Genital infections: dapagliflozin versus placebo.
3.5.2. Hypoglycemia
Three studies[13,16,18] (n = 1100) included in the meta-analysis reported the data on the incidence of hypoglycemia. The result was shown in Supplementary Appendix 2. Dapagliflozin monotherapy did not cause hypoglycaemia (RR: 1.13; 95%CI: 0.62, 2.07; P = .68, I2 = 0%). Other study[8] has shown that dapagliflozin may increase the risk of hypoglycemia when combined with other hypoglycaemic drugs.
3.5.3. Headache
There are 4 trials[13,16,18] (n = 1379) included in this study reported the data of headache. The dapagliflozin group was similar to the placebo group in the risk of headache (RR: 0.96; 95%CI: 0.69, 1.35; P = .83, I2 = 0%). (Supplementary Appendix 3).
3.5.4. Nasopharyngitis
Five trials[13–17] involving 1700 patients reported adverse event of nasopharyngitis. This meta-analysis showed that dapagliflozin treatment did not increase the risk of nasopharyngitis (RR: 1.00; 95%CI: 0.78, 1.27; P = .97, I2 = 0%). (Supplementary Appendix 4).
3.5.5. Gastrointestinal symptoms
Different gastrointestinal symptoms like diarrhea, constipation, gastritis, gastroenteritis, and nausea were reported in the trials included in this study. There are 3 studies[15,16,18] (n = 1211) reported diarrhea, the result was shown in Supplementary Appendix 5, it showed that dapagliflozin monotherapy in patients with T2DM did not increase the proportion of diarrhea (RR: 1.33; 95%CI: 0.77, 2.30; P = .31, I2 = 18%), and other gastrointestinal symptoms cannot be meta-analyzed because of the limitation of the data.
3.5.6. Hypovolaemia
Three studies[13,16,18] (n = 1100) included in the meta-analysis reported the data on the signs, symptoms, and events suggestive of hypovolaemia such as dehydration. The dapagliflozin did not increase the risk of hypovolaemia versus placebo (RR: 0.74; 95%CI: 0.32, 1.71; P = .47, I2 = 0%). (Supplementary Appendix 6).
3.5.7. Other adverse events
Some other adverse events were mentioned in the articles included in this study. There is a study[13] included in this meta-analysis reported pharyngitis, dizziness, hypertriglyceridemia, and hypercholesterolemia; other 2 studies[14,15] reported renal impairment; 1 study[14] reported dental caries and another study[15] reported toothache, back pain, cough, thrombocytopenia, increased N-acetyl-β-D-glucosaminidase and blood creatine phosphokinase. The above symptoms occurred in both dapagliflozin groups and placebo groups except thrombocytopenia and increased blood creatine phosphokinase which only occurred in dapagliflozin groups. At present, there is no enough data to support the difference between the dapagliflozin and placebo about these adverse events. There is a study[19] reported a case on dapagliflozin tablets associated allergic reaction and fluid filled lesion on legs in a diabetic patient, and the causality of the event was analyzed by Naranjo's ADR probability scale and the score was 5, which can be a probable event. There are also reports on the increased risk of bladder cancer and breast cancer with dapagliflozin.
4. Discussion
There are numerous hypoglycemic drugs in clinical use, but most of them are associated with adverse effects such as hypoglycemia and weight gain, almost half of patients with T2DM still do not meet glycaemic targets.[20,21] SGLT2 inhibitors are a new therapeutic class of oral agents for the treatment of T2DM. The sodium-glucose cotransporter (SGLT) is a glucose transporter with multiple subtypes including SGLT1 to SGLT6,[22] SGLT1 and SGLT2 are involved in the glucose reabsorption process.[23–25] SGLT1 is a high-affinity, low-capacity glucose carrier, which mainly located in the small intestine brush border and the S3 segment of the renal distal tubule, responsible for 10% filtered glucose reabsorption.[25] SGLT2 is a low-affinity, high-capacity glucose transporter that is mainly expressed in the S1 segment of the renal proximal tubule, mediating 90% filtered glucose reabsorption.[6] Dapagliflozin, a novel inhibitor of renal sodium-glucose cotransporter 2, reduces renal glucose reabsorption, leading to urinary glucose excretion and a reduction in blood glucose levels.
Some studies have shown that dapagliflozin was well tolerated and effective as combination therapy, the drug can significantly reduce HbA1c, FPG and body weight without increase risk of hypoglycemia in patients with T2DM,[7,26–28] furthermore, it can reduce blood pressure levels through weight loss and its action as osmotic diuretic.[29–31] However, there are few reviews on dapagliflozin as monotherapy for type 2 diabetes, the aim of this study was to systematically evaluate the efficacy and safety of different doses of dapagliflozin monotherapy in the treatment of type 2 diabetes. The result of this study showed that dapagliflozin was associated with a significant decrease in HbA1c (P < .00001), FPG (P < .00001), and body weight (P < .00001) compared with placebo, it is consistent with previous clinical trials. In the case of dapagliflozin lowering blood pressure, no meta-analysis was performed in this study due to limited data. In terms of drug safety, this study showed that dapagliflozin increased the risk of UTI (P = .003) and genital infections (P < .00001), the mechanism by which dapagliflozin increase UTI and genital infections is unclear, it may be related to the increase in urine sugar, which provides a growth environment for bacteria and microorganisms. These studies showed that infections are usually mild to moderate and respond to standard antimicrobial treatment. While dapagliflozin was similar to placebo in increasing the risk of other adverse events including hypoglycemia (P = .68), headache (P = .83), nasopharyngitis (P = .97), diarrhea (P = .31), and hypovolaemia (P = .47). It has been reported that patients experienced a higher rate of hypoglycemic episodes when receiving dapagliflozin added to glimepiride or insulin versus placebo added to them,[27,32] but no patient discontinued study treatment as a result of hypoglycaemia. However, our study showed that dapagliflozin monotherapy does not increase the risk of hypoglycemic. It is considered that the increased risk of hypoglycemia may be related to insulin and glimepiride itself when dapagliflozin is combined with these drugs. Some studies have shown that patients with dapagliflozin have developed bladder cancer and breast cancer,[33,34] but this conclusion still needs further clinical trials to evaluate.
However, the literatures searched in this study were limited to Chinese and English, which leads to publication bias in the included studies. Since dapagliflozin is a new type of hypoglycemic agent, there are few studies on dapagliflozin monotherapy for type 2 diabetes, a total of 6 RCTs were included in the meta-analysis, and the sample size was small, the follow-up time was shorter, and the probability of bias was higher. The above conclusions still need more large-sample, long-course, multi-center clinical randomized controlled trials to evaluate.
5. Conclusion
Our meta-analysis indicates that dapagliflozin was well tolerated and significantly lowered HbA1c, FPG, and body weight as monotherapy in patients with T2DM for the first time. Patients with T2DM take a higher risk of genital infection, treatment with dapagliflozin increased urine glucose excretion, and increased the risk of genitourinary infections. However, considering the limitations of this study, further high quality clinical trials are needed to evaluate the relevance of adverse events and the safety of application in specific populations.
Author contributions
Conceptualization: Xia Xu.
Data curation: Wenyi Lyu.
Formal analysis: Miao Feng, Jue Wang.
Methodology: Haihong Lv, Songbo Fu.
Project administration: Haihong Lv.
Resources: Xia Xu, Jue Wang, Songbo Fu.
Software: Wenyi Lyu.
Writing – original draft: Miao Feng.
Writing – review & editing: Miao Feng, Haihong Lv.
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, FPG = fasting plasma glucose, HbA1c = glycosylated hemoglobin A1c, RCT = randomized controlled trial, RR = relative risk, SGLT-2 = sodium-glucose cotransporter-2, T2DM = type 2 diabetes, UTI = urinary tract infection, WMD = weighted mean difference.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271–81. [DOI] [PubMed] [Google Scholar]
- [2].Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest 2006;116:1802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].DeFronzo RA, Hompesch M, Kasichayanula S, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 2013;36:3169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med 2010;27:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vallon V, Platt KA, Cunard R, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 2011;22:104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kaku K, Maegawa H, Tanizawa Y, et al. Dapagliflozin as monotherapy or combination therapy in Japanese patients with Type 2 diabetes: an open-label study. Diabetes Ther 2014;5:415–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang M, Zhang L, Wu B, et al. Dapagliflozin treatment for type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Res Rev 2014;30:204–21. [DOI] [PubMed] [Google Scholar]
- [9].Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia 2017;60:215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wilson C. Diabetes: Dapagliflozin: an insulin-independent, therapeutic option for type 2 diabetes mellitus. Nat Rev Endocrinol 2010;6:531. [DOI] [PubMed] [Google Scholar]
- [11].Gilor C, Niessen SJ, Furrow E, et al. What's in a name? Classification of diabetes mellitus in veterinary medicine and why it matters. J Vet Intern Med 2016;30:927–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bailey CJ, Iqbal N, T’Joen C, et al. Dapagliflozin monotherapy in drug-naive patients with diabetes: a randomized-controlled trial of low-dose range. Diabetes Obes Metab 2012;14:951–9. [DOI] [PubMed] [Google Scholar]
- [14].Kaku K, Kiyosue A, Inoue S, et al. Efficacy and safety of dapagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise. Diabetes Obes Metab 2014;16:1102–10. [DOI] [PubMed] [Google Scholar]
- [15].Ji L, Ma J, Li H, et al. Dapagliflozin as monotherapy in drug-naive Asian patients with type 2 diabetes mellitus: a randomized, blinded, prospective phase III study. Clin Ther 2014;36:84.e9–100.e9. [DOI] [PubMed] [Google Scholar]
- [16].Ferrannini E, Ramos SJ, Salsali A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 2010;33:2217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kaku K, Inoue S, Matsuoka O, et al. Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: a phase II multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 2013;15:432–40. [DOI] [PubMed] [Google Scholar]
- [18].List JF, Woo V, Morales E, et al. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009;32:650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shibil Pk, Shinu C, Joseph E, et al. Case report on dapagliflozin tablet associated allergic reaction and fluid filled lesion on limbs in a tertiary care referral hospital – kerala. World J Pharm Res 2014;3:915–9. [Google Scholar]
- [20].Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006-2013. Diabetes Care 2017;40:468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–206. [DOI] [PubMed] [Google Scholar]
- [22].Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr 2003;89:3–9. [DOI] [PubMed] [Google Scholar]
- [23].Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res 2015;12:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rieg T, Masuda T, Gerasimova M, et al. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 2014;306:F188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vallon V. Molecular determinants of renal glucose reabsorption. Focus on “Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2”. Am J Physiol Cell Physiol 2011;300:C6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wieland D, Kellerer M, Cypryk K, et al. Efficacy and safety of dapagliflozin or dapagliflozin plus saxagliptin versus glimepiride as add-on to metformin in patients with type 2 diabetes. Diabetes Obes Metab 2018;20:2598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Araki E, Onishi Y, Asano M, et al. Efficacy and safety of dapagliflozin in addition to insulin therapy in Japanese patients with type 2 diabetes: results of the interim analysis of 16-week double-blind treatment period. J Diabetes Investig 2016;7:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rosenstock J, Vico M, Wei L, et al. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care 2012;35:1473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Weber MA, Mansfield TA, Cain VA, et al. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol 2016;4:211–20. [DOI] [PubMed] [Google Scholar]
- [30].Baker WL, Smyth LR, Riche DM, et al. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens 2014;8:262–75. [DOI] [PubMed] [Google Scholar]
- [31].Maliha G, Townsend RR. SGLT2 inhibitors: their potential reduction in blood pressure. J Am Soc Hypertens 2015;9:48–53. [DOI] [PubMed] [Google Scholar]
- [32].Yang YY, Chen S, Pan H, et al. Safety and efficiency of SGLT2 inhibitor combining with insulin in subjects with diabetes. Systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Reilly TP, Graziano MJ, Janovitz EB, et al. Carcinogenicity risk assessment supports the chronic safety of dapagliflozin, an inhibitor of sodium-glucose co-transporter 2, in the treatment of type 2 diabetes mellitus. Diabetes Ther 2014;5:73–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lin HW, Tseng CH. A review on the relationship between SGLT2 inhibitors and cancer. Int J Endocrinol 2014;2014:719578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


