Abstract
The new Tumor Node Metastasis staging system does not recognize fissure status with respect to adjacent lobe invasion (ALI) in lung cancer. Furthermore, no specific surgical strategies have been recommended for lymph node dissections around adjacent nontumor-bearing lobes (NTBLs) according to fissure status. Therefore, this study was undertaken to investigate the necessity of removing additional adjacent lobe lymph nodes in patients with nonsmall cell lung cancer (NSCLC) for lesions limited to in the vicinity of the interlobar fissure.
From August 2013 to March 2015, the records of 332 patients, who underwent systematic mediastinal lymph node dissection, were reviewed in this retrospective study. The bronchial lymph nodes had been subjected to pathological examination, and the status of the fissures was also recorded. A statistical analysis was performed to identify the significant predictors of lymph node metastasis.
The patients were divided into a nonadjacent lobe invasion (NALI) group (n = 295) and an ALI group (n = 37). There was a significant difference in tumors with pN2 disease between the ALI and NALI groups (37.8% vs 8.8%, P = .001). ALI tumors had significantly more frequent pleural involvement than NALI tumors (62.2% vs 43.1%, P = .035). The frequency of N2 involvement among tumors invading across the complete fissure was higher than that of the tumors invading across the incomplete fissure (44.4% vs 14.3%, P = .015). However, the frequency of N1 involvement among tumors invading across the incomplete fissure was not statistically different than that of tumors not invading across incomplete fissure (32.1% vs 24.2%, P = .357). Regarding lymph node metastasis in NTBL, 15 (12.7%) patients had lymph node metastases in NTBLs. Pleural involvement was an independent predictor of lymph node metastasis in an NTBL.
A greater frequency of N2 lymph nodes existed in NSCLC with invading adjacent lobe across complete fissure, extensive lymphatic resection within the hilum, and NTBL in tumors with pleural involvement are justifiable and necessary.
Keywords: adjacent lobe invasion, interlobar fissure, lymph node, nonsmall cell lung cancer, surgery
1. Introduction
Lung cancer is the most commonly diagnosed cancer clinically and is also one of the leading causes of death.[1] Lobectomy with systematic nodal dissection has been a standard surgery for nonsmall cell lung cancer (NSCLC).[2] In the 8th Tumor Node Metastasis (TNM) classification, currently, tumors invading the surface of the interlobar pleura are still classified as T2, unless other T factors are identified that induce a higher category. This classification is made regardless of fissure status at the site of tumor invasion (interlobar pleura only or transfissural and into the parenchyma of the adjacent lobe).[3] Although the new TNM staging system does not consider fissure status in the diagnosis of adjacent lobe invasion (ALI), previous results have indicated that interlobar fissure status affects survival in patients with ALI.[4] Furthermore, no specific surgical strategies have been recommended when this condition occurs. The feasibility of anatomical or nonanatomical resections (lobectomy plus segmentectomy for tumor-bearing tissue, or limited wedge resection of the adjacent lobe) is currently controversial. Moreover, the optimal resection method for lymph node dissection is unclear. Incomplete fissures indicating partial fusion between lobes may also alter the spread of disease within the lung.[5] Because of anatomical variations, the extent and frequency of lymph node metastases into an adjacent lobe (nontumor-bearing lobe [NTBL]) are unclear and can ultimately affect the success of the surgical procedure. After completing the anatomical lobectomy of a tumor-bearing lobe, removal of highly suspicious N1 nodes around the artery and bronchus of the adjacent (nontumor-bearing) lobe and segment should be considered. However, this could cause excess bleeding, air leakage, and incidental injury to the bronchovascular structures, even though it would provide a more definitive staging and further guidance for prognosis and target adjuvant treatments.
To the best of our knowledge, this is the first study on lymph node metastasis outside of a tumor-bearing lobe in primary lung cancer and the status of interlobar fissures. The aim of this study was to better understand the frequency of regional lymph nodes metastasis in NSCLC and to tentatively explore the necessity of removing lymph nodes around the adjacent (nontumor-bearing) lobe according to the status of the interlobar fissure.
2. Materials and methods
2.1. Patients
This study was approved by the Ethics Committee of Shandong Cancer Hospital and Institute, Jinan, Shandong Province, China. The need to obtain informed consent from each patient was waived due to the retrospective nature of the study. We retrospectively evaluated 786 patients, from August 2013 to March 2015 whose pathologically examinations indicated a diagnosis of lung cancer in the Cancer Hospital. The patients subsequently underwent pulmonary resection for treatment of lung cancer. TNM staging and lymph node station numbers were determined and classified according to the description proposed by the International Association for the Study of Lung Cancer (IASLC).[3] Patients with extrathoracic metastases and those who received prior chemotherapy or radiotherapy were excluded. Patients with nonsystematic sampling or dissection intraoperatively, those with pathologic findings consistent with small cell lung cancer or rare histologic type, those with ipsilateral multiple lesions or benign disease, and those with incomplete records were also excluded.
2.2. Collection of clinicopathological information
Preoperative radiologic findings and tumor locations in all patients were determined on chest computed tomography (CT) scans. Brain magnetic resonance imaging (MRI), positron emission tomography (PET), abdominal ultrasonography, and whole body radionuclide bone scanning were selectively performed at the physician's discretion. Mediastinoscopy and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) were selectively performed in patients with mediastinal lymph nodes >1 cm on chest CT or an increased maximum standard uptake value in the mediastinal nodes on PET scan. We reviewed the medical records of each patient for their clinicopathological information, including age, sex, smoking history, preoperative serum carcinoembryonic antigen (CEA) level (with a cutoff at the normal upper limit of 3.4 ng/mL), mode of resection, histology, tumor size, pathological node status, lymphatic permeation, vascular invasion, and pleural invasion as defined in the TNM classification, 8th edition.[3]
2.3. CT assessment
Tumors were assessed via CT preoperatively by 2 or more experienced radiologists using a diagnostic picture archiving and communication system (PACS) station. The PACS station was used to obtain interactive multiplanar reconstructions and assigned to the following assessments based on axial CT images presented in a preoperative conference attended by all general thoracic surgeons and radiologists. The locations of the lesions were determined from the transverse, sagittal, and coronal planes on CT imaging. We assessed the presence, extension, localization, and type of incompleteness of the oblique interlobar fissures and the horizontal fissures. The extensions of incompleteness were expressed as percentages of the entire fissure area and were divided into 5 categories (1%–20%, 21%–40%, 41%–60%, 61%–80%, and ≥81%). Two localizations of fissure discontinuity were defined, parahilar and other. The following radiographic findings were used as diagnostic criteria for incomplete interlobar fissures: the interlobar line was not observed; the zone was avascular; vessels in the adjacent lobes crossed over the interlobar region; pulmonary blood vessels, particularly the pulmonary vein, penetrated the interlobar region; the pulmonary vein was observed in the interlobar region and was related to the vessels in the adjacent lobes.[6] When multiple types of incompleteness were present simultaneously, the type with a higher number was prioritized.[7] In the present study, we defined a complete interlobar fissure as one with entirely clear and continuous interlobar pleura and separate lobes and an incomplete interlobar fissure was defined by the 5 categories as mentioned above. Ground glass opacity (GGO) was defined as a misty increase in lung attenuation that did not obscure the underlying vascular markings. A pure ground grass opacity (pGGO) type in the study was defined by a 100% GGO appearance; the solid type was defined by a 100% solid appearance without any GGO components; and the mixed GGO (mGGO) type was defined by any other patterns of combined solid and GGO components.
2.4. Surgical procedure
Decisions regarding the type of resection for each patient were based on the surgeon's preference according to the pulmonary reserve of the patient and the localization of the tumor, if it was eligible for resection. Patients with pGGO types who underwent sublobar resection, such as a wedge resection or segmentectomy, were excluded. Intraoperative frozen pathology examinations that assessed tumor invasiveness may have helped to identify patients in whom systematic lymph node dissection or sampling could be omitted. All the patients underwent systematic mediastinal lymph node dissection, At least 6 nodes were removed from all patients and included intrapulmonary, hilar, and mediastinal nodes. The criteria for systemic lymphadenectomy was defined by the European Society of Thoracic Surgeons guidelines.[8] After completing a primary tumor-bearing lobectomy, pneumonectomy and bilobectomy, lobectomy plus wedge resection or segmentectomy, and dissection or sampling of bronchial lymph nodes of the adjacent lobe, including hilar nodes and intersegmental nodes, were performed and recorded separately.
2.5. Pathologic examination
After identifying each bronchus and the intersegmental veins delineating the segmental borders in the resected specimens, the location of intrapulmonary nodes, including the intersegmental and segmental nodes, was dissected by a well-trained thoracic surgeon immediately after resection of the specimen. The lung parenchyma was dissociated from the bronchial wall until subsegmental bronchioles were exposed. Meanwhile, intersegmental and segmental nodes were identified and separately recorded as being inside or outside of the tumor-bearing segment (TBS) and were then subjected to pathological examination. Extrapulmonary nodes, including hilar and mediastinal nodes, were separately recorded and classified as lymph nodes in either a TBS or a nontumor-bearing segment (NTBS). Dissection or sampling of bronchial lymph nodes in an adjacent lobe was recorded as NTBL nodes. We defined a skip N2 metastasis as a mediastinal nodal metastasis with no hilar, lobar, or intrapulmonary nodes.[9]
The integrity of the fissures in the specimens was recorded, and the anatomical classification based on the degree of fissure completeness, proposed by Craig and Walker, was followed to determine the presence and completeness of the fissures.[10] Fissure completeness was graded in 4 stages: grade 1, complete fissure with entirely separate lobes; grade 2, complete visceral cleft but parenchymal fusion at the base of the fissure; grade 3, visceral cleft evident for part of the fissure; and grade 4, complete fusion of the lobes with no evident fissure line. In the present study, we classified complete interlobar fissures as grades 1 and 2 and incomplete interlobar fissures as grades 3 and 4. Directly adjacent lobar invasion was defined by a contiguous extension of the primary tumor across the fissure and into another lobe according to the histopathologic examination. For tumors adjacent to an incomplete pulmonary fissure, it was also necessary to determine the authenticity of the tumors invading the fissure or the intersegmental and interlobar plane, and into the adjacent lobe according to their relationship to the interlobar veins or intersegmental veins. To determine whether the tumor invaded a complete lobar fissure or not, elastic fiber staining was used to determine whether the adjacent pleura was involved.[11,12]
2.6. Statistical analysis
In a univariate analysis, we compared the characteristics of patients with ALI and those with nonadjacent invasion and analyzed them using Pearson χ2 test or Fisher exact test and the independent samples t test. Relationships between the clinicopathologic factors and nodal metastasis were analyzed using the Kruskal–Wallis test. Pearson χ2 test or Fisher exact test was used to compare the quantitative parameters. In the multivariate analysis, categorical variables and continuous variables were assessed to identify independent predictors. A multivariate logistic regression analysis using a backward stepwise procedure was performed to identify the significant predictors of lymph node metastasis in NTBL. Receiver-operating characteristic (ROC) curves were created to determine the optimal cutoff value for tumor size that would be predictive of lymph node metastasis. All P values are based on a 2-tailed statistical analysis and P < .05 was considered statistically significant. All analyses were performed using SPSS 19.0 statistical software (SPSS II for Windows, standard version 19.0, SPSS Inc., Chicago, IL).
3. Results
3.1. Compare of patient characteristics
A total of 786 patients received surgical treatment during the study period. Based on the exclusion criteria, 454 patients were omitted from the analysis for the following reasons: nonsystematic sampling or dissection intraoperatively (n = 139), small cell lung cancer (n = 10) or rare histologic type (n = 18), multiple ipsilateral lesions (n = 27), and benign disease (n = 22). We selected only those tumors that were located in the vicinity of the fissures. Forty-three patients (5.67%) with tumors that were located away from the area of the fissures were excluded from our study. Patients with CT scans showing major accessory fissures (n = 5) were also excluded. Consequently, 332 patients with an average age of 62 years were subjected to the analysis (Fig. 1). These characteristics of the patients are summarized in Table 1. There were 209 male patients and 123 female patients. Tumor size was ≤3 cm in 124 patients and ranged from >3.0 to 10.5 cm in the remaining 208 patients according to long-axis measurements in the CT lung window assessment on a diagnostic PACS station. The tumor consistency was solid in 311 patients (93.7%) and showed mGGO appearance in 21. A total of 158 tumors were right-sided and the other 174 were left-sided. In our series of 332 consecutive patients, 60 (18.1%) had complete lung fissures and 272 (81.9%) had incomplete fissures. The pathological findings after surgery included 198 adenocarcinomas, 85 squamous cell carcinomas, 14 large cell carcinomas, and 35 adenosquamous carcinomas. At total of 5810 lymph nodes were harvested in our study. There were 875 metastasized lymph nodes in 1620 (27.8%) harvested lymph nodes from 126 patients who showed lymph node metastasis. The pathologic stages included 40 stage IA, 71 stage IB, 64 stage IIA, 95 stage IIB, 57 stage IIIA, and 5 stage IIIB. All patients were divided into nonadjacent lobe invasion (NALI) group (n = 295) and ALI groups (n = 37) according to the presence or absence of interlobar fissure invasion. The ALI group comprised those patients with direct invasion to the adjacent lobe, but without invasion of the chest wall, diaphragm, mediastinal pleura, or parietal pericardium. These characteristics of the patients are summarized in Table 2.
Figure 1.
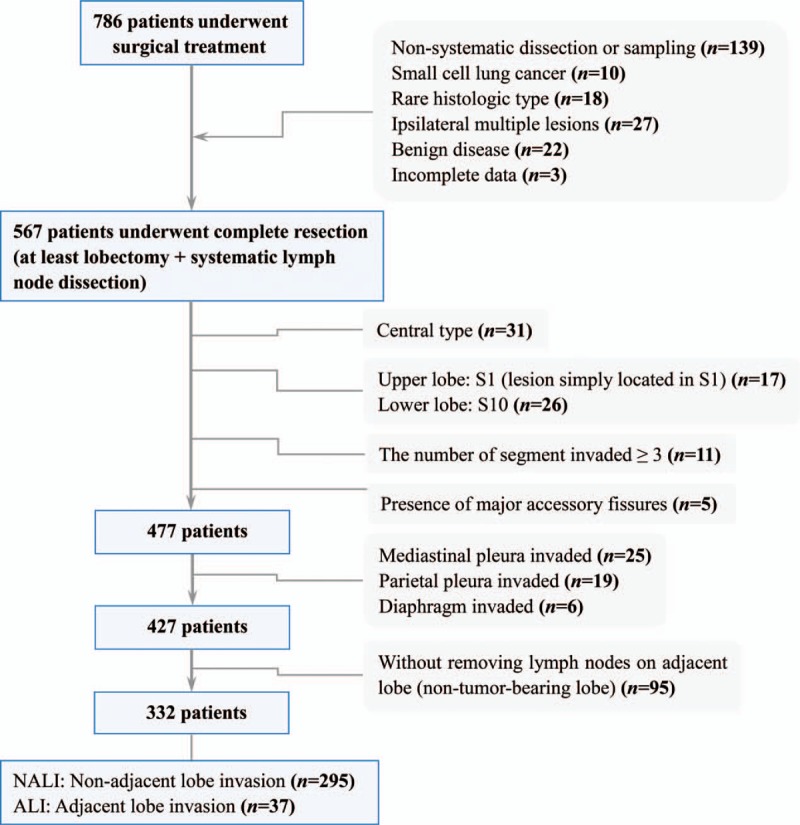
Patients enrolled in the study.
Table 1.
Patient characteristics.
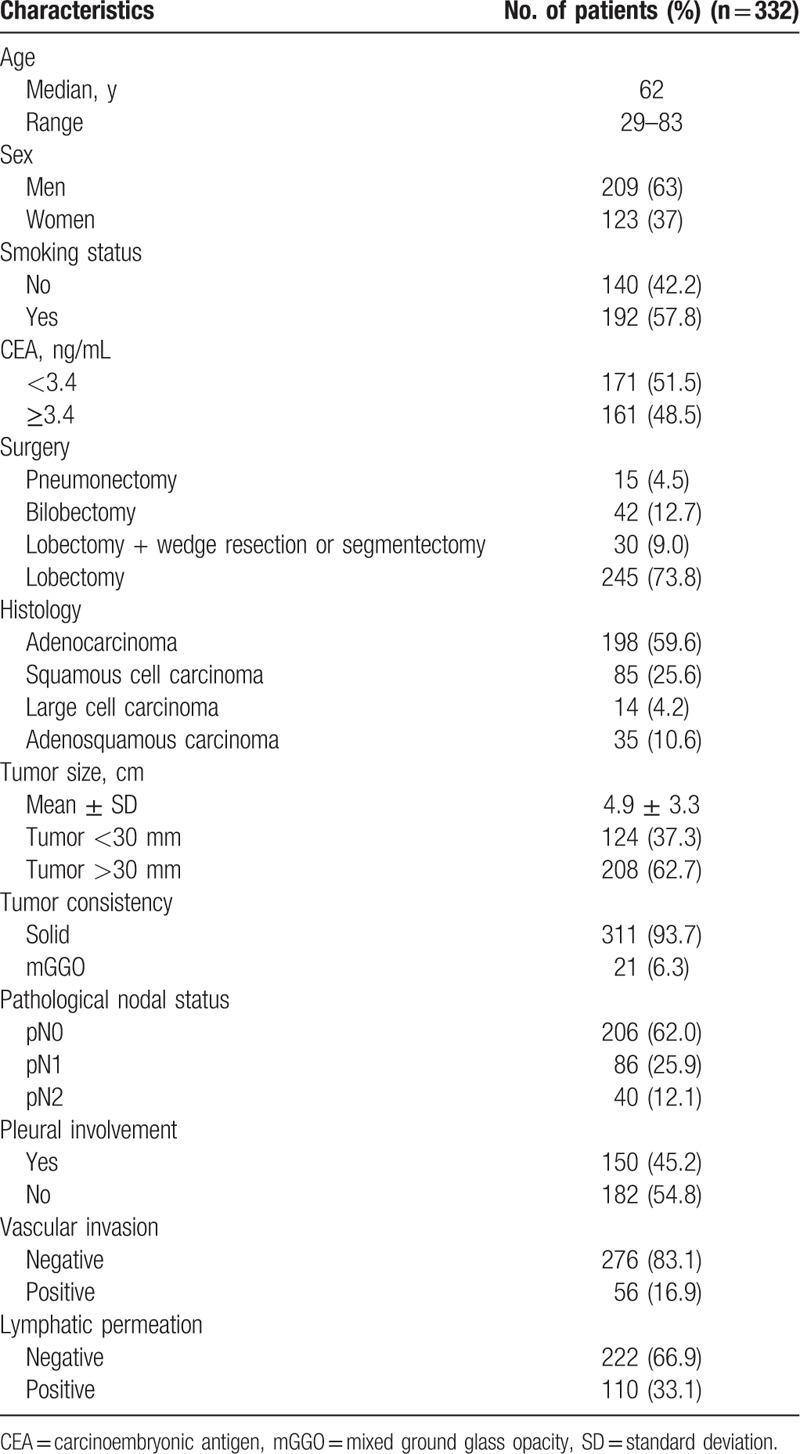
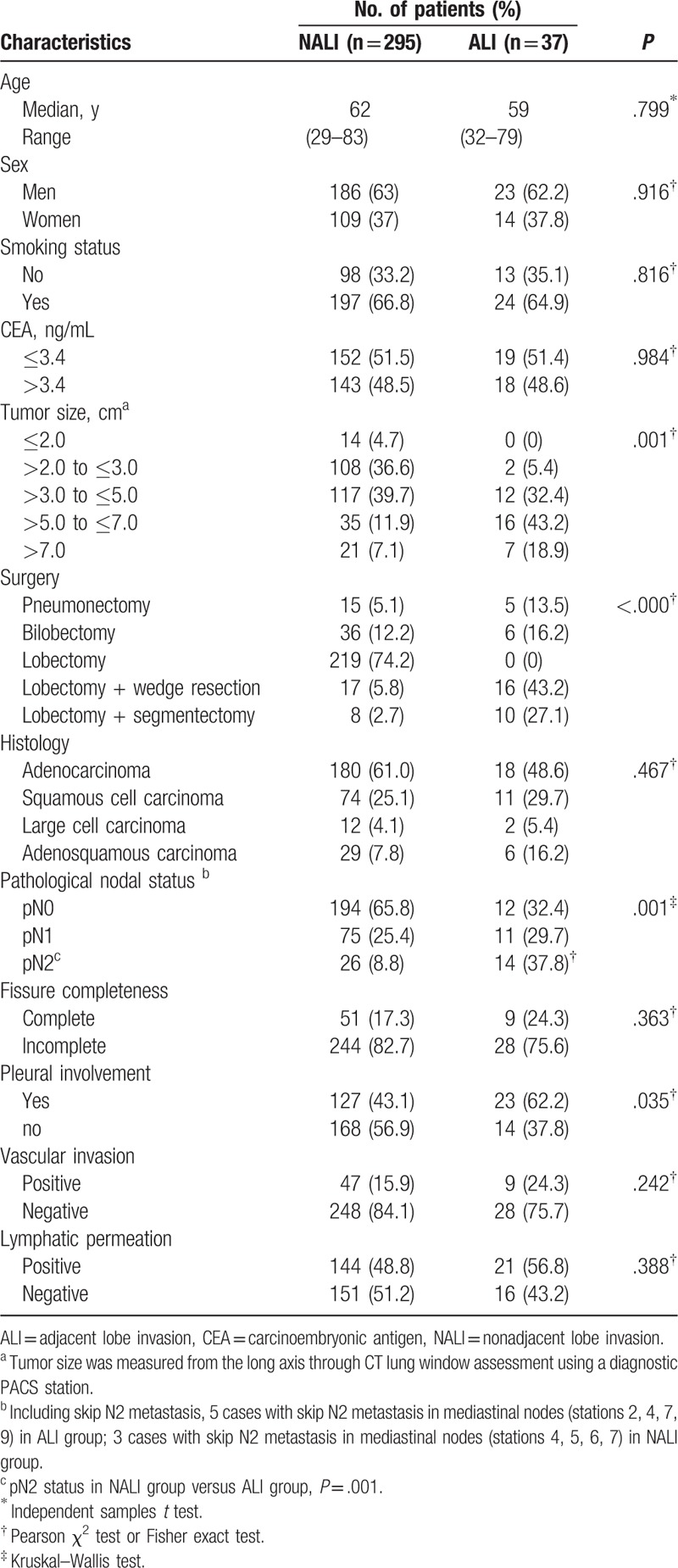
Table 2.
Comparison of patient characteristics between the adjacent lobe invasion and nonadjacent lobe invasion groups.
3.2. Lymph node metastases in patients by ALI, NALI, and fissure status
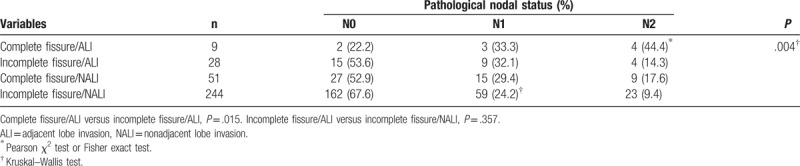
In all, 5 patients underwent pneumonectomy and lobectomy plus limited resection (wedge resection [n = 16] and segmentectomy [n = 10]) and 6 underwent bilobectomy for ALI. Patients with ALI underwent more aggressive approaches than those with NALI according to a higher number of lobes and segments invaded (P < .000). There were no significant differences in the characteristics of patients with ALI and NALI tumors based on age, sex, preoperative CEA, and histology. There was a significant difference in pN2 tumors in the ALI group compared with the NALI group (37.8% vs 8.8%, P = .001). ALI tumors had significantly more pleural involvement than NALI tumors (62.2% vs 43.1%, P = .035). Lymphatic permeation was more frequent with ALI tumors than with NALI tumors, although this was not statistically significant (56.8% vs 48.8%, P = .388). The pathological nodal status of the patients, according to fissure status and ALI, is shown in Table 3. The frequency of N2 involvement in the tumors invading across complete fissures was higher than that of tumors invading across incomplete fissures (44.4% vs 14.3%, P = .015). However, the frequency of N1 involvement in the tumors with invasion across incomplete fissures was not statistically different from that of tumors without invasion across incomplete fissures (32.1% vs 24.2%, P = .357).
Table 3.
Comparison between lymph node metastases according to fissure status and adjacent lobe invasion.
3.3. Lymph node metastases in TBL and NTBL at each tumor size
Forty-seven patients (14.2%) had segmental lymph node metastasis. Seventeen of these 47 patients showed intrapulmonary metastasis relative to the primary tumors that were in the T3 category. With increased tumor size, greater lymph node involvement was observed. Lymph node metastases of the patients are summarized in Table 4. Metastasis outside the TBS was observed in 4 patients, which represented 1.2% all lesions. Twelve patients had associated lymph node metastases outside the TBS accompanied by extrapulmonary metastasis. Of the 47 patients with segmental lymph node metastases, the frequency of segmental node involvement was higher in TBS than in NTBS. Of 319 patients with tumors 2.0 cm or more in diameter, 15 (4.51%) had lymph node metastases in NTBL. Skip metastases were detected in 8 patients (2.4%). An ROC curve for tumor size on CT was established to determine the optimal cutoff value for predicting lymph node metastasis. The area under the ROC curve (AUC) was 0.697 (95% confidence interval [CI], 0.638–0.757) and 3.05 cm was selected as the cutoff value (sensitivity 74.5%, specificity 51.7%), as shown in Figure 2.
Table 4.
The details of lymph node metastases in each tumor size.
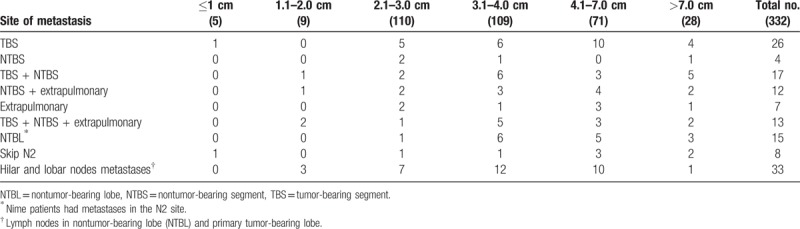
Figure 2.
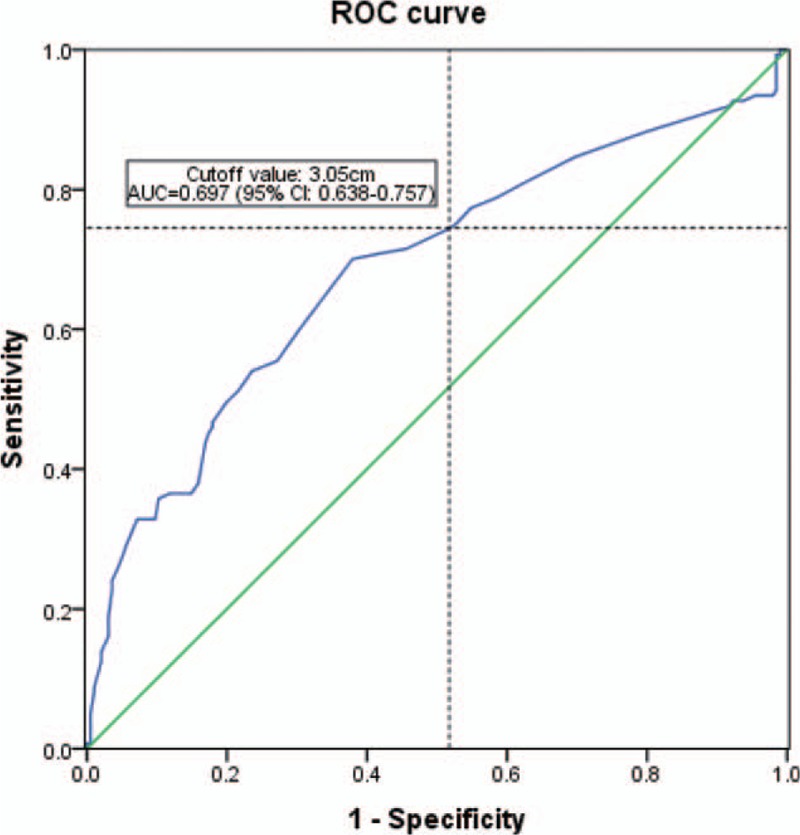
The receiver-operating characteristic (ROC) curve for tumor size on computed tomography was established to determine the optimal cutoff value for predicting lymph node metastasis. Details of lymph node metastases in each tumor size are summarized in Table 4. Based on the material, a database was created without considering the location of lymph node involvement.
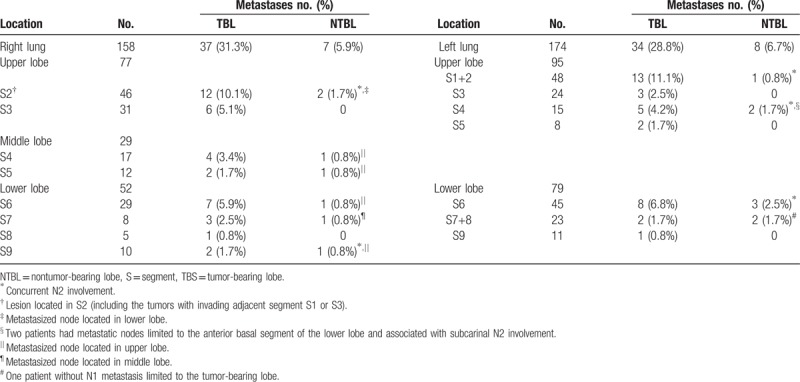
3.4. Tumor location and status of N1 involvement
The details of tumor location are shown in Table 5. Among 86 patients with N1 involvement and 32 with N1 and N2 involvement, 15 (12.7%) had lymph node metastases in an NTBL. Of those 15 patients, 9 had metastases in an N2 site. Considering the direction of lymph node drainage and location of the tumor, a greater number of lymph node metastases from adjacent upper lobe and segments were observed in 6.25% of lesions (10/160) in the upward-invasion group. These represented lesions primarily located in the lower and middle lobes, regardless of fissure completeness or tumor patterns extending beyond the fissure. Five of these 15 patients with lymph node metastasis in an adjacent NTBL were observed in the downward-invasion group. Interestingly, all primary lesions were located in the upper lobes and were associated with concurrent N2 disease. Lymph node metastasis of the apico-posterior and lingual segments of the upper lobe of the left lung were observed with 5 primary lesions in the lower lobe of the left lung.
Table 5.
Primary tumor location and status of N1 metastases.
3.5. Lymph node metastases in the NTBL
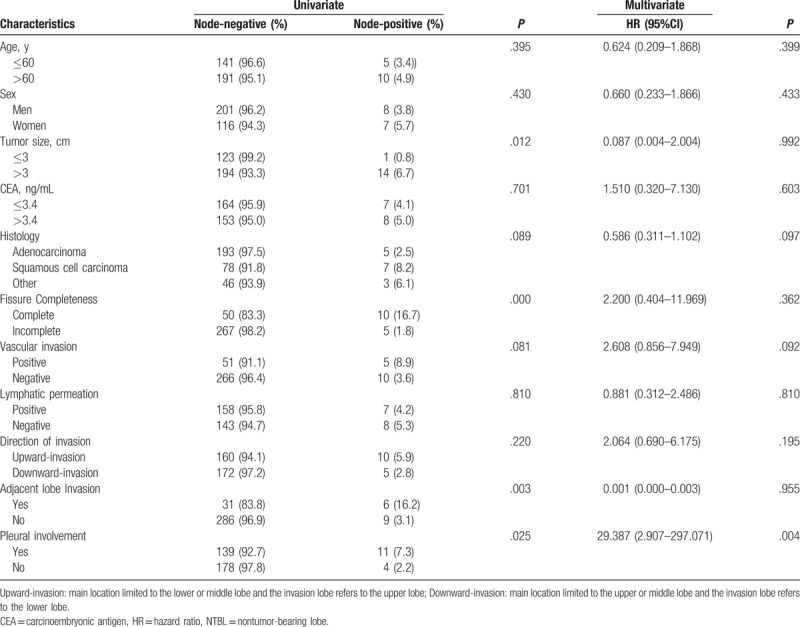
Follow-up and therapy of patients with lymph node metastases in NTBL are summarized in Table 6. Both univariate and multivariate logistic analyses were performed to evaluate the impact of potential predictors of lymph node metastasis in NTBL. As shown in Table 7, the univariate analysis identified that pleural involvement, ALI, complete fissure, and tumor size were statistically significant predictors. The result of the multivariate analysis revealed that pleural involvement was an independent predictor of lymph node metastasis in NTBL.
Table 6.
Follow-up and therapy in patients with lymph node metastases in NTBL.
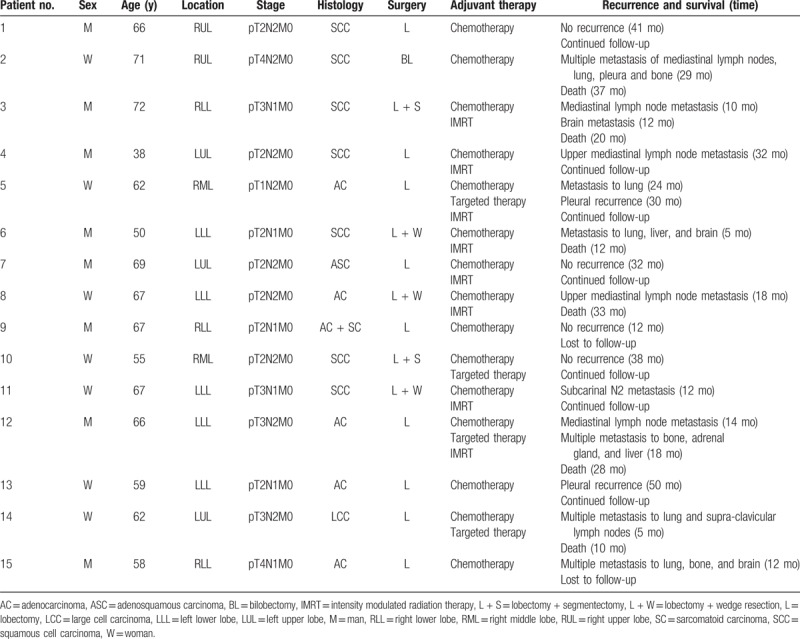
Table 7.
Univariate and multivariate analyses of lymph node metastases in NTBL.
4. Discussion
Some previous reports have described the appropriateness of selective nodal dissection based on the extent of lobe-specific nodal spread.[13] Pleural invasion indicates biological tumor invasiveness and is associated with a poor outcome.[12] From an anatomical point of view, small vascular structures, including lymphatic ducts and blood vessels, are abundant in the subpleural space.[13] Theoretically, tumors invade adjacent segments and lobes, or directly extend from the primary lesion into adjacent lobes beyond the complete fissure through 2 layers of the visceral pleura. This increases lymphatic drainage and the probability of nodal involvement in adjacent lung segments and lobes. Giovanni Leuzzi et al[14] reported a pN0 rate of 58%, whereas other authors have reported that pN0 generally occurs in <50% of cases. However, nodal involvement is present in more than two-thirds of patients because the invading tumor drains into the adjacent lobe lymphatic system.[15,16] Although the frequency of pleural invasion occurred in 45.2% of patients in our study, the N involvement rate was 37.9% and the lymphatic invasion rate was 33.1%. There is not yet a clear definition of ALI as a potential factor affecting node involvement.[15–18] In this study, ALI differentiated tumors that invaded beyond the intersegmental and interlobar planes into the adjacent lobe. Moreover, controversies exist as to the treatment of tumors with fissure invasion. Yang et al[17] reported that, in their study, the patients who underwent pneumonectomy or bilobectomy had better survival rates than those who underwent nonanatomical resections. On the contrary, Okada et al[18] and Haam et al[19] reported that survival did not differ between the patients who underwent lobectomy plus partial resection and those who underwent an anatomical resection. Actually, N1 involvement was observed far less than N2 involvement, which is attributed to the tumor cells exfoliating from the visceral pleura, disseminating into the pleural cavity, and being reabsorbed by the parietal pleural and diaphragmatic lymph vessels to move toward the mediastinal lymph nodes.[20] pN2 involvement occurred more frequently than pN1 involvement in tumors invading the visceral pleura. This finding that was also reported by Miura et al,[15] who found that 30% of pN2 and 13.3% of pN1 occurred with visceral pleural involvement. In this study, the frequency of N2 involvement in the tumors invading across complete fissures was higher than that of tumors invading across incomplete fissures, which is in accordance with previous reports.[21] However, the frequency of N1 metastases in the tumors invading across incomplete fissures was not statistically different from that of tumors without invasion across incomplete fissures. The side of the primary tumor was thought to influence the extent of lobe-specific nodal spread; however, there might be other specific mechanisms.
The incidence of metastasis to segmental lymph nodes in TBS was higher than that in NTBSs, as had been expected. Metastasis outside the TBS was observed in 4 patients, which represented 1.2% all lesions. Of 47 patients with segmental lymph node metastases, 26 had metastases limited to TBSs, 17 had metastases to both TBSs and NTBSs, and 4 had metastases to NTBSs alone, as similarly described by some authors.[9,22] Solid type tumors comprise 100% with metastases to NTBSs. In the literature, 16% of solid type tumors of 20 mm or less are associated with nodal metastasis.[23] However, these investigations rarely noted the impact of clinical characteristics on lymph node metastases in NTBL. The present study found that 15 (12.7%) patients had lymph node metastases in NTBL and 9 had metastases in N2 sites. As reported in the literature,[24] whether or not LN metastases occurred in NTBL did not affect staging or treatment. We demonstrated a significantly higher frequency of lymph node metastases in NTBL among tumors invading across complete fissures and in tumors sized 3.0 cm or more in diameter. Moreover, the multivariate analysis revealed that pleural involvement was an independent predictor of lymph node metastasis in NTBL. However, these results are questionable considering the number of samples and statistical bias.
Regional lymph node metastases of among the tumors with invading across fissures were probably associated with a variety of factors, including histology, tumor size and variations of the lymphatic drainage pathways. In addition, different recurrence patterns were identified in 15 patients whose N1 diseases were observed in the NTBLs. In our study, regional mediastinal lymph node or distant metastases were common recurrences. It was a lower probability event and different from the multi-N1 metastases subgroup in the same lobe, which was likely more aggressive in tumors invading across the fissure. However, it will be necessary to investigate in further studies with more cases and long-term follow-up to confirm the consideration.
Tumor size was an important factor in the classification of T staging in the 8th edition of the TNM classification,[3] and the relationship between T and N staging was not clearly correlated, as shown by Warren and Faber.[25] We observed that the ratio of N1 and N2 staging increased significantly with tumor sizes of 3.0 cm or more in diameter, especially among tumors that invaded into the adjacent lobe beyond the fissure. In fact, even limited to the patients with a tumor 2.0 cm or less, N1 to N2 cases occurred frequently.[26] It is probably that the tumor needs to be larger to have a greater chance of invading through the fissure. Based on the relationship between tumor location and the adjacent areas of the lung lobes and segments, the upward-invasion group was prone to more lymph nodes metastasizing to the adjacent lobe and apico-posterior segments, which lesions in the lower lobe, similarly, and it also appeared lymph metastasis in posterior segment of upper lobe in the lesions located in right lung dorsal segment of lower lobe regardless of fissures completeness and patterns of tumor extending beyond fissure. Consistent with the literature,[27] a similar phenomenon was observed in the right lobe. Some studies suggested that the T1-2 group in NSCLC showed no significant difference in prognosis regardless of fissure completeness.[28] Saeteng et al[29] and Maniwa et al[30] reported that tumor location was not a precise predictor of the pattern of nodal metastasis. However, Xiong et al[27] reported that lymph node metastasis varied according to tumor location and T-stage, which were independent factors influencing N2 station lymph node metastasis. Therefore, the systematic lymphatic resection, including lymph node dissection of NTBL and NTBS, was more significant and could accurately determine pathological staging, guidance for prognosis, and target adjuvant treatments.
Lymph node metastases can appear in the NTB segments, lobes, and even skip metastasis, with regard to lymph drainage on the abnormal anatomy of pulmonary segments. Zuo et al[31] found that pulmonary segmental anatomy was relatively independent, owing to continuous interval segments, with no alveolar hole sections. We hypothesized that tumors invading adjacent parenchyma across the interval would be hindered and that the interval might shut down the spread pathway of spread for tumor cells along the alveolar hole. Incomplete fissures indicating partial fusion between lung lobes might also alter the spread of the tumor within the lung. It is even probable that tumor cells can spread through air spaces (STAS).[32] This indicates that fissure status could influence lymphatic spread and that abnormal lymphatic drainage could possibly occur through multiple pulmonary metastases. However, it was difficult to determine fissure completeness from the hypovascular zones. Many methods reported in the literature cannot be accurately evaluated,[7] especially because tumors with extension of the primary tumor across incomplete lung fissures could spread to adjacent lobes through the parenchyma. Currently, there are few similar diagnostic criteria for incomplete interlobar fissures.[6,10] There were 60 (18.1%) complete fissures and 272 (81.9%) incomplete fissures in our study. The frequency of complete fissures was lower than previously reported.[33] A possible reason for this difference might lie in the selection of tumors that were only located in the vicinity of part of the fissure, without considering the fissure type.
This study had some limitations. First, it was difficult to avoid selection bias. This study was carried out in a single-center, with a small number of patients, and included some deficiencies. Lymph node dissection of the adjacent lobes and segments could have been affected by tumor degree, spread pattern, anatomical structure of the lobe, fissure status, and the surgical approach. For example, with middle lobe lesions of undeveloped horizontal fissures, because of the excessive dissection of the deep lung parenchymal tissue it was difficult to accurately perform lymph node dissection around the bronchus in the anterior segment of the upper lobe. This was especially true with wedge resections of the lung, which were limited by poor pulmonary function, the integrity and thoroughness of the lymph node dissection, or representative nodes that were one sided. Second, the study selected only tumors located in the vicinity of the fissures. Our hypothesis was that the lymphatic drainage in different areas of the lobe might be different. In 5.67% (43 cases) of the patients who were excluded from the study, whose diseases away from the area of the fissures were not in this study. Third, the operative procedure and surgical type might be altered according to tumor size, lesion location, fissure structure, patient pulmonary function, and the preference and technical ability of the surgeons. Furthermore, it seemed more likely that anatomical resections could obtain more accurate assessments of N1 involvement than wedge resections of the adjacent invaded lobes, with lymph node sampling as a limited procedure to be performed with curative intent.
Our study, conducted at a single cancer institution, demonstrated that a higher frequency of N2 metastases on adjacent lobes existed in tumors with invasion across complete fissures. With regard to the status of interlobar fissures, no difference in N1 involvement existed in the tumors with and without invasion of the incomplete fissure. Removing lymph nodes in the NTBL did not seem to improve the accuracy of detecting N1 involvement. However, lymphatic metastasis of NTBL was a lower probability event that was likely to be more aggressive in tumors crossing complete fissures. Therefore, extensive lymphatic resection within the hilum and NTBL for tumors with pleural involvement is justifiable and necessary unless there are other technical reasons that hinder such extensive lymphatic resection of the NTBL. Further prospective studies with large samples in multicenter environments could help to address the limitations of this study in the future.
Acknowledgments
We appreciate Dr. Ruimin Wang for reviewing and editing the manuscript, and also thank Editage (www.editage.com) for English language editing.
Author contributions
Conceptualization: Hui Li, Ruimin Wang, Qi Liu, Jiajun Du.
Data curation: Hui Li, Ruimin Wang, Qi Liu, Jiajun Du.
Formal analysis: Hui Li, Ruimin Wang, Qi Liu, Jiajun Du.
Funding acquisition: Hui Li, Ruimin Wang, Qi Liu, Jiajun Du.
Investigation: Dexian Zhang, Yongming Zhang, Wanhu Li, Qi Liu, Jiajun Du.
Methodology: Dexian Zhang, Yongming Zhang, Wanhu Li, Qi Liu, Jiajun Du.
Project administration: Dexian Zhang, Yongming Zhang, Wanhu Li, Qi Liu, Jiajun Du.
Resources: Dexian Zhang, Yongming Zhang, Wanhu Li, Qi Liu, Jiajun Du.
Software: Dexian Zhang, Yongming Zhang, Wanhu Li, Qi Liu, Jiajun Du.
Supervision: Baijiang Zhang, Qi Liu, Jiajun Du.
Validation: Baijiang Zhang, Qi Liu, Jiajun Du.
Visualization: Baijiang Zhang, Qi Liu, Jiajun Du.
Writing – original draft: Baijiang Zhang, Qi Liu, Jiajun Du.
Writing – review and editing: Baijiang Zhang, Qi Liu, Jiajun Du.
Footnotes
Abbreviations: ALI = adjacent lobe invasion, CEA = carcinoembryonic antigen, NALI = nonadjacent lobe invasion, NSCLC = nonsmall cell lung cancer, NTBL = nontumor-bearing lobe, NTBS = nontumor-bearing segment.
This study was supported by the Natural Science Foundation of Shandong (ZR2013HL046) and by the research fund of Shandong Academy of Medical Sciences (no. 2016-21).
The authors have no conflicts of interest to disclose.
References
- [1].Hashim D, Boffetta P, La Vecchia C, et al. The global decrease in cancer mortality: trends and disparities. Ann Oncol 2016;27:926–33. [DOI] [PubMed] [Google Scholar]
- [2].Cahan WG. Radical lobectomy. J Thorac Cardiovasc Surg 1960;39:555–72. [PubMed] [Google Scholar]
- [3].Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193–203. [DOI] [PubMed] [Google Scholar]
- [4].Lee S, Lee JG, Lee CY, et al. Pulmonary fissure development is a prognostic factor for patients with resected stage I lung adenocarcinoma. J Surg Oncol 2016;114:848–52. [DOI] [PubMed] [Google Scholar]
- [5].Raasch BN, Carsky EW, Lane EJ, et al. Radiographic anatomy of the interlobar fissures: a study of 100 specimens. AJR Am J Roentgenol 1982;138:1043–9. [DOI] [PubMed] [Google Scholar]
- [6].Mahmut M, Nishitani H. Evaluation of pulmonary lobe variations using multidetector row computed tomography. J Comput Assist Tomogr 2007;31:956–60. [DOI] [PubMed] [Google Scholar]
- [7].Heřmanová Z, Ctvrtlík F, Heřman M. Incomplete and accessory fissures of the lung evaluated by high-resolution computed tomography. Eur J Radiol 2014;83:595–9. [DOI] [PubMed] [Google Scholar]
- [8].Lardinois D, Leyn PD, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787–92. [DOI] [PubMed] [Google Scholar]
- [9].Sakairi Y, Yoshino I, Yoshida S, et al. Pattern of metastasis outside tumor-bearing segments in primary lung cancer: rationale for segmentectomy. Ann Thorac Surg 2014;97:1694–700. [DOI] [PubMed] [Google Scholar]
- [10].Craig SR, Walker WS. A proposed anatomical classification of the pulmonary fissures. J R Coll Surg (Edin) 1997;42:233–4. [PubMed] [Google Scholar]
- [11].Hammar SP. Dail DH, Hammar SP. Common tumors. Pulmonary Pathology 2nd ed.New York: Springer-Verlag; 1994. 1138. [Google Scholar]
- [12].Travis WD, Brambilla E, Rami-Porta R, et al. Visceral pleural invasion:pathologic criteria and use of elastic stains: proposal for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol 2008;3:1384–90. [DOI] [PubMed] [Google Scholar]
- [13].Riquet M, Rivera C, Pricopi C, et al. Is the lymphatic drainage of lung cancer lobe-specific? A surgical appraisal. Eur J Cardiothorac Surg 2015;47:543–9. [DOI] [PubMed] [Google Scholar]
- [14].Leuzzi G, Cesario A, Cafarotti S, et al. Surgical treatment in patient with non-small-cell lung cancer with fissure involvement anatomical versus nonanatomical resection. J Thorac Oncol 2013;9:97–108. [DOI] [PubMed] [Google Scholar]
- [15].Miura H, Taira O, Uchida O, et al. Invasion beyond interlobar pleura in non-small cell lung cancer. Chest 1998;114:1301–4. [DOI] [PubMed] [Google Scholar]
- [16].Demir A, Gunluoglu MZ, Sansar D, et al. Staging and resection of lung cancer with minimal invasion of the adjacent lobe. Eur J Cardiothorac Surg 2007;32:855–8. [DOI] [PubMed] [Google Scholar]
- [17].Yang HX, Hou X, Lin P, et al. Peripheral direct adjacent lobe invasion non-small cell lung cancer has a similar survival to that of parietal pleural invasion T3 disease. J Thorac Oncol 2009;4:1342–6. [DOI] [PubMed] [Google Scholar]
- [18].Okada M, Tsubota N, Yoshimura M, et al. How should interlobar pleural invasion be classifed? Prognosis of resected T3 non-small cell lung cancer. Ann Thorac Surg 1999;68:2049–52. [DOI] [PubMed] [Google Scholar]
- [19].Haam SJ, Park IK, Paik HC, et al. T-stage of non-small cell lung cancer directly invading an adjacent lobe. Eur J Cardiothorac Surg 2012;42:807–10. [DOI] [PubMed] [Google Scholar]
- [20].Manac’h D, Riquet M, Medioni J, et al. Visceral pleura invasion by non-small cell lung cancer: an underrated bad prognostic factor. Ann Thorac Surg 2001;71:1088–93. [DOI] [PubMed] [Google Scholar]
- [21].Riquet M, Berna P, Arame A, et al. Lung cancer invading the fissure to the adjacent lobe: more a question of spreading mode than a staging problem. Eur J Cardiothorac Surg 2012;41:1047–51. [DOI] [PubMed] [Google Scholar]
- [22].Yamanaka A, Hirai T, Fujimoto T, et al. Analyses of segmental lymph node metastases and intrapulmonary metastases of small lung cancer. Ann Thorac Surg 2000;70:1624–8. [DOI] [PubMed] [Google Scholar]
- [23].Hattori A, Suzuki K, Matsunaga T, et al. Is limited resection appropriate for radiologically “solid” tumors in small lung cancers? Ann Thorac Surg 2012;94:212–5. [DOI] [PubMed] [Google Scholar]
- [24].Li N, Tan F, Li J, et al. Blind spot in lung cancer lymph node metastasis: cross-lobe peripheral lymph node metastasis in early stage patients. Thorac Cancer 2018;9:480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg 1994;107:1087–94. [PubMed] [Google Scholar]
- [26].Takizawa T, Terashima M, Koike T, et al. Lymph node metastasis in small peripheral adenocarcinoma of the lung. J Thorac Cardiovasc Surg 1998;116:276–80. [DOI] [PubMed] [Google Scholar]
- [27].Xiong J, Wang R, Sun Y, et al. Lymph node metastasis according to primary tumor location in T1 and T2 stage non-small cell lung cancer patients. Thorac Cancer 2016;7:304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kamiyoshihara M, Kawashima O, Sakata S, et al. Does an incomplete interlobar fissure influence survival or recurrence in resected non-small-cell lung cancer? Lung Cancer 1999;25:33–8. [DOI] [PubMed] [Google Scholar]
- [29].Saeteng S, Tantraworasin A, Euathrongchit J, et al. Nodal involvement pattern in resectable lung cancer according to tumor location. Cancer Manag Res 2012;4:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Maniwa T, Okumura T, Isaka M, et al. Recurrence of mediastinal node cancer after lobe-specific systematic nodal dissection for non-small-cell lung cancer. Eur J Cardiothorac Surg 2013;44:e59–64. [DOI] [PubMed] [Google Scholar]
- [31].Zuo Y, Li L, Liu S. Kohn's pores are not responsible for collateral ventilation between inflated and deflated segments: a microscopic study of pulmonary intersegmental septa in the human lung. J Anat 2015;226:381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kadota K, Nitadori Ji, Sima Cs, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol 2015;10:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Unver Dogan N, Uysal II, Demirci S, et al. Major anatomic variations of pulmonary fissures and lobes on postmortem examination. Acta Clin Croat 2015;54:201–7. [PubMed] [Google Scholar]


