Abstract
Background:
Recently, many studies have been carried out to investigate the clinicopathological significance of E-cadherin expression in thyroid cancer. However, the results remained inconsistent. In the present study, we performed a meta-analysis to evaluate the associations of E-cadherin expression with susceptibility and clinicopathological characteristics of thyroid cancer.
Methods:
Eligible studies were searched from Medicine, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), and Wanfang databases. The strength of associations between E-cadherin expression and susceptibility and clinicopathological features of thyroid cancer were assessed by pooled odds ratios (ORs) and 95% confidence intervals (CIs).
Results:
Forty-six studies with 1700 controls and 2298 thyroid cancer patients were included for this meta-analysis. Pooled results indicated that E-cadherin expression was significantly associated with susceptibility of papillary cancer and follicular cancer (papillary cancer, ORs = 14.31, 95% CIs = 3.42–59.90; follicular cancer, ORs = 10.14, 95% CI = 4.52–22.75). Significant association between E-cadherin expression and thyroid cancer risk was also observed in the subgroup analysis based on control group (normal thyroid tissue, ORs = 28.28, 95% CI = 8.36–95.63; adjacent thyroid tissue, ORs = 8.83, 95% CI = 3.27–23.85; benign thyroid tissue, ORs = 43.96, 95% CI = 9.91–194.95). In addition, E-cadherin expression was significantly correlated with lymph node metastasis, differentiation, and tumor-node-metastasis (TNM) stage of thyroid cancer (lymph node metastasis, ORs = 3.21, 95% CI = 1.98–5.20; differentiation, ORs = 0.25, 95% CI = 0.07–0.82; TNM stage, ORs = 4.85, 95% CI = 2.86–8.25).
Conclusions:
The present study showed that E-cadherin expression was significantly associated with susceptibility and clinicopathological characteristics of thyroid cancer, which suggested that E-cadherin expression might be a potential predictive factor for clinical progression of thyroid cancer.
Keywords: E-cadherin, expression, meta-analysis, thyroid cancer
1. Introduction
Thyroid carcinoma is the most common endocrine malignancy, and accounts for approximately 1% of human cancers.[1] In the past few years, the incidence of thyroid cancer has tripled.[2] Thyroid malignancies were classified into papillary thyroid cancer (PTC), poorly differentiated thyroid cancer, follicular thyroid cancer (FTC), and anaplastic thyroid cancer. Most thyroid cancer patients were PTC and FTC, which were classified as differentiated thyroid cancer (DTC).[3] In the treatment of DTC, conventional therapies such as surgery, radioactive iodine, thyroid hormone therapy, and chemotherapy, were commonly used. However, some of these therapeutic options were harmful for the human body.[4] Therefore, many studies have been carried out to explore the molecular pathogenesis mechanism of thyroid cancer, and establish relevant targeted therapies. It has been reported that epigenetic factors, genetic factors, age, gender, radiation exposure, geographical region, and histological type increased the susceptibility of thyroid cancer.[5] Many genetic alterations have been identified and played fundamental role in the tumorigenesis of thyroid cancer. A prominent example was that T1799A mutation of BRAF gene was found in 45% of PTC, which leaded to the expression of BRAFV600E protein and caused the activation of serine/threonine kinase.[6] And rare mutations of BRAE gene were also detected in some thyroid cancer patients. BRAFV600E protein maintained the growth of thyroid tumor cells, which was demonstrated in xenograft tumor model.[7,8] The results of clinical studies showed V600E mutation of BRAF gene was significantly associated with clinicopathological outcomes of PTC, which was demonstrated in a large meta-analysis.[9,10] Second in prevalence to BRAF mutations of thyroid cancer was RAS mutation, which caused the inactivation of GTPase. When RAS was bounded to GTP, the complex leaded to the hydrolysis of GTP and converted GTP into GDP. Published studies have found RAS activated the signal pathway of PI3K–AKT in thyroid cancer, and had significant associations with AKT phosphorylation.[11,12] Mutations of PTEN and AKT gene were common genetic alterations of PI3K–AKT signal pathway, and were often observed in thyroid tumorigenesis.[13,14] In addition, other genes such as: β-catenin (CTNNB1), epidermal growth factor receptor, isocitrate dehydrogenase 1, TP53, and anaplastic lymphoma kinase were also detected in the studies of thyroid carcinoma.[15–19] These genes mostly were involved in PI3K–AKT signaling pathway, NF-κB signaling pathway, RASSF1–MST1–FOXO3 signaling pathway, WNT–β-catenin signaling pathway, and HIF1α pathway, which revealed dysregulation of proliferation, apoptosis, and metabolism of thyroid tumor cells. In recent years, in order to clarify molecular mechanism of thyroid cancer and develop molecular targeted therapies, many studies have focused on the epigenetics modifications of thyroid cancer. Promoter hypermethylation of PTEN gene was found in about 50% papillary carcinomas and almost 100% of follicular carcinomas, which suggested that it was complicated in the thyroid tumorigenesis.[20] In addition, aberrant methylation of TIMP3, DAPK, RARβ2, and SLC5A8 were correlated with extrathyroidal invasion, tumor stage, and lymph node metastasis of thyroid neoplasms.[21] In addition to aberrant gene methylation, some studies have found aberrant pattern of histone modifications was associated with the clinical progression of thyroid cancer. For instance, EZH2 and SMYD3 were overexpressed in the tissue of thyroid cancer, and EZH2 could lead to trimethylation of histone H3 lysine 27.[22] In the meantime, histone methyltransferase encoding by SMYD3, was involved in the growth of cancer cells and was correlated with metastasis of cancer cells.[23] Although no structural mutations were detected in these genes, the levels of the genes expression have changed. In the past few years, many studies were carried out to explore the association between relevant gene expression and hallmarks of thyroid cancer. However, no effective biomarkers were observed and used in the early diagnosis and treatment of thyroid cancer.
E-cadherin protein, a single-pass transmembrane glycoprotein, was transcribed from CDH1 gene and was responsible for the adhesion of epithelial cells.[24] Calcium binded to the interdomain junctions such as: DXD, DXNDNAPXF, DRE, and further rigidified the ectodomain of E-cadherin protein, which stabilized the proteins and allowed its proper localization.[25] In recent years, many literatures have developed the notion that E-cadherin played an important role in the invasion and metastasis of tumor cells.[26,27] From study of breast cancer, scientists have found expression of E-cadherin protein in breast cancer tissues with metastases was higher than their primary counterparts.[28] Furthermore, the repression of E-cadherin protein was also observed in metastatic cells of prostate cancer when these cancer cells colonized another site.[29] Several studies have found that E-cadherin protein was involved in the invasion and metastasis of thyroid cancer, and the associations have been investigated in some published literatures. However, these results were still inconsistent. Thus, we performed this meta-analysis to explore the effect of E-cadherin protein in the clinical progression of thyroid cancer.
2. Materials and methods
2.1. Literature searching
In order to acquire available information from eligible literatures, we carried out a comprehensive electronic search on PubMed, Web of Science, Embase, Chinese National Knowledge Infrastructure (CNKI), and Wanfang database on the basis of PRISMA guideline.[30] The following searching strategy was used: (“E-cadherin” or “CDH1” or “ECAD”) and (“Thyroid cancer” or “Thyroid carcinoma” or “thyroid adenocarcinoma”). In addition, other search terms were also used such as: “prognosis,” “survival,” “outcome,” “susceptibility,” “risk,” and “prognostic.” The references of included studies and relevant review were scanned to identify additional eligible studies. The literature searching ended on August 20, 2018. Two investigators independently searched eligible studies, and relevant divergences were resolved by discussion with the 2 researchers.
2.2. Inclusion and exclusion criteria
In the present study, the included studies should meet the following inclusion criteria: relevant pathologically diagnosis was used in thyroid cancer patients; E-cadherin expression levels were detected in tissues with immunohistochemistry (IHC); cases or controls should divided into 2 groups according to E-cadherin expression level; enough data was provided to calculate odds ratios (ORs) and 95% confidence intervals (CIs); and literatures were written with English or Chinese language. Exclusion criteria were used to eliminate irrelevant studies: reviews, meta-analysis, or letters; conference abstracts; samples were not human tissues; and relevant data could not be extracted with fuzzy description.
2.3. Data extraction and quality assessment
All relevant data were extracted by 2 researchers independently, and the 2 investigators reached a consensus by discussion. The following information was extracted from eligible studies: first author, study location, ethnicity, number of cases expressing E-cadherin, year of publication, tumor type, detection method of E-cadherin expression, Newcastle–Ottawa scale (NOS) score, cutoff value, and clinical features of thyroid tumor. The methodological quality of included studies was assessed with NOS.[31] NOS scores were calculated from 3 parts: selection, comparability, and exposure of thyroid cancer patients. In addition, studies of ≥6 scores were considered as high quality.[32]
2.4. Statistical methods
Stata software (version 14.0; Stata Corporation, College Station, TX) was used to all statistical analysis. To determine whether E-cadherin expression was associated with clinical features of thyroid cancer, ORs and 95% CI were calculated for quantitative assessment of associations between E-cadherin expression and thyroid cancer.[33] Cochran's Q and I2 statistics were applied to evaluate the heterogeneity among eligible studies, and I2 > 50% or P < .05 were considered as a sign of significant heterogeneity.[34,35] If significant heterogeneity was found, the random-effect model was used, otherwise the fixed-effect model was chosen to calculate the pooled results.[36,37] In addition, subgroup analysis and meta-regression were conducted to explore the source of heterogeneity and obtained a more accurate result. The publication bias was assessed using Begg test and Egger test.[38] Finally, in order to observe the effect of each study in the overall pooled results, sensitivity analysis was performed. P ≤ .05 was considered as statistically significant differences Table 1 .
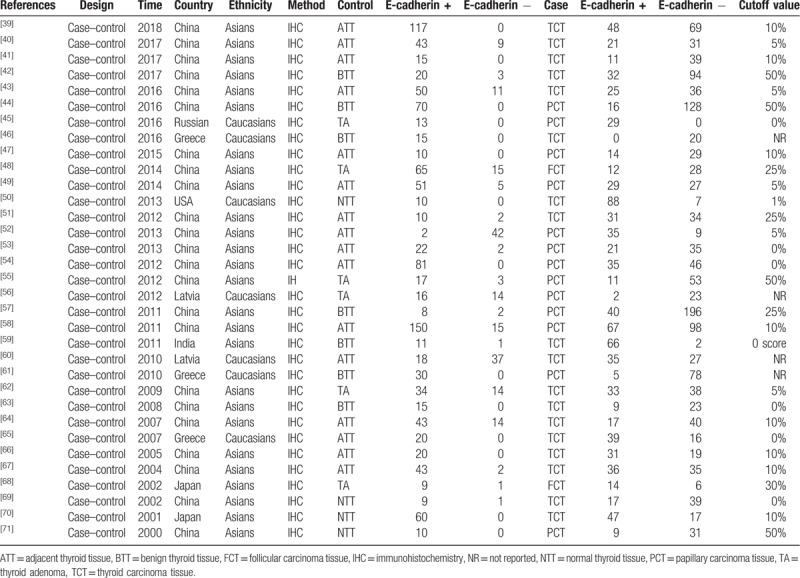
Table 1.
Main characteristics of the included studies for the analysis of thyroid cancer risk.
3. Results
3.1. Study characteristics
In the present study, a total of 890 literatures were initially retrieved, then duplicates (n = 694) were eliminated. In the remaining articles, 5 articles were reviews and 1 article was meta-analysis. After removing reviews and meta-analysis, 190 literatures were obtained. These obtained articles were screened by reading titles and abstracts, and we acquired 113 studies. In order to further get available articles, the full-text of literatures were read and 67 studies did not have enough data or were not related with E-cadherin expression and thyroid cancer. Finally, 46 articles were included for the meta-analysis.[39–84] Thirty-two case–control studies were included for the analysis of correlation between E-cadherin expression and susceptibility of thyroid cancer. And 15 literatures were included to investigate the role of E-cadherin expression in the tumor-node-metastasis (TNM) stage (n = 17), lymph node metastasis (n = 30), differentiation (n = 2), T-stage (n = 4), distant metastasis (n = 5), and capsular invasion (n = 5) of thyroid cancer. IHC was applied to detect the E-cadherin expression level in all eligible studies. It was worth mentioning that the cutoff definitions were different, and therefore cutoff values were different in the eligible studies (Fig. 1, Tables 2 and 3).
Figure 1.
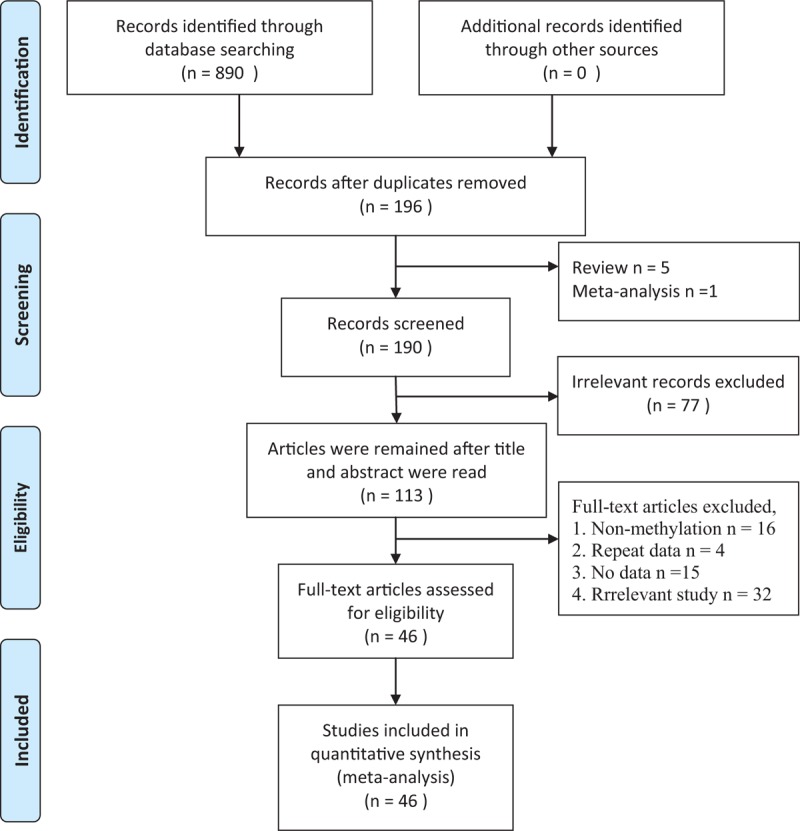
Flow diagram of searching eligible studies.
Table 2.
Characteristics of included studies for the analysis of clinical characteristics of thyroid cancer.
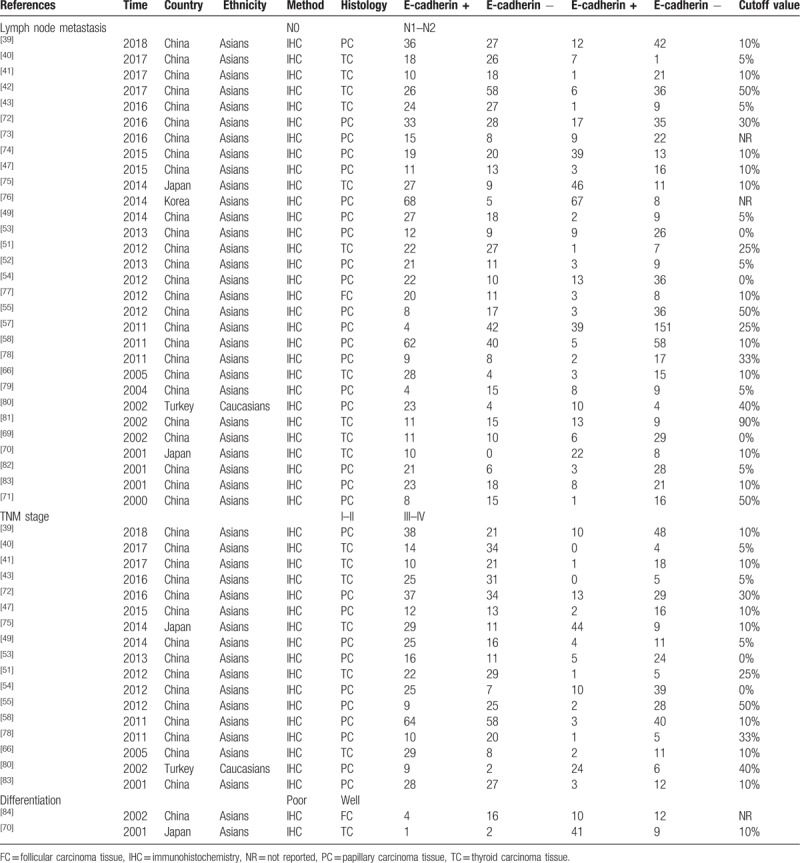
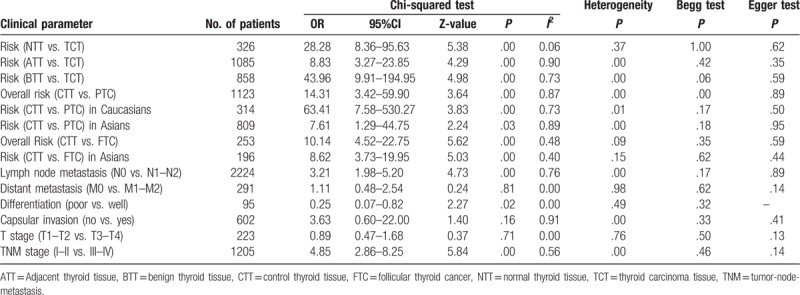
Table 3.
Main results of association between E-cadherin expression and clinical parameters of thyroid cancer.
4. Results of meta-analysis
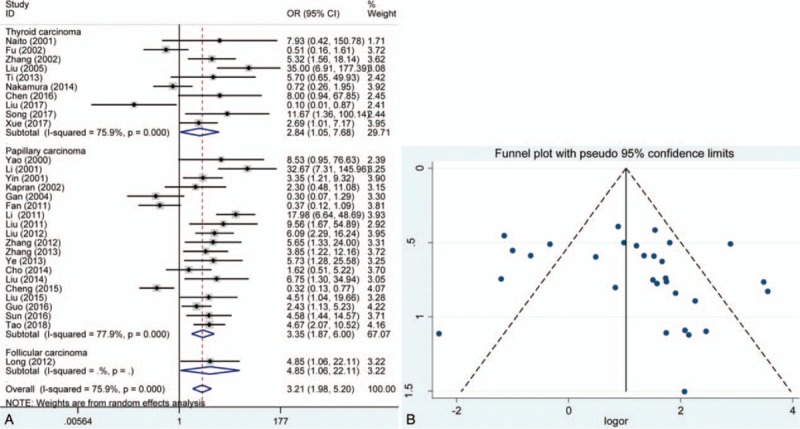
The pooled results showed that E-cadherin negative expression was significantly associated with increased risk of thyroid cancer in different control sample types (normal thyroid tissue, ORs = 28.28, 95% CI = 8.36–95.63; adjacent thyroid tissue, ORs = 8.83, 95% CI = 3.27–23.85; benign thyroid tissue, ORs = 43.96, 95% CI = 9.91–194.95). Subgroup analysis based on thyroid tumor subtypes revealed that E-cadherin negative expression significantly increased the risk of papillary cancer and follicular cancer (papillary cancer, ORs = 14.31, 95% CIs = 3.42–59.90; follicular cancer, ORs = 10.14, 95% CI = 4.52–22.75). Significant heterogeneity among studies was found in the analysis for the susceptibility of thyroid cancer, thus the random effects model was used. Furthermore, significant associations of E-cadherin expression with lymph node metastasis, differentiation, and TNM stage of thyroid cancer were detected (lymph node metastasis, ORs = 3.21, 95% CI = 1.98–5.20; differentiation, ORs = 0.25, 95% CI = 0.07–0.82; TNM stage, ORs = 4.85, 95% CI = 2.86–8.25). In addition to differentiation of thyroid cancer, significant heterogeneity was observed in the analysis of lymph node metastasis and TNM stage of thyroid cancer, and the fixed effect model was applied. According to the results of meta-regression, publication year and race was not the main source of heterogeneity among included studies (P > .05) (Figs. 2–6, Table 3). In order to investigate the role of cutoff values in the heterogeneity among studies which evaluated the risk of thyroid cancer, we calculated the P value of different cutoff values in the meta-regression analysis. The results indicated that 5% cutoff values contributed to a significant heterogeneity (P = .017 < .05).
Figure 2.
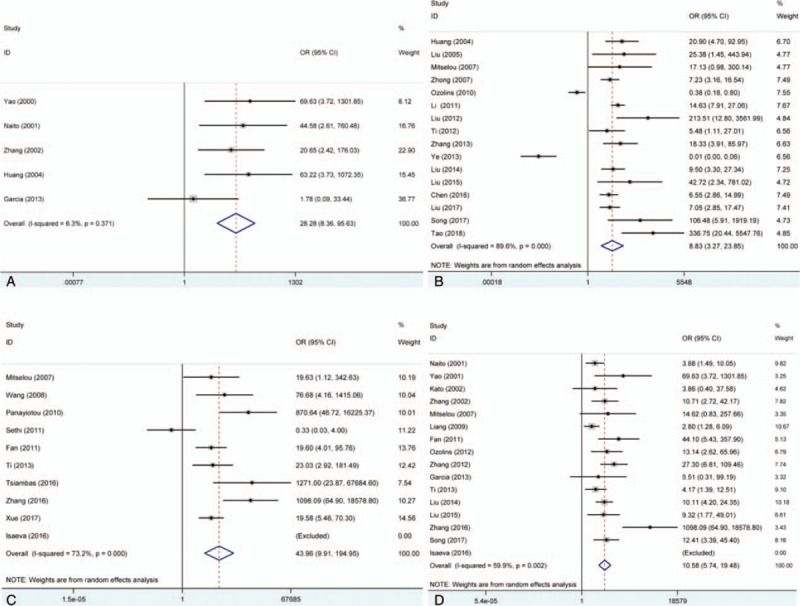
Forest plot of the association between E-cadherin expression and risk of thyroid cancer. (A) Normal thyroid tissue vs. thyroid cancer tissue; (B) adjacent thyroid tissue vs. thyroid cancer tissue; (C) benign thyroid tissue vs. thyroid cancer tissue; and (D) thyroid adenoma tissue vs. thyroid cancer. CI = confidence intervals, OR = odds ratios.
Figure 6.
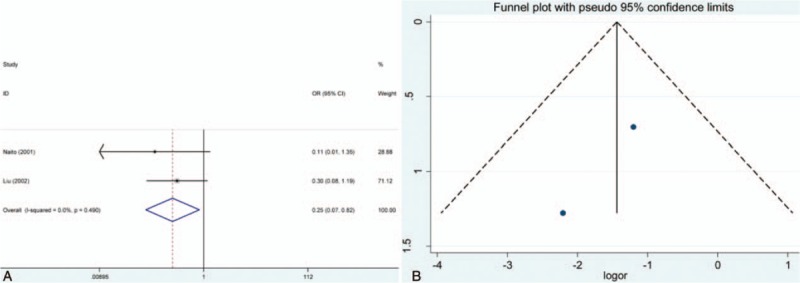
Meta-analysis of the effect of E-cadherin expression in differentiation of thyroid cancer patients. (A) Forest plot; (B) funnel plot. CI = confidence intervals, OR = odds ratios.
Figure 3.
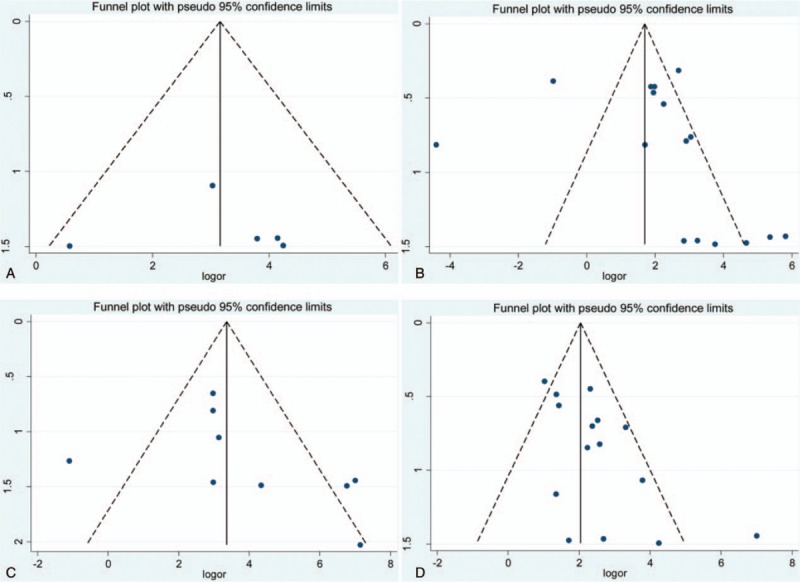
Funnel plot of association between E-cadherin expression and risk of thyroid cancer. (A) Normal thyroid tissue vs. thyroid cancer tissue; (B) adjacent thyroid tissue vs. thyroid cancer tissue; (C) benign thyroid tissue vs. thyroid cancer tissue; and (D) thyroid adenoma tissue vs. thyroid cancer.
Figure 4.
Meta-analysis of the effect of E-cadherin expression in lymph node metastasis of thyroid cancer patients. (A) Forest plot and (B) funnel plot. CI = confidence intervals, OR = odds ratios.
Figure 5.
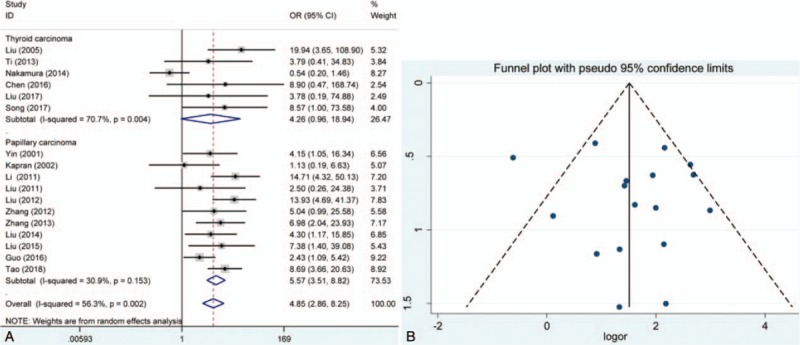
Meta-analysis of the effect of E-cadherin expression in tumor-node-metastasis stage of thyroid cancer patients. (A) Forest plot; (B) funnel plot. CI = confidence intervals, OR = odds ratios.
4.1. Publication bias and sensitivity analysis
In the overall analysis of exploring the effect of E-cadherin expression in the susceptibility of papillary cancer, significant publication bias was detected in Begg test. However, subgroup analysis based on ethnicity suggested that no significant publication bias was found in Caucasians and Asians. No significant publication bias was found in other analysis. In addition, the results of sensitivity analysis revealed that the pooled overall result was stable by eliminating each individual study (Figs. 2–6).
5. Discussion
In addition to conventional therapeutic methods, some molecular-targeted agents have been developed which inhibited tyrosine kinases receptors. Tyrosine kinase was involved in the proliferation and tumorous differentiation of thyroid cancer cells, and these tyrosine kinases inhibitors could block tyrosine kinases receptors and repress the growth and angiogenesis of thyroid cancer.[85] Cabozantinib, lenvatinib, sorafenib, and vandetanib were approved by the European Medical Agency and Food and Drug Administration (FDA) to treat advanced intolerance of differentiated thyroid carcinoma (RAI-R) and medullary thyroid carcinoma.[86] These agents were developed according to different biological targets, and influenced the function of relevant protein or signal pathway. Moreover, some studies were conducted to develop relevant targeted agents such as: axitinib, bevacizumab, imatinib, motesanib, nintedanib, pazopanib, ponatinib, selumetinib, sunitinib, vemurafenib, everolimus, and temsirolimus. The molecular targets of these drugs included dual PI3K/mTOR, MET, RET-KIF5B rearrangement, Bcr-Abl, RET-KIF5B, CCDC6-RET, NcoA4-RET rearrangement, FLT3, KIT, MEK, Raf, BRAFV600E, and CRAF.[2] From published clinical study, aberrant signaling pathways were involved in the progression and invasiveness of thyroid cancer. Mutations in BRAF gene and RAS gene and rearrangement of the RET proto-oncogene were common in thyroid cancer.[87] In fact, these genes mutations were also significantly associated with reduced expression of some genes. For example, the BRAF V600E mutation was related with the low expression of iodine-metabolism genes.[88] The lower expression of NIS was observed in Ki-ras-transformed rat thyroid cells.[89] In addition, several lines of evidence demonstrated that epigenetics was complicated in the thyroid tumorigenesis. Some findings have found the promoter hypermethylation and silencing of TSHR gene in thyroid cancer.[90] A large meta-analysis has found that methylation of CDH1 gene was significantly associated with risk of thyroid cancer, which might change the level of E-cadherin protein expression in thyroid cancer.[91] Therefore, clarifying the levels of E-cadherin protein expression in different stage of thyroid cancer was important for the diagnosis and treatment of thyroid carcinoma.
In this meta-analysis, a systematic review and pooled analysis was performed and the result suggested that E-cadherin negative expression had a worse impact on the susceptibility of thyroid cancer. The type of control group of eligible studies included thyroid adenoma, adjacent thyroid tissue, normal thyroid tissue, and benign thyroid tissue. Subgroup analysis was carried out according to these control groups, and significant association between E-cadherin expression and risk of thyroid cancer was still observed. The results of Begg test and Egger test did not show significant publication bias, and sensitivity analysis indicated that the overall OR was stable. However, significant heterogeneity among studies was observed. Subgroup analysis based on ethnicity suggested that Caucasians and Asians were not the main source of heterogeneity. In order to explore the influence of E-cadherin expression in papillary carcinoma and follicular carcinoma, we conducted stratified analysis according to type of thyroid carcinoma. The results indicated that E-cadherin negative expression was a risk factor for susceptibility of thyroid cancer. The similar results were also found in breast cancer and colorectal cancer.[92,93] However, the significant heterogeneity was still detected, which indicated that some factors might cause the heterogeneity and affect the accuracy of results. The results of meta-regression showed ethnicity and publication year other than cutoff value were not the main source. Thus, relevant unified evaluation criterion of IHC should be established and applied in the future studies.
Additionally, it was found that E-cadherin negative expression significantly correlated with lymph node metastasis, differentiation, and TNM stage of thyroid cancer. The forest plot revealed that these results of included studies were inconsistent, and negative results and positive results could be found in the forest plot. As mentioned above, E-cadherin could promote the cell adhesion, and the loss of E-cadherin might lead to the tumor metastasis and invasion of cancer cells which was consistent with the results of the meta-analysis. Interestingly, we did not find that E-cadherin expression was associated with capsular invasion of thyroid cancer. However, 2 included studies still found E-cadherin negative expression had a significant association with capsular invasion of thyroid cancer,[20,44] while 1 included study obtained opposite result.[19] Considering only 5 studies were included for the analysis of association of capsular invasion of thyroid cancer with E-cadherin expression, the result might be taken into consideration carefully. Furthermore, E-cadherin negative expression significantly repressed differentiation of cancer cells according to the pooled results. As we all know, cells with lower differentiation were more likely to transform into cancer cells and metastasize other sites. Thus, E-cadherin positive expression might prevent normal cells from transformation of cancer cells. Unexpectedly, E-cadherin expression did not have a significant association with distant metastasis of thyroid cancer. After reading the eligible studies, all included 5 studies have found that there was no significant association between E-cadherin expression and distant metastasis of thyroid cancer. One conventional theory was that distant metastasis originated from lymph node metastasis, but a study which was published on Science journal has observed that independent subclones resulted in lymphatic and distant metastases in colorectal cancer.[94] In this published study, common variants in hypermutable DNA regions of 213 biopsy samples were used to reconstruct high-confidence phylogenetic trees, and 2 different lineage relationships between distant and lymphatic metastases were found. The results of our meta-analysis might reflect the similar phenomenon in thyroid cancer. Although significant heterogeneity was found in most studies, almost no obvious publication bias was observed, which revealed the overall results were stable and credible.
In the present study, some limitations should be noted. First, the significant heterogeneity might influence the accuracy of results. However, the main source of heterogeneity was not observed, so other studies with more clinical or therapeutic information should be conducted to acquire a more accurate result. Second, information of differentiation, TNM stage, T-stage, distant metastasis, capsular invasion, and hormone level of thyroid cancer patients were still insufficient. Third, most population of eligible studies were from Chinese, so the result might tend to be more accurate to Chinese population. Fourth, evaluation criterion of E-cadherin expression by IHC was not inconsistent, which might partly cause heterogeneity among eligible studies.
Conclusively, our meta-analysis first systematically investigated the associations of E-cadherin expression with susceptibility and clinical progression of thyroid carcinoma. According to the pooled data, we find that E-cadherin expression is significantly associated with susceptibility, lymph node metastasis, differentiation, and TNM stage of thyroid cancer. However, considering the limitation of the present study, more studies with large population, clinical information, multicenter design, and high quality should be carried out to confirm these findings.
Author contributions
Conceptualization: Changlin Zhou.
Data curation: Changlin Zhou, Chunsheng Yang.
Formal analysis: Changlin Zhou.
Funding acquisition: Daoqun Chong.
Investigation: Changlin Zhou, Chunsheng Yang.
Methodology: Changlin Zhou.
Software: Changlin Zhou.
Supervision: Daoqun Chong.
Visualization: Chunsheng Yang.
Writing – original draft: Changlin Zhou, Chunsheng Yang.
Writing – review & editing: Daoqun Chong.
Footnotes
Abbreviations: ATT = adjacent thyroid tissue, BTT = benign thyroid tissue, CI = confidence interval, CTT = control thyroid tissue, DTC = differentiated thyroid cancer, FC = follicular carcinoma tissue, FCT = follicular carcinoma tissue, FDA = Food and Drug Administration, FTC = follicular thyroid cancer, IHC = immunohistochemistry, NOS = Newcastle–Ottawa scale, NR = not reported, NTT = normal thyroid tissue, PC = papillary carcinoma tissue, PCT = papillary carcinoma tissue, OR = odds ratio, PTC = papillary thyroid cancer, TA = thyroid adenoma, TCT = thyroid carcinoma tissue, TNM = tumor-node-metastasis.
C.Z. and C.Y. contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Patel KN, Shaha AR. Poorly differentiated and anaplastic thyroid cancer. Cancer Control 2006;13:119–28. [DOI] [PubMed] [Google Scholar]
- [2].Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 2009;115:3801–7. [DOI] [PubMed] [Google Scholar]
- [3].Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 2013;13:184–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Orlandi F, Caraci P, Berruti A, et al. Chemotherapy with dacarbazine and 5-fluorouracil in advanced medullary thyroid cancer. Ann Oncol 1994;5:763–5. [DOI] [PubMed] [Google Scholar]
- [5].Pellegriti G, Frasca F, Regalbuto C, et al. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013;2013:965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst 2003;95:625–7. [DOI] [PubMed] [Google Scholar]
- [7].Trovisco V, Soares P, Preto A, et al. Type and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients’ age but not with tumour aggressiveness. Virchows Arch 2005;446:589–95. [DOI] [PubMed] [Google Scholar]
- [8].Liu D, Liu Z, Condouris S, et al. BRAF V600E maintains proliferation, transformation, and tumorigenicity of BRAF-mutant papillary thyroid cancer cells. J Clin Endocrinol Metab 2007;92:2264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 2005;90:6373–9. [DOI] [PubMed] [Google Scholar]
- [10].Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev 2007;28:742–62. [DOI] [PubMed] [Google Scholar]
- [11].Abubaker J, Jehan Z, Bavi P, et al. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J Clin Endocrinol Metab 2008;93:611–8. [DOI] [PubMed] [Google Scholar]
- [12].Liu Z, Hou P, Ji M, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab 2008;93:3106–16. [DOI] [PubMed] [Google Scholar]
- [13].Beadnell TC, Nassar KW, Rose MM, et al. Src-mediated regulation of the PI3K pathway in advanced papillary and anaplastic thyroid cancer. Oncogenesis. 201;7(2):23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gustafson S, Zbuk KM, Scacheri C, et al. Cowden syndrome. Semin Oncol 2007;34:428–34. [DOI] [PubMed] [Google Scholar]
- [15].Garcia-Rostan G, Tallini G, Herrero A, et al. Frequent mutation and nuclear localization of β-catenin in anaplastic thyroid carcinoma. Cancer Res 1999;59:1811–5. [PubMed] [Google Scholar]
- [16].Fagin JA, Matsuo K, Karmakar A, et al. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest 1993;91:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Murugan AK, Bojdani E, Xing M. Identification and functional characterization of isocitrate dehydrogenase 1 (IDH1) mutations in thyroid cancer. Biochem Biophys Res Commun 2010;393:555–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Murugan AK, Xing M. Anaplastic thyroid cancers harbor novel oncogenic mutations of the ALK gene. Cancer Res 2011;71:4403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Murugan AK, Dong J, Xie J, et al. Uncommon GNAQ, MMP8, AKT3, EGFR, and PIK3R1 mutations in thyroid cancers. Endocr Pathol 2011;22:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Alvarez-Nunez F, Bussaglia E, Mauricio D, et al. PTEN promoter methylation in sporadic thyroid carcinomas. Thyroid 2006;16:17–23. [DOI] [PubMed] [Google Scholar]
- [21].Xing M. Gene methylation in thyroid tumorigenesis. Endocrinology 2007;148:948–53. [DOI] [PubMed] [Google Scholar]
- [22].Bae WK, Hennighausen L. Canonical and non-canonical roles of the histone methyltransferase EZH2 in mammary development and cancer. Mol Cell Endocrinol 2014;382:593–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Colón-Bolea P, Crespo P. Lysine methylation in cancer: SMYD3-MAP3K2 teaches us new lessons in the Ras-ERK pathway. Bioessays 2014;36:1162–9. [DOI] [PubMed] [Google Scholar]
- [24].Ogou SI, Yoshida-Noro C, Takeichi M. Calcium-dependent cell–cell adhesion molecules common to hepatocytes and teratocarcinoma stem cells. J Cell Biol 1983;97:944–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yagi T, Takeichi M. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev 2000;14:1169–80. [PubMed] [Google Scholar]
- [26].Frixen UH, Behrens J, Sachs M, et al. E-cadherin-mediated cell–cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol 1991;113:173–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Luo M, Li Z, Wang W, et al. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett 2013;333:213–21. [DOI] [PubMed] [Google Scholar]
- [28].Chen L, Jian W, Lu L, et al. Elevated expression of E-cadherin in primary breast cancer and its corresponding metastatic lymph node. Int J Clin Exp Med 2015;8:11752–8. [PMC free article] [PubMed] [Google Scholar]
- [29].Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev 2013;27:2192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [32].Wong WC, Cheung CS, Hart GJ. Development of a quality assessment tool for systematic reviews of observational studies (QATSO) of HIV prevalence in men having sex with men and associated risk behaviours. Emerg Themes Epidemiol 2008;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [34].Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101–29. [Google Scholar]
- [35].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [37].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [38].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tao XF, Liu C, Fu MJ, et al. Association of HMGA2 and E-Cadherin with invasion of thyroid cancer. Chin J Gerontol 2018;38:560–2. [Google Scholar]
- [40].Liu WM. Association of CDH1 and MLH1 with lymphatic metastasis of thyroid cancer. Inner Mongolia Med J 2017;49:1478–80. [Google Scholar]
- [41].Song CY, Zhao S, Mao H, et al. Expression of HSP70, E-cadherin and CyclinD1 in differentiated thyroid carcinoma and their relationship with clinicopathological features. Anti-tumor Pharm 2017;7:336–74. [Google Scholar]
- [42].Xue Y, Li DM, Zhang J, et al. Expression of Stat5 in thyroid carcinoma and its relationship with EMT. J Pract Med 2017;33:905–8. [Google Scholar]
- [43].Chen YT, Wang WJ, Zhang P. The clinical significance of CDH1 and MLH1 expression in thyroid carcinomas. Chin J Integr Surg Chin Western Med 2018;22:328–31. [Google Scholar]
- [44].Zhang HW, Han XC, Xiong YJ, et al. Expression and significance of Periostin, Slug, E-cadherin in papillary thyroid carcinoma. J Pract Med 2016;32:2383–5. [Google Scholar]
- [45].Isaeva AV, Zima AP, Saprina TV, et al. Comparative evaluation of β-Catenin and E-Cadherin expression in liquid aspiration biopsy specimens of thyroid nodules. Bull Exp Biol Med 2016;161:288–91. [DOI] [PubMed] [Google Scholar]
- [46].Tsiambas E, Ragos V, Georgakopoulos G, et al. E-cadherin/α-catenin deregulated co-expression in thyroid carcinoma based on tissue microarray digital image analysis. J BUON 2016;21:450–5. [PubMed] [Google Scholar]
- [47].Liu Y, Miao YH, Li XM. The expression and clinical significance of EphA2 and E-cadherin in papillary thyroid carcinoma. J Clin Otorhinolaryngol Head Neck Surg (China) 2015;29:1020–3. [PubMed] [Google Scholar]
- [48].Liu FZ, Zhang Y, Fan YM, et al. Significance of β-galectin-3 and E-cadherin expressions in diagnosis of atypical thyroid adenoma. Jiangsu Med J 2014;40:2004–206. [Google Scholar]
- [49].Liu L, Wu ZH, Zhang ZL, Dong W, Lei L. Expression of ARHI and E-cadherin Proteins in Papillary Thyroid Carcinoma and Its significance. 2014; 23(4):333–337 [Google Scholar]
- [50].Montemayor-Garcia C, Hardin H, Guo Z, et al. The role of epithelial mesenchymal transition markers in thyroid carcinoma progression. Endocr Pathol 2013;24:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Halimulati M, Ssilike M, Hua T, et al. Expression and clinicopathological significances of CDH1 and MLH1 protein in thyroid carcinoma. Chin Oncol 2012;22:329–35. [Google Scholar]
- [52].Ye XG, Zhang YY, Ren WM, et al. Expression of epidermal growth factor receptor in papillary thyroid carcinoma and its relationship with epithelial-mesenchymal transition. Chin J Clin Med 2013;20:118–21. [Google Scholar]
- [53].Zhang ZQ, Bai Y, Li P, et al. Relationship between activated STAT3 protein and epithelial-mesenchymal transition in papillary thyroid carcinoma. J Clin Otorhinolaryngol Head Neck Surg (China) 2013;27:1265–8. [PubMed] [Google Scholar]
- [54].Liu C, Chen X, Gao ZN, et al. The study of the correlation of papillary thyroid carcinoma's invasion toward Ezrin and E-Cadherin. J Clin Otorhinolaryngol Head Neck Surg (China) 2012;26:789–95. [PubMed] [Google Scholar]
- [55].Zhang WD, Mao R. Expressions of Snail and E-cadherin proteins in thyroid papillary carcinoma and their clinical significance. Chin J Clin Res 2012;25:843–5. [Google Scholar]
- [56].Ozolins A, Narbuts Z, Strumfa I, et al. Immunohistochemical expression of HBME-1, E-cadherin, and CD56 in the differential diagnosis of thyroid nodules. Medicina (Kaunas) 2012;48:507–14. [PubMed] [Google Scholar]
- [57].Fan YW, Zhang Y, Zhao WP, et al. Expression and significance of galectin-3 and e-cadherin in the thyroid papillary carcinoma. J Southeast Univ (Med Sci Edi) 2011;30:598–602. [Google Scholar]
- [58].Li Association of expression of E-cadherin in papillary thyroid carcinoma tissues with neck lymph node metastasis. Basic Clin Res 2011;18:102–4. [Google Scholar]
- [59].Sethi K, Sarkar S, Das S, et al. Expressions of CK-19, NF-kappaB, E-cadherin, beta-catenin and EGFR as diagnostic and prognostic markers by immunohistochemical analysis in thyroid carcinoma. J Exp Ther Oncol 2011;9:187–99. [PubMed] [Google Scholar]
- [60].Ozolins A, Narbuts Z, Strumfa I, et al. Diagnostic utility of immunohistochemical panel in various thyroid pathologies. Langenbecks Arch Surg 2010;395:885–91. [DOI] [PubMed] [Google Scholar]
- [61].Pazaitou-Panayiotou K, Mygdakos N, Boglou K, et al. The immunocytochemistry is a valuable tool in the diagnosis of papillary thyroid cancer in FNA's using liquid-based cytology. J Oncol 2010;2010:963926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Liang HS, Zhong YH, Luo ZJ, et al. Comparative analysis of protein expression in differentiated thyroid tumours: a multicentre study. J Int Med Res 2009;37:927–38. [DOI] [PubMed] [Google Scholar]
- [63].Wang XJ, Li JT, Li JH, et al. Expression and significance of high molecularweight cytokeratin 34βE12 and E-cadherin in the thyroid papillary carcinoma. Clin Med J Chin 2008;15:886–8. [Google Scholar]
- [64].Zhong YH, Liang HS, Lin HD, et al. Significance of expressions of E-cadherin and MMP7 in thyroid carcinoma. Acta Acad Med Jiangxi 2007;47:10–2. [Google Scholar]
- [65].Mitselou A, Ioachim E, Peschos D, et al. E-cadherin adhesion molecule and syndecan-1 expression in various thyroid pathologies. Exp Oncol 2007;29:54–60. [PubMed] [Google Scholar]
- [66].Liu GL, Qi FY, Tian JL, et al. Clinical significance and expression of vascular endothelial growth factor matrixmetalloproteinase-9, E-cadherin in human thyroid carcinoma. Clin Med Chin 2005;21:454–6. [Google Scholar]
- [67].Huang HW, Li DJ. Expression of E-cadherin in thyroid papillary carcinoma and its significant. Anthol Med 2004;23:131–3. [Google Scholar]
- [68].Kato N, Tsuchiya T, Tamura G, et al. E-cadherin expression in follicular carcinoma of the thyroid. Pathol Int 2002;52:13–8. [DOI] [PubMed] [Google Scholar]
- [69].Zhang ZC, Sun C, Shi GS. The expression of CD44V6, PCNA, E-cadherin and EGFR in thyroid tumor. J Henan Oncol 2002;15:161–3. [Google Scholar]
- [70].Naito A, Iwase H, Kuzushima T, et al. Clinical significance of E-cadherin expression in thyroid neoplasms. J Surg Oncol 2001;76:176–80. [DOI] [PubMed] [Google Scholar]
- [71].Yao QY, Zou Q, Ni QX, et al. Expression of E-cadherin in papillary thyroid carcinoma and its clinical significance. Chin Oncol 2000;10:234–6. [Google Scholar]
- [72].Guo WR, Chen YF, Chen SC, et al. The relative analysis of HER-2/neu, E-cadherin protein and clinicopathological characteristics of papillary thyroid carcinoma. J Med Theor Prac 2016;29:563–6. [Google Scholar]
- [73].Sun L, Zhang WJ, Zhang J, et al. Expression and mechanism of activated leukocyte cell adhesion molecule, E-cadherin and β-catenin in papillary thyroid carcinoma. Acta Anat Sin 2016;47:221–7. [Google Scholar]
- [74].Cheng Y, Meng YX, Liang ZY, et al. Expression of EpCAM and E-cadherin in papillary thyroid carcinoma and its clinicopathologic significance. Chin J Pathol 2015;44:189–94. [PubMed] [Google Scholar]
- [75].Nakamura M, Onoda N1, Noda S, et al. E-cadherin expression and cell proliferation in the primary tumor and metastatic lymph nodes of papillary thyroid microcarcinoma. Mol Clin Oncol 2014;2:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cho SW, Kim YA, Sun HJ, et al. Therapeutic potential of Dickkopf-1 in wild-type BRAF papillary thyroid cancer via regulation of β-catenin/E-cadherin signaling. J Clin Endocrinol Metab 2014;99:E1641–9. [DOI] [PubMed] [Google Scholar]
- [77].Long FY, Yang JY, Liu ZM, et al. Expression and clinical significance of hypoxia-inducible factor-1α and epithelial mesenchymal transition-related protein in human follicular thyroid carcinoma. Chin J Biologicals 2012;25:1671–9. [Google Scholar]
- [78].Liu ZB, Wang L, Ye XG, et al. Expression of E-cadherin, β-catenin and cyclinD1 proteins in papillary thyroid carcinoma and their clinical significance. Chin J Clin Exp Pathol 2011;27:146–9. [Google Scholar]
- [79].Gan XY, Xiao LZ, Lao SL, et al. Associations of E-cadherin, CD44V3, and nm23 expression with lymph node metastasis of thyroid cancer. J Clin Exp Pathol 2004;20:754–5. [Google Scholar]
- [80].Kapran Y, Ozbey N, Molvalilar S, et al. Immunohistochemical detection of E-cadherin, alpha- and beta-catenins in papillary thyroid carcinoma. J Endocrinol Invest 2002;25:578–85. [DOI] [PubMed] [Google Scholar]
- [81].Fu YF, Yao ZX. Expression and significance of E-cadherin in thyroid cancer. Chin J Gen Surg 2002;11:300–1. [Google Scholar]
- [82].Li ZN, Qiu JL, Wu CH, et al. Adhesive molecule expression in relation to invasion and metastasis in thyroid papillary carcinoma. Chin J Bases Clin General Surg 2001;8:317–8. [Google Scholar]
- [83].Yin DS, Wang L, Wang QZ. Expression of E-cadherin and nm23 in papillary thyroid carcinoma and its clinical significance. Chin Oncol 2001;11:243–5. [Google Scholar]
- [84].Liu Y, Jiang CX, Tan YB. Pathological study on the expression of cell adhesion molecules and metastasis suppressor gene in thyroid follicular carcinoma and papillary carcinoma. Chin J Pathol 2002;31:322–6. [PubMed] [Google Scholar]
- [85].Viola D, Valerio L, Molinaro E, et al. Treatment of advanced thyroid cancer with targeted therapies: ten years of experience. Endocr Relat Cancer 2016;23:R185–205. [DOI] [PubMed] [Google Scholar]
- [86].Valerio L, Pieruzzi L, Giani C. Targeted therapy in thyroid cancer: state of the art. Clin Oncol (R Coll Radiol) 2017;29:316–24. [DOI] [PubMed] [Google Scholar]
- [87].Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014;159:676–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Puxeddu E, Durante C, Avenia N, et al. Clinical implications of BRAF mutation in thyroid carcinoma. Trends Endocrinol Metab 2008;19:138–45. [DOI] [PubMed] [Google Scholar]
- [89].Trapasso F, Iuliano R, Chiefari E, et al. Iodide symporter gene expression in normal and transformed rat thyroid cells. Eur J Endocrinol 1999;140:447–51. [DOI] [PubMed] [Google Scholar]
- [90].Hoque MO, Rosenbaum E, Westra WH, et al. Quantitative assessment of promoter methylation profiles in thyroid neoplasms. J Clin Endocrinol Metab 2005;90:4011–8. [DOI] [PubMed] [Google Scholar]
- [91].Khatami F, Larijani B, Heshmat R, et al. Meta-analysis of promoter methylation in eight tumor-suppressor genes and its association with the risk of thyroid cancer. PLoS One 2017;12:e0184892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Horne HN, Sherman ME, Garcia-Closas M, et al. Breast cancer susceptibility risk associations and heterogeneity by E-cadherin tumor tissue expression. Breast Cancer Res Treat 2014;143:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Govatati S, Singamsetty GK, Nallabelli N, et al. Contribution of cyclin D1 (CCND1) and E-cadherin (CDH1) alterations to colorectal cancer susceptibility: a case-control study. Tumour Biol 2014;35:12059–67. [DOI] [PubMed] [Google Scholar]
- [94].Naxerova K, Reiter JG, Brachtel E, et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science 2017;357:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


