Abstract
Background:
Perioperative bleeding during total knee arthroplasty (TKA) is an ongoing problem for surgeons. Intravenous or topical application of tranexamic acid (TXA) can effectively stop bleeding, but there is still no uniform standard for the best method of administration and dose.
Methods:
From October 2016 to September 2018, 218 patients with unilateral primary knee osteoarthritis requiring knee replacement were enrolled and randomly divided into four groups. Group 1 (n = 55) received intra-articular injection (IAI) of TXA and peri-articular injection (PAI) of placebo, group 2 (n = 55) received IAI of placebo and PAI of TXA, group 3 (n = 51) received IAI of TXA and PAI of TXA, and group 4 (n = 57) received double placebo (IAI of placebo and PAI of placebo). The demographic characteristics, surgical indices, hematological indices, wound healing history, and thromboembolic events were investigated.
Results:
Eight patients were lost to follow-up and 210 patients were included in the analysis. The median TBLs in patients who received IAI of TXA and PAI of placebo and those who received IAI of placebo and PAI of TXA were 470.81 ml and 481.54 ml, respectively. These TBL levels were significantly higher compared to those in patients who received IAI of TXA and PAI of TXA (359.18 ml, P ≤ .001), but significantly lower compared to those in patients who received the double placebo (522.71 ml, P ≤ .001). Compared to other groups, more patients in the double placebo group needed a blood transfusion (P = .013). In the short-term, the double placebo group had higher VAS pain scores and less ROM after surgery (P = .011 and P = .001, respectively). In the long-term (6-month follow-up), there were no significant differences in ROM, VAS, DVT, PE, or wound-related complications.
Conclusion:
The combined use of IAI and PAI of TXA can significantly reduce the TBL and the need for blood transfusion without delaying wound healing or increasing the risk of DVT and PE. In the short-term after surgery, this combined method reduces the pain VAS scores and improves the ROM; however, there are no long-term effects on VAS and ROM.
Keywords: blood loss, multi-route applications, total knee arthroplasty, tranexamic acid
1. Introduction
Control of the massive bleeding that occurs during total knee arthroplasty (TKA) has always been a difficult problem for surgeons. The total amount of bleeding can reach 1000 to 2000 ml.[1] The complications and economic burden associated with blood transfusions should not be ignored.[2] Therefore, control of blood loss during TKA is very important. Reports suggest that tranexamic acid (TXA) has a strong effect on reducing blood loss by blocking the lysine binding sites of plasminogen.[3]
The common methods of TXA administration are intravenous drip and local administration.[4] Although intravenous infusion of TXA can significantly reduce postoperative blood loss, the associated risk of deep vein thrombosis (DVT) and pulmonary embolism (PE) makes its use controversial.[5,6] Although some studies have proven the safety and effectiveness of topical application of TXA,[7–9] the optimal route and method of administration of TXA is yet to be determined.[10,11] A large number of studies have shown that combined use of TXA is more effective in hemostasis than single use.[12–14] Most of the combined applications involve an intravenous drip combined with intra-articular injection (IAI), or an intravenous drip combined with peri-articular injection (PAI).[13,15] There are no reports on the combined application of an intravenous drip, IAI, and PAI. Therefore, we designed a randomized controlled trial to compare the effects of different TXA administration methods on blood loss after TKA in order to find the best way to stop bleeding during TKA.
2. Materials and methods
2.1. Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the local Ethics Committee of the Zaozhuang Municipal Hospital in Shandong. Informed written consent was obtained from all patients or their guardians before their enrollment in this study. Registration number: 16-0920; Name of trial registry: study on the hemostatic effect of TXA after knee arthroplasty.
2.2. Study design
From October 2016 to September 2018, 246 patients with knee osteoarthritis requiring unilateral TKA in Zaozhuang Municipal Hospital were included. The exclusion criteria were as follows:
-
1.
patients with hemorrhagic diseases,
-
2.
patients treated with anticoagulation or with a history of thrombosis,
-
3.
patients with malignant tumors, and
-
4.
patients who were reluctant to participate in the study.
Twenty-eight patients were excluded for the following reasons. Seven patients refused to participate, 15 patients had a history of thrombosis or anticoagulation therapy, 5 patients had cancer, and 1 patient had a hematological disease. Therefore, 218 patients were enrolled in the study. Eight patients were lost to follow-up and the data of 210 patients was included in the analysis (Fig. 1).
Figure 1.
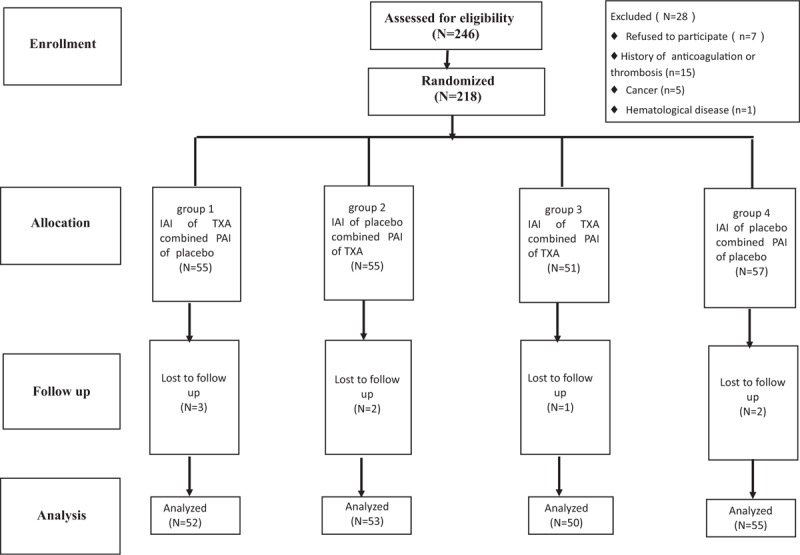
The flow diagram shows the number of patients assessed and included at each stage of the trial. IAI = intra-articular injection, PAI = peri-articular injection, TXA = tranexamic acid,.
2.3. Randomization and masking
Patients were randomly divided into 4 groups: group 1 (n = 55) received IAI of TXA and PAI of placebo, group 2 (n = 55) received IAI of placebo and PAI of TXA, group 3 (n = 51) received IAI of TXA and PAI of TXA, and group 4 (n = 57) received double placebo (IAI of placebo and PAI of placebo). Randomization sequence was created using Stata 9.0 (StataCorp, College Station, TX) statistical software and was stratified by center with a 1:1 allocation using random block sizes of 2, 4, and 6. All investigators, staff, and participants were kept masked to outcome measurements and trial results.
All patients had the same preoperative preparation and were operated on by the same team. LPS-FLEX prostheses (Zimmer Inc., Warsaw, IN) were used in all patients. Atourniquet (Zimmer ATS 2000; Zimmer, Warsaw, IN) was used in all patients and the pressures were set at 100 mmHg higher than the systolic pressure of the patients. All patients were injected intravenously with 20 mg/kg TXA (TXA Injection; Reyong Pharmaceutical Factory, Shandong, China)15 minutes before tourniquet compression.[16] To stop bleeding, the tourniquet was loosened and electric coagulation was used after prosthesis installation. A peri-articular injection of TXA solution (1 g TXA, 20 ml saline) comprising 5 ml to the medial capsule, 5 ml to the lateral capsule, and10 ml to the soft tissue around quadriceps femoris.[17] The TXA solution of 20 ml (TXA 1 g + 20 ml saline) was injected into the articular cavity after suture incision. All placebos involved injection of the same amount of saline at the same place.
After surgery, a drainage tube was routinely placed and clamped for 4 hours, and then removed 24 hours after surgery. Elastic bandages were wrapped around the lower limbs and removed 24 hours after surgery. Quadriceps femoris training was performed on the first day after surgery. All patients walked on the ground using walking aids on the third day after surgery. Considering the serious consequences of DVT and PE, all patients were injected with 1 ml of low molecular weight heparin calcium subcutaneously (Fraxiparine; GlaxoSmithKline, Brentford, UK) on the first day after surgery. After discharge, 10 mg of rivaroxaban (Xarelto; Bayer Schering Pharma AG, Leverkusen, Germany) was taken orally per day up to 5 weeks after the operation. Patients with lower extremity swelling underwent bilateral lower extremity venous ultrasonography to detect DVT.
2.4. Data collection
The observed variables included:
-
1.
demographic variables such as age, sex, and body mass index (BMI);
-
2.
preoperative measurements such as Kellgren–Lawrence classification, visual analog scale (VAS), range of motion (ROM), hemoglobin (Hb) level, hematocrit (Hct) level, platelet count (PLT), prothrombin time (PT), and activated partial thromboplastin time (APTT);
-
3.
surgical measures such as operation time, intraoperative blood loss, blood transfusion volume, total blood loss (TBL), tourniquet time, intraoperative blood loss, and drainage volume;
-
4.
postoperative measures such as Hb and Hct levels on the first day after surgery and ROM and VAS on the second day after surgery; and
-
5.
complications such as delayed wound healing and incidence of DVT and PE.
At the 6-month follow-up, assessments included ROM, VAS, wound healing, and incidence of DVT and PE. The patient's total blood volume was calculated according to the following formula[18]: male blood volume (ml) = 366.9 × height (m)3 + 32.19 × weight (kg) + 604.1 OR female blood volume (ml) = 356.1×height (m)3 +33.08 × weight (kg) +183.3. The TBL was calculated according to the followingformula[19]: total blood loss/theoretical blood loss (ml) = total blood volume (ml) × (Hct 1 day before surgery–Hct 1 day after surgery). For patients receiving blood transfusion therapy, the TBL was calculated according to the following formula: TBL (ml) = theoretical blood loss (ml) + red blood cell transfusion (U). The unit of transfusion volume was U, which is the equivalent to 200 ml of whole blood. Hidden blood loss (HBL) (ml)= actual blood loss (ml) – intraoperative blood loss (ml).
2.5. Statistical analysis
Data were analyzed using SPSS24.0 analysis software (IBM, Chicago, IL) and a Shapiro–Wilk test was used to evaluate the normal distribution of quantitative data. Indicators conforming to normal distribution were expressed in as mean ± standard deviation. A 1-way ANOVA was used for comparisons within groups, and least significant difference Statistics (LSD) was used for comparison between the two groups. Indicators that did not conform to normal distribution were described as median and quartile. A multiple independent sample rank sum test (Kruskal–Wallis H test) was used for comparison within groups. An independent sample rank sum test (Mann–Whitney U test) was used for comparison between the 2 groups. A Chi-square test or Fisher exact test was used for counting data. A P value <.05 was considered statistically significant.
3. Results
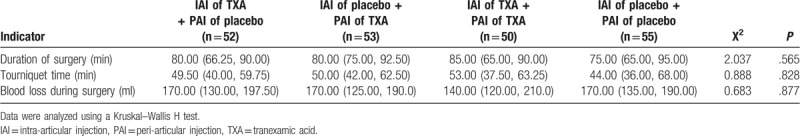
Preoperatively, there were no significant differences in age, sex, height, weight, BMI, Kellgren–Lawrence classification, or other measures (VAS, ROM, Hb, HCT, PLT, PT, and APTT) among the 4 groups (all P > .05). Because of the high incidence of osteoarthritis in female patients, the male/female ratios in each group were 14/38, 16/37, 11/39, and 12/43, respectively; however, there was no significant difference in gender distribution among the groups (P = .709) (Table 1). All operations were performed by the same surgical team. There were no significant differences in operation time, tourniquet time, or intraoperative blood loss (all P > .05) (Table 2).
Table 1.
Preoperative demographics.
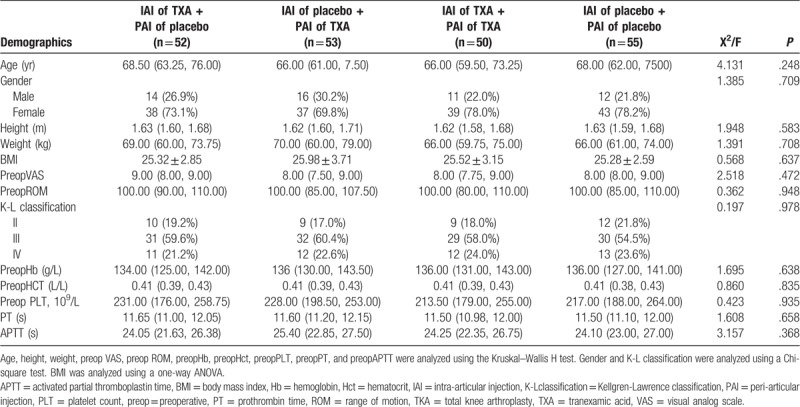
Table 2.
Operative procedure.
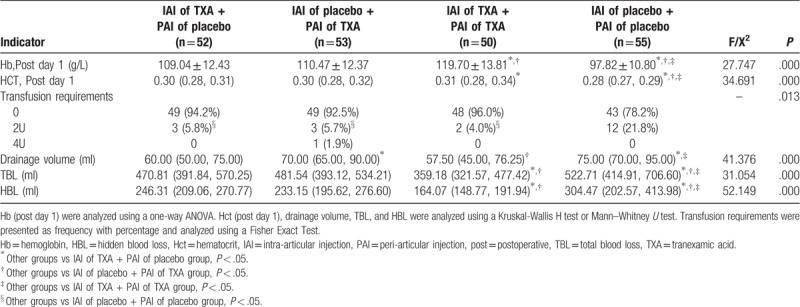
Patients who received IAI of TXA and PAI of TXA showed a better hemostatic effect compared to the other patients. On postoperative day 1, there were significant differences in Hb and HCT between the four groups (P < .001). The mean values of Hb in patients who received IAI of TXA and PAI of placebo and those who received IAI of placebo and PAI of TXA were 109.04 g/L and 110.47 g/L, respectively, and these values were lower compared to those in patients who received IAI of TXA and PAI of TXA (119.70 g/l) (P ≤ .001), but higher compared to those in patients who received the double placebo (IAI of placebo and PAI of placebo) (97.82 g/L) (P ≤ .001). Significantly lower HCTs were observed in patients who received IAI of TXA and PAI of placebo and those who received IAI of placebo and PAI of TXA compared to those in patients who received IAI of TXA and PAI of TXA (P = .02); however, all of these HCTs were higher compared to patients who received double placebo (P < .001). TBL and HBL were higher in patients who received IAI of TXA and PAI of placebo (470.81 ml and 246.31 ml, respectively) and those who received IAI of placebo and PAI of TXA (481.54 ml and 233.15 ml, respectively) compared to those in patients who received both IAI and PAI of TXA (359.18 ml and 164.07 ml, respectively) (all P ≤ .001), but lower compared to those in patients who received the double placebo (522.71 ml and 304.47 ml, respectively) (P ≤ .001 and P = .03, respectively). The hemostatic effects in patients who received IAI of TXA and PAI of placebo and those who received IAI of placebo and PAI of TXA were similar because there were no differences in Hb, Hct, TBL, or HBL (all P > .05).
The indications for transfusion in this study were as follows: hemoglobin <80 g/L, Hct ≤ 20%, or symptoms of severe anemia (such as dizziness and syncope). Although there were no significant differences in the other 3 groups, the transfusion requirements in those groups were significantly lower compared to patients who received the double placebo (P = .013). The drainage volumes in patients who received IAI of TXA and PAI of placebo and those who received IAI of placebo and PAI of TXA were significantly lower than the other 2 groups (P < .001), but there were no other significant differences between groups (all P > .05) (Table 3).
Table 3.
Hemostatic effect.
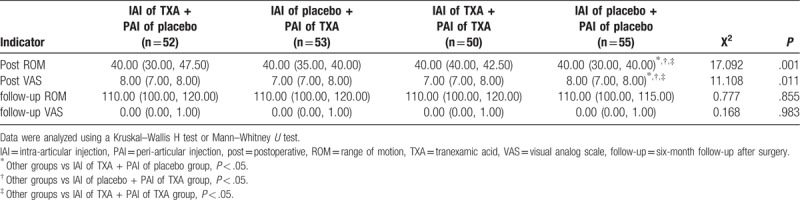
On postoperative day 2, there were no significant differences in ROM and VAS in the treatment groups; however, the VAS scores were significantly higher (P = .011) and the ROMs were significantly lower (P = .001) in the patients who received the double placebo. Despite these findings, there were no significant differences in VAS (P = .983) or ROM (P = .855) in the 4 groups at the 6-month follow-up (Table 4).
Table 4.
Pain and function.
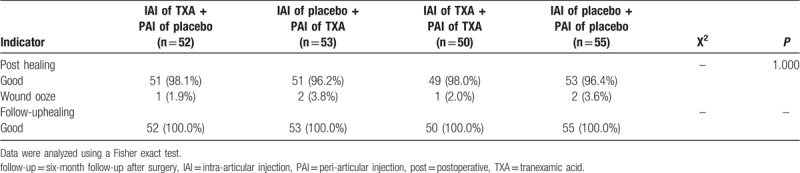
One case of asymptomatic DVT was detected by Doppler ultrasound examination 7 days after surgery in the group of patients who received IAI of TXA and PAI of placebo (P = .386). There were no cases of postoperative PE, DVT at the 6-month follow-up, or PE at the 6-month follow-up (Table 5). The rates of wound-related complications were similar in all groups (P = .99). All wound-related complications were ultimately cured on follow-up (Table 6).
Table 5.
Thromboembolic events.
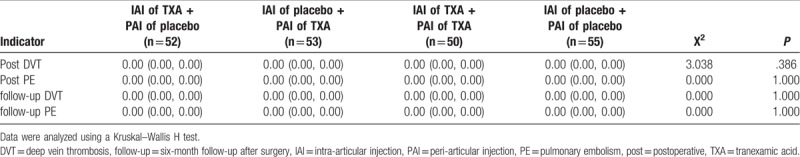
Table 6.
Wound-related complications.
4. Discussion
Patients undergoing TKA experience massive bleeding. At present, TXA is the main drug used to reduce bleeding.[20] TXA is a synthetic amino acid that competes with fibrinogen for lysine binding sites and inhibits fibrinolysis to achieve hemostasis.[21] Because fibrinolytic activation is an enzyme cascade process, it can be inhibited quite easily in the early stage.[22]
There is no doubt about the hemostatic effect of TXA.[23] In theory, TXA prevents fibrinolysis and supports ongoing coagulation without increasing thrombosis.[22] Some studies and meta-analyses have suggested that TXA reduces blood loss without increasing the risk of DVT and PE.[24] However, there are also opposing reports.[25] Ravendran[26] argued that the effect of TXA on thromboembolic events remains uncertain. Intravenous drip is the most commonly used method at present. Other drug delivery methods include intramuscular injection, oral administration, and intra-articular injection.[27] Studies have shown that only a small portion of intravenous TXA solution reaches the target tissue.[28] In view of the high coagulation state of the whole body and the serious consequences of thrombosis complications, local application is a better choice. Compared with intravenous drip, systemic TXA plasma concentration decreases by 70% after topical administration, and the hemostasis effect is similar.[29] In terms of overall impact, if the effect is the same, local application is more advantageous than systemic application.[30] A recent meta-analysis by Dai also found no difference in transfusion rates or TBL according to whether local application or intravenous infusion of TXA was used.[31] However, in the above studies, the doses of topical application and intravenous drip were different in each study group.[29] There is still no uniform standard recommendation for the minimum dose to achieve the best results.[10,11]
Local applications currently include PAI and IAI. Most literature has shown that PAI of TXA is less risky than intravenous drip.[32,28] TXA has a half-life of about 3 hours in articular fluid and can effectively stop bleeding during the peak period of bleeding after surgery.[33] However, many studies dispute this conclusion because the amount of intra-articular injection is 5 to 25 ml, which is not enough to immerse the anterior tissue of the knee joint in a supine position after surgery. In addition, in order to balance the force line of the lower limbs, a large amount of soft tissue release is sometimes required, which may lead to leakage of injected TXA solution.[28,34] We believe that although the injection dose may be low, the joint cavity is filled with blood and TXA due to the clamping of drainage tube, which can effectively stop bleeding.
In our study, the median Hct value (0.30) and the mean Hb value (109.04 g/L) in patients who received IAI of TXA + PAI of placebo on the first postoperative day were significantly higher than compared to patients who received the double placebo (IAI of placebo + PAI of placebo) (0.28, 97.82 g/L) (all P < .001). The median TBL (470.81 ml) in patients who received IAI of TXA + PAI of placebo was significantly lower compared to patients who received the double placebo (522.71 ml) (P = .03).
A total of 21.8% of patients who received the double placebo required a blood transfusion, and this rate was significantly higher compared to patients who received IAI of TXA + PAI of placebo (5.8%) (P = .02). The median TBL (481.54 ml) in patients who received IAI of placebo + PAI of TXA was significantly lower compared to that in patients who received the double placebo (522.71 ml) (P = .04). We know that PAI involves injecting TXA solution into soft tissue and this creates a risk of bleeding around the joint before the incision is closed. Compared with intravenous drip, local injection can reduce systemic toxicity, directly affect injured tissues, last longer, have better hemorrhage control, and reduce complications related to the risk of thrombosis events during systemic application.[35,36,16]
At present, research on combined applications mainly includes PAI combined with intravenous drip, or IAI combined with intravenous drip. It has been reported that intravenous drip combined with IAI is more effective for hemostasis than each of these two in isolation, and there is no increased risk of thrombosis.[12–14] Intravenous drip combined with PAI has the same effect.[37] Some authors believe that intravenous drip combined with local medication and intravenous drip in isolation are equally as effective in reducing blood transfusion rates and TBL, but the combined regimen achieves good results for knee joint pain, knee joint swelling, hospitalization time, and short-term satisfaction after surgery.[38] In our study, the median TBLs in patients who received IAI of TXA + PAI of placebo and patients who received IAI of placebo + PAI of TXA (470.81 ml and 481.54 ml, respectively) were significantly higher compared to patients who received IAI of TXA + PAI of TXA (359.18 ml) (P < .001). However, there was no statistically significant difference in the number of patients needing blood transfusion in the 3 groups (P = .92).
TKA is usually associated with high HBL.[39] HBL is caused by extravasation of blood from tissues, residual blood in joints, and hemolysis. It also includes hidden losses not assessed during and after surgery. HBL accounts for a large part of TBL and nearly two-thirds of TBL comes from HBL.[40] Sehat confirmed that the average HBL after TKA accounted for 49% of the TBL.[41] In this study, the median HBL/median TBL in the 4 groups was 246.31/470.81 (52%), 233.15/481.54 (48%), 164.07/359.18 (46%), and 304.47/522.71 (58%), respectively. Therefore, strategies aimed at reducing HBL are the key to reduction of bleeding. The application of a tourniquet can maintain a clear operative field of vision and reduce intraoperative blood loss; however, it cannot prevent postoperative blood loss.[42] The causes of massive blood loss during TKA are not limited to bone incision and soft tissue injury. Tissue ischemia caused by the tourniquet and/or surgical trauma can cause up-regulation of plasmin activation, and this aggravates bleeding.[43,44] Local injection is effective for soft tissue hemorrhage, but not for intra-articular hemorrhage, such as hemorrhage from the bone after osteotomy. For hemorrhage of the medullary cavity, especially bleeding resulting from fibrinolytic enhancement caused by surgical trauma and/or the tourniquet, the hemostasis effect of PAI and IAI is minimal. It has been reported that inhibition of fibrinolysis at the beginning of surgery is more effective than during the peak of fibrinolysis.[45] Therefore, in this study, preoperative intravenous infusion of TXA was used as a basic drug in all groups. Based on the low dose intravenous drip, and the combination of PAI and IAI, theoretically, this study could achieve a good hemostatic effect.
Regarding the dosage of TXA, the best dose for single use reported at present is 3 g for local use and 10 to 20 mg/kg for intravenous use.[46] Regarding the timing of medication, studies have shown that in order to achieve maximum plasma concentration, an intravenous drip takes 5 to 15 minutes, and intramuscular and intra-articular injection takes about 30 minutes.[47] Therefore, we intravenously infused TXA 15 minutes before applying tourniquet pressure and clamped the drainage tube for 4 hours after surgery so that we could obtain the highest concentration in the operative area. The placebo in the control group was saline, and according to the current medical view, this should not cause harm to patients. There was no significant difference in wound healing complications in the 4 groups (P = .04).
In theory, by creating a filling effect in the joint, clamping drainage can lead to temporary hemostasis, thus effectively reducing blood loss.[48] However, the hemostatic effect is not directly proportional to the clamping time.[49] Prolonged clamping time can increase the risk of infection and lead to intra-articular hematoma.[50] Because most TKA blood loss occurs on the first day after surgery, the drainage volume in the first 8 hours after surgery accounts for 65% of the total drainage volume.[51] Therefore, we chose to clip the drainage tube for 4 hours and pull out the drainage tube 24 hours after surgery. There were no cases of hematoma.
We found that the median drainage volume in patients who received IAI of TXA + PAI of placebo (60.00 ml) was significantly lower compared to patients who received IAI of placebo + PAI of TXA and those who received the double placebo (70.00 ml and 75.00 ml, respectively) (P < .001). The median drainage volume in patients who received IAI of TXA + PAI of TXA (57.50 ml) was significantly lower compared to patients who received IAI of placebo + PAI of TXA and patients who received the double placebo (70.00 ml and 75.00 ml, respectively) (P < .001).
About 0.1% to 2% of patients who undergo arthroplasty have symptomatic or asymptomatic thrombosis.[52] Reports on whether the use of TXA increases the risk of thrombotic events are inconsistent.[24–26] Considering the catastrophic consequences of thrombosis complications, we must pay special attention to the prevention of thrombosis. The treatment includes informing patients about adherence to a low-fat diet and rehabilitative exercises, administration of low molecular weight heparin after surgery, and administration of oral rivaroxaban after discharge and up to 5 weeks after surgery. Only one case of DVT occurred in a patient who received IAI of TXA + PAI of placebo and this occurred on the 7th postoperative day.
Our results showed that the dosage and method of TXA used in our study, combined with postoperative management, proved to be safe and efficient. Of course, we must state that interpretation of these results should be done with caution as we did not conduct thrombosis screening in every patient after surgery. In theory, asymptomatic thrombosis patients may have been neglected.
Early postoperative pain can seriously affect patient satisfaction. It is reported that the pain after TKA is severe in about 60% of patients and moderate in about 30% of patients. Many patients reject undergoing TKA for fear of the resulting pain.[53] The VAS pain scores in patients who received IAI of TXA + PAI of placebo, those who received IAI of placebo + PAI of TXA, and those who received IAI of TXA + PAI of TXA on the second day after surgery were significantly lower compared to patients who received the double placebo (P = .011). Early HBL after surgery can obviously aggravate lower limb swelling.[54] This may be related to the local application of TXA to reduce the postoperative swelling, and thus, relieve the pain caused by swelling. Related to the VAS pain score, the ROM was significantly lower on the second day after surgery in patients who received the double placebo (P = .001). Despite this, at the 6-month follow-up, there were no significant differences in VAS and ROM between the 4 groups (P = .983 and P = .855, respectively). Therefore, TXA can alleviate pain and improve the ROM in the early stages after surgery, but it has no obvious effect the ability to perform rehabilitation exercises in later stages.
There were several limitations to this study. First, the effect of anticoagulants on this trial was not considered. In view of the serious consequences of thrombosis complications, anticoagulant therapy was started on the first day after surgery for all patients. Second, some patients with hypotension after surgery fail to meet the transfusion standard, and they are given fluid replacement treatment. At this time, there may be inaccuracies in the calculation of blood loss. Third, the sample may have significant selection bias. In our study, other diseases with large amount of bleeding after operation were excluded, such as rheumatoid arthritis, synovial chondromatous arthritis and so on. Because these diseases may involve extensive synovectomy and increase the amount of bleeding, further studies on the control of hemorrhage in these diseases are required. Because of the mechanisms behind this morbidity, the proportion of men and women is obviously different, and the influence of drugs on sex was not taken into account. However, there were no significant sex differences among the groups.
In conclusion, we have shown that IAI of TXA and PAI of TXA have the same hemostatic effect. Compared with their use in isolation, the combined use of IAI of TXA and PAI of TXA can significantly reduce the TBL and blood transfusion requirements without delaying wound healing or increasing the risk of DVT and PE. In the short-term after surgery, this method can reduce the VAS pain scores and improve the ROM; however, it has no effect on long-term VAS pain scores and ROM.
Acknowledgments
The authors would like to thank all the editors and the reviewers for their constructive comments that have significantly contributed to the improvement of our manuscript.
Author contributions
Conceptualization: Shenqi Zhang, Chengbin Wang, Qingyun Xue.
Data curation: Shenqi Zhang, Lei Shi.
Formal analysis: Shenqi Zhang, Qingyun Xue.
Investigation: Shenqi Zhang, Lei Shi.
Methodology: Qingyun Xue.
Project administration: Chengbin Wang, Qingyun Xue.
Resources: Chengbin Wang.
Supervision: Chengbin Wang.
Writing – original draft: Shenqi Zhang, Lei Shi.
Writing – review & editing: Shenqi Zhang.
Footnotes
Abbreviations: APTT = activated partial thromboplastin time, BMI = body mass index, DVT = deep vein thrombosis, Hb = hemoglobin, HBL = hidden blood loss, Hct = hematocrit, IAI = intra-articular injection, PAI = peri-articular injection, PE = pulmonary embolism, PLT = platelet count, PT = prothrombin time, ROM = range of motion, TBL = total blood loss, TKA = total knee arthroplasty, TXA = tranexamic acid, VAS = visual analog scale.
The authors have no conflicts of interest to disclose.
References
- [1].Kalairajah Y, Simpson D, Cossey AJ, et al. Blood loss after total knee replacement: effects of computer-assisted surgery. J Bone Joint Surg (Br) 2005;87:1480–2. [DOI] [PubMed] [Google Scholar]
- [2].Kim JL, Park JH, Han SB, et al. Allogeneic blood transfusion is a significant risk factor forsurgical-site infection following total hip and knee arthroplasty: a meta-analysis. J Arthroplasty 2017;32:320–5. [DOI] [PubMed] [Google Scholar]
- [3].Zhang LK, Ma JX, Kuang MJ, et al. The efficacy of tranexamic acid using oral administration in total knee arthroplasty: a systematic review and meta-analysis. J Orthop Surg Res 2017;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gómez-Barrena E, Ortega-Andreu M. Widespread of total knee arthroplasty perioperative blood management techniques based on tranexamic acid: barriers and opportunities. Ann Transl Med 2015;3:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Georgiadis AG, Muh SJ, Silverton CD, et al. A prospective double-blind placebo controlled trial of topical TXA in total knee arthroplasty. J Arthroplasty 2013;28:78–82. [DOI] [PubMed] [Google Scholar]
- [6].Yang ZG, Chen WP, Wu LD. Effectiveness and safety of TXA in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am 2012;94:1153–9. [DOI] [PubMed] [Google Scholar]
- [7].Kim TK, Chang CB, Koh IJ. Practical issues for the use of TXA in total knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc 2014;22:1849–58. [DOI] [PubMed] [Google Scholar]
- [8].Konig G, Hamlin BR, Waters JH. Topical TXA reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty 2013;28:1473–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, et al. Topical intra-articular compared with intravenous TXA to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am 2014;96:1937–44. [DOI] [PubMed] [Google Scholar]
- [10].Jain NP, Nisthane PP, Shah NA. Combined administration of systemic and topical TXA for total knee arthroplasty: can it be a better regimen and yet safe? A randomized controlled trial. J Arthroplasty 2016;31:542–7. [DOI] [PubMed] [Google Scholar]
- [11].Shin YS, Yoon JR, Lee HN, et al. Intravenous versus topical TXA administration in primary total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc 2017;25:3585–95. [DOI] [PubMed] [Google Scholar]
- [12].Yang L, Du S, Sun Y. Is combined topical and intravenous tranexamic acid superior to single use of tranexamic acid in total joint arthroplasty? A meta-analysis from randomized controlled trials. Medicine (Baltimore) 2017;96:e7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lin SY, Chen CH, Fu YC, et al. The efficacy of combined use of intraarticular and intravenous TXA on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty 2015;30:776–80. [DOI] [PubMed] [Google Scholar]
- [14].Lee SY, Chong S, Balasubramanian D, et al. What is the ideal route of administration of TXA in TKA? A randomized controlled trial. Clin Orthop Relat Res 2017;475:1987–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pinsornsak P, Rojanavijitkul S, Chumchuen S. Peri-articular tranexamic acid injection in total knee arthroplasty: a randomized controlled trial. BMC Musculoskelet Disord 2016;17:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Patel JN, Spanyer JM, Smith LS, et al. Comparison of intravenous versus topical TXA in total knee arthroplasty: a prospective randomized study. J Arthroplasty 2014;29:1528–31. [DOI] [PubMed] [Google Scholar]
- [17].Pinsornsak P, Rojanavijitkul S, Chumchuen S. Peri-articular TXA injection in total knee arthroplasty: arandomized controlled trial. BMC Musculoskelet Disord 2016;17:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery 1962;51:224–32. [PubMed] [Google Scholar]
- [19].Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology 1983;58:277–80. [DOI] [PubMed] [Google Scholar]
- [20].Pitta M, Zawadsky M, Verstraete R, et al. Intravenous administration of TXA effectively reduces blood loss in primary total knee arthroplasty in a 610-patient consecutive case series. Transfusion 2016;56:466–71. [DOI] [PubMed] [Google Scholar]
- [21].Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of TXA. Biochim Biophys Acta 1981;673:75–85. [PubMed] [Google Scholar]
- [22].Benoni G, Lethagen S, Fredin H. The effect of TXA on local and plasma fibrinolysisduring total knee arthroplasty. Thromb Res 1997;85:195–206. [DOI] [PubMed] [Google Scholar]
- [23].Fu DJ, Chen C, Guo L, et al. Use of intravenous TXA in total knee arthroplasty: a meta-analysis of randomized controlled trials. Chin J Traumatol 2013;16:67–76. [PubMed] [Google Scholar]
- [24].Wei Z, Liu M. The effectiveness and safety of TXA in total hip or knee arthroplasty: a meta-analysis of 2720 cases. Transfus Med 2015;25:151–62. [DOI] [PubMed] [Google Scholar]
- [25].Kim C, Park SS, Davey JR. TXA for the prevention and management of orthopedicsurgical hemorrhage: current evidence. J Blood Med 2015;6:239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Raveendran R, Wong J. TXA reduces blood transfusion in surgical patients while itseffects on thromboembolic events and mortality are uncertain. Evid Based Med 2013;18:65–6. [DOI] [PubMed] [Google Scholar]
- [27].Schulman S. Pharmacologic tools to reduce bleeding in surgery. Hematology Am Soc Hematol Educ Program 2012;2012:517–21. [DOI] [PubMed] [Google Scholar]
- [28].Ishida K, Tsumura N, Kitagawa A, et al. Intra-articular injection of TXA reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop 2011;35:1639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fu Y, Shi Z, Han B, et al. Comparing efficacy and safety of 2 methods of TXA administration in reducing blood loss following total knee arthroplasty: a meta-analysis. Medicine (Baltimore) 2016;95:e5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wong J, Abrishami A, El Beheiry H, et al. Topical application of TXA reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am 2010;92:2503–13. [DOI] [PubMed] [Google Scholar]
- [31].Dai WL, Zhou AG, Zhang H, et al. Most effective regimen of TXA for reducing bleeding and transfusions in primary total knee arthroplasty: a meta-analysis of randomized controlled trials. J Knee Surg 2018;31:654–63. [DOI] [PubMed] [Google Scholar]
- [32].Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) TXA reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am 2013;95:1961–8. [DOI] [PubMed] [Google Scholar]
- [33].Hiippala ST, Strid LJ, Wennerstrand MI, et al. TXA radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg 1997;84:839–44. [DOI] [PubMed] [Google Scholar]
- [34].Roy SP, Tanki UF, Dutta A, et al. Efficacy of intra-articular TXA in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2012;20:2494–501. [DOI] [PubMed] [Google Scholar]
- [35].Pinsornsak P, Rojanavijitkul S, Chumchuen S. Peri-articular TXA injection in total knee arthroplasty: a randomized controlled trial. BMC Musculoskelet Disord 2016;17:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen TP, Chen YM, Jiao JB, et al. Comparison of the effectiveness and safety of topical versus intravenous TXA in primary total knee arthroplasty: a meta-analysis of randomized controlled trials. J Orthop Surg Res 2017;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lin CM, Qi YM, Li J, et al. Is combined topical with intravenous TXA superior than topical, intravenous TXA alone and control groups for blood loss controlling after total knee arthroplasty: a meta-analysis. Medicine (Baltimore) 2016;95:e5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Huang Z, Ma J, Shen B, et al. Combination of intravenous and topical application of tranexamic acid in primary total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty 2014;29:2342–6. [DOI] [PubMed] [Google Scholar]
- [39].Kagoma YK, Crowther MA, Douketis J, et al. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res 2009;123:687–96. [DOI] [PubMed] [Google Scholar]
- [40].Gao FQ, Li ZJ, Zhang K, et al. Impact factors for hidden blood loss after primary total knee arthroplasty. Chin J Surg 2011;49:419–23. [PubMed] [Google Scholar]
- [41].Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br 2004;86:561–5. [PubMed] [Google Scholar]
- [42].Alcelik I, Pollock RD, Sukeik M, et al. A comparison of outcomes with and without a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty 2012;27:331–40. [DOI] [PubMed] [Google Scholar]
- [43].Kambayashi J, Sakon M, Yokota M, et al. Activation of coagulation and fibrinolysis during surgery, analyzed by molecular markers. Thromb Res 1990;60:157–67. [DOI] [PubMed] [Google Scholar]
- [44].Aglietti P, Baldini A, Vena LM, et al. Effect of tourniquet use on activation of coagulation in total knee replacement. Clin Orthop Relat Res 2000;371:169–77. [DOI] [PubMed] [Google Scholar]
- [45].Tanaka N, Sakahashi H, Sato E, et al. Timing of the administration of TXA for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br 2001;83:702–5. [DOI] [PubMed] [Google Scholar]
- [46].Melvin JS, Stryker LS, Sierra RJ. TXA in hip and knee arthroplasty. J Am Acad Orthop Surg 2015;23:732–40. [DOI] [PubMed] [Google Scholar]
- [47].Sano M, Hakusui H, Kojima C, et al. Absorption and excretion of TXA following intravenous, intramuscular and oral administrations in healthy volunteers. Jpn J Clin Pharmacol Therapeutics 1976;7:375–82. [Google Scholar]
- [48].Kiely N, Hockings M, Gambhir A. Does temporary clamping of drains following knee arthroplasty reduce blood loss? A randomised controlled trial. Knee 2001;8:325–7. [DOI] [PubMed] [Google Scholar]
- [49].Yamada K, Imaizumi T, Uemura M, et al. Comparison between 1-hour and 24-hour drain clamping using diluted epinephrine solution after total knee arthroplasty. J Arthroplasty 2001;16:458–62. [DOI] [PubMed] [Google Scholar]
- [50].Kang Y, Zhang ZJ, Fu M, et al. Blood transfusion and drainage catheter clamping are associated with ecchymosis formation at the surgical site after total knee arthroplasty: an analysis of 102 unilateral cases. Eur J Orthop Surg Traumatol 2013;23:219–24. [DOI] [PubMed] [Google Scholar]
- [51].Prasad N, Padmanabhan V, Mullaji A. Comparison between two methods of drain clamping after total knee arthroplasty. Arch Orthop Trauma Surg 2005;125:381–4. [DOI] [PubMed] [Google Scholar]
- [52].Bosque J, Jr, Coleman SI, Di Cesare P. Relationship between deep vein thrombosis and pulmonary embolism following THA and TKA. Orthopedics 2012;35:228–33. [DOI] [PubMed] [Google Scholar]
- [53].Fu P, Wu Y, Wu H, et al. Efficacy of intra-articular cocktail analgesic injection in total knee arthroplasty: a randomized controlled trial. Knee 2009;16:280–4. [DOI] [PubMed] [Google Scholar]
- [54].Gao FQ, Li ZJ, Zhang K, et al. Risk factors for lower limb swelling after primary total knee arthroplasty. Chin Med J (Engl) 2011;124:3896–9. [PubMed] [Google Scholar]


