Abstract
Arterial hypertension is considered to be an inflammatory condition with low intensity. Therefore, an elevated concentration of inflammatory cytokines can be expected in patients with systemic arterial hypertension, including tumor necrosis factor (TNF).
The study included a group of 96 persons aged 18 to 65 years: 76 patients with primary arterial hypertension and 20 healthy individuals (control group). Blood pressure was measured in all individuals using the office and ambulatory blood pressure monitoring (ABPM) measurement, blood was collected for laboratory tests [tumor necrosis factor (TNF), tumor necrosis factor receptor 1 (TNFR1)], and 24-hour urine collection was performed in which albuminuria and TNF concentration were assessed. Moreover, assessment of the intima-media thickness (IMT) in ultrasonography and left ventricular mass index (LVMI) in echocardiography were carried out.
Statistically elevated TNF concentration in the blood serum (P = .0001) and in the 24-hour urine collection (P = .0087) was determined in patients with hypertension in comparison with the control group. The TNF and TNFR1 concentration in the serum and TNF in the 24-hour urine in the group of patients with arterial hypertension and organ damages and without such complications did not differ statistically significantly.
We observed a positive and statistically significant correlation between TNFR1 concentration in the serum and TNF urine excretion in patients with hypertension (r = 0.369, P < .05)
Patients with arterial hypertension are characterized by higher TNF concentrations in blood serum and higher TNF excretion in 24-hour urine than healthy persons.
TNF and TNFR1 concentration in blood serum and TNF excretion in 24-hour urine in patients with early organ damages due to arterial hypertension do not differ significantly from those parameters in patients with arterial hypertension without organ complications.
There is a positive correlation between TNFR1 concentration in the serum and TNF urine excretion in patients with hypertension.
Keywords: early organ damages, primary hypertension, tumor necrosis factor
1. Introduction
Due to its prevalence and complications, arterial hypertension constitutes a serious health and economic issue. Hypertension can now be diagnosed in more than 12.5 million adult Poles (42.7% of the population aged 19–99 years) and it is one of the most important causes of cardiovascular complications and mortality due to cardiovascular and cerebrovascular disease. On the basis of the results of the WOBASZ (2003–2005) and WOBASZ II (2013–2014) studies, it is known that the prevalence of hypertension in Poland is on an upward trend (it has changed by 12% within a decade).[1] It is estimated that more than over 7 million deaths per year worldwide could be caused by hypertension, and according to WHO, elevated blood pressure is the primary cause of deaths globally.[2,3] In approx. 90%, arterial hypertension has a primary character. Pathogenesis of primary hypertension is complex and its direct etiology has not been fully explained. It has been assumed that its genesis is influenced by a series of genetic, environmental, and behavioral factors.[4–6] Interventional and clinical studies prove that arterial hypertension is also an inflammatory condition with low intensity.[7,8] The inflammatory condition is characterized by an increased concentration of certain proteins in the blood serum, which include, among others C-reactive protein (CRP), TNF, fibrinogen, and interleukins 6 and 8.[9] Among those, TNF is one of the major cytokines of inflammatory and immunologic response, regulating the differential and growth of cells. It is primarily produced by mastocytes, macrophages as well as granulocytes, endothelial cells, fibroblasts, lymphocytes T, smooth muscle myocytes.[10] This cytokine can be also secreted in pathological conditions by malignant cells such as breast cancer, pancreatic cancer, ovarian cancer, kidney cancer, melanoma, and chronic B-cell lymphoid leukemia.[11] Metabolic functions of TNF include, among others, influence on the increase of leptin concentration, which may lead to insulin resistance.[12,13] TNF exerts a biological effect on 2 receptor types (TNFR1 and TNFR2), which differ in their glycation degree, molecular mass, and type of transduced signal.[14] Increased concentration of proinflammatory cytokines, including TNF, is observed in conditions such as heart failure, obesity (and its associated insulin resistance), diabetes, or rheumatoid arthritis.[12,13,15–17] Increasing evidence has been presented that TNF is indeed associated with arterial hypertension, yet we still do not know whether it only constitutes a marker for the disease, or it is a significant etiopathogenetic factor of primary hypertension.[18] Arterial hypertension constitutes a risk factor for other diseases. The most frequent cardiovascular complications include ischemic heart disease, arrhythmia, peripheral arterial disease, heart failure, strokes, and dementia.[19–21] Untreated arterial hypertension may also lead to the development of numerous renal complications manifested by creatinine elevation, proteinuria, up to end-stage kidney failure.[22]
The objective of the study was comparison of TNF concentration in the blood serum and assessment of TNF excretion level in the urine of patients with arterial hypertension and normotensive individuals, comparison of serum TNF concentration and assessment of the level of TNF excretion level in the urine in patients with arterial hypertension with and without early organ damages, and comparison of serum soluble receptor 1 concentration for TNF in the serum of patients with arterial hypertension with and without early organ damages.
2. Material and method
The study included a group of patients with arterial hypertension diagnosed at the Department of Hypertensiology, Angiology and Internal Diseases, Poznan University of Medical Sciences in the period 2012 to 2015. The study design was submitted to the Bioethics Committee at the Poznan University of Medical Sciences and an approval for its conduct was obtained (decision no. 42/10 of 2010). All patients were informed of the objective and principles of the study and provided written consent to participate in the study.
The study involved 96 individuals aged 18 to 65 years, including 76 patients with primary arterial hypertension (mild and moderate, thus far untreated) and 20 healthy individuals with normal values of blood pressure (control group). A 2-day study protocol was applied. On the first day, patients visited the clinic in morning hours, between 8:00 AM and 9:00 AM, on an empty stomach. An interview and physical examination were carried out, blood was collected for laboratory tests, blood pressure was measured using office measurement, the ambulatory blood pressure monitor (ABPM) was applied, and 24-hour urine collection was ordered. On the second day, heart echocardiography was performed and the IMT of the carotid arteries was measured.
The blood pressure was performed according to ESC 2013 (European Society of Cardiology/European Society of Hypertension)[23] and PTNT 2015 (Polish Society of Hypertension)[24] guidelines using electronic apparatus Omron-705IT (Omron Corporation, Kyoto, Japan). The arterial blood pressure was measured on the left arm in a sitting position after a 10-minute rest; moreover, at least 30 minutes before the measurement, patient refrained from drinking coffee and smoking tobacco. Three measurements at 2-minute intervals were preformed using a standard cuff (measuring 12–13 cm width and 35 cm length). In the case of a larger or smaller arm circumference, the cuff size was adjusted accordingly. The value of arterial blood pressure was determined as a mean from 3 subsequent measurements.
ABPM was carried out using a 2430TM apparatus by A&D (A&D Company, Limited, Tokyo, Japan) and Mobil-O-Graph (IEM GmbH, Stolberg, Germany) by IEM. Cuff of suitable size was applied on the left arm of the patient. The measurement frequency was set at 15 minutes during the day and 30 minutes at night. Daytime hours were set at the period between 6.00 and 22.00 and nighttime hours from 22.00 to 6.00. Having completed an interview on the nighttime rest, the actual sleep time was corrected for each patient. Switching the measurement preview off eliminated the potential stress-inducing factor. The following parameters were determined based on the obtained measurements: mean SBP and DBP for the entire day (SBP24h, DBP24h), mean SBP and DBP for daytime measurements (SBPd, DBPd) and nighttime measurements (SBPn, DBPn).
Venous blood was collected on an empty stomach for laboratory testing. The tests were carried out at the Laboratory of the Poznan University of Sciences Hospital of Lord's Transfiguration. Serum and urine TNF were determined at the Laboratory of Nuclear Medicine. TNF concentration was determined using commercial sets by DIAsource ImmunoAssays, Louvain-la-Neuve, Belgium. Urine TNF concentration was determined in samples obtained from 24-hour urine collection. The concentration of soluble receptor TNF R1 was performed using ELISA method (it was performed only in 40 patients).
The testing of 24-hour urine collection was commenced on obtaining the first portion of morning urine, and it was completed with the morning urine portion of the following day. After 24 hours, the total volume of collected urine was recorded. From the obtained 24-hour urine collection, approximately 40 mL of urine was preserved, in which creatinine, sodium, potassium, and total protein concentration were determined, as well as approx. Twenty milliliter of urine was collected to determine TNF. Microalbumin urine was assessed using a semi-quantitative method by means of a Clinitek apparatus by Siemens (Siemens Healthcare GmbH, Kemnath, Germany) (bar result >150 mg/L indicated clinical albuminuria).
The carotid arteries examinations were performed in patients in the recumbent posture, with head tilted back, directed slightly in the opposite direction to the tested side, after a several minute rest. Testing in all patients was performed using the ultrasonographic apparatus Esaote MyLab 60 (Esaote S.p.A., Genova, Italy) with linear head LA 523 with changeable frequency in the range 4 to 13 MHz. The assessment of the intima and media complex was performed within carotid arteries approximately 1 to 2 cm below the carotid sinus, using 2-dimensional ultrasound. The IMT thickness in excess of 0.9 mm was determined as abnormal.
Echocardiography was performed using Vivid 6 apparatus by GE Medical System (GE Vingmed Ultrasound AS, Norway), equipped with a sector head M4S RS with frequency changeable in the range 1.5 to 3.6 MHz, in 2-dimensional presentation (2D) and M-mode and with the application of a color Doppler. The images were obtained in views: parasternal long and short axis and apical: 4, 2, and 3-chamber. For the assessment of the left ventricular size, images obtained from parasternal long axis view were used.
In arterial hypertension, the left ventricular remodeling was assessed based on the LVMI; standard for men <125 g/m2, for women <110 g/m2).
Left ventricular mass index (LVMI) was calculated based on the formulas recommended by the American Echocardiography Society in Devereux modification.[25]
2.1. Statistical analysis
The analyzed parameters were described, in the case of a normal distribution, by an arithmetic mean and standard deviation (SD), otherwise with a median and median absolute deviation. In the case of data with normal distribution, Student t test was used for unrelated data, otherwise Mann–Whitney test was used. For the correlation between TNFR1 concentration in the serum and TNF urine excretion, the nonparametric Spearman correlation coefficient was determined. Statistical calculations were performed with Statistica 10 by StatSoft (CSS Statistica v. 10.0 Stat Soft, Inc., Oklahoma, USA). The significance level was assumed at P < .05.
3. Results
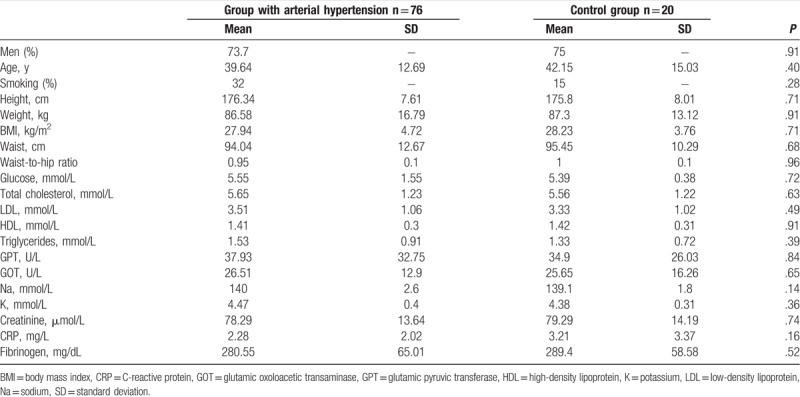
Patients qualified to the study were classified into 2 experimental groups. The first group is 76 patients (56-M, 20-F, mean age 39.64 ± 12.69 years) with untreated arterial hypertension. The second group is control comprising of 20 healthy persons (15-M, 5-F, mean age 42.15 ± 15.03 years). Anthropometric and biochemical parameters of both groups are presented in Table 1.
Table 1.
Anthropometric and biochemical parameters in the group of patients with arterial hypertension and in control group.
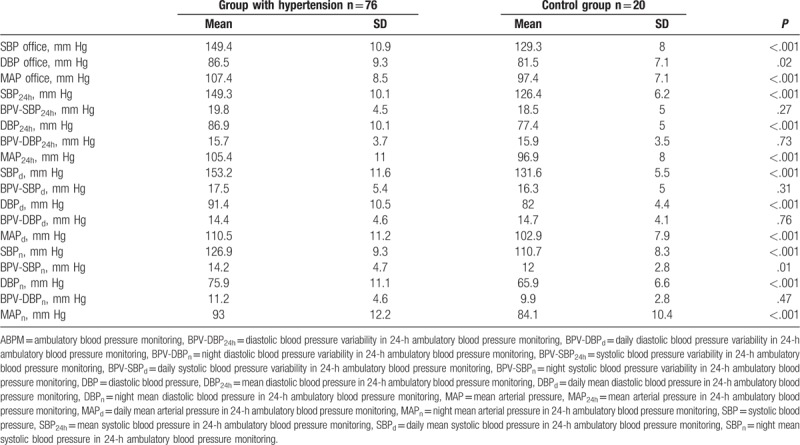
The mean values of arterial blood pressure obtained through office measurement in the experimental group amounted for systolic blood pressure (SBP) 149.4 mm Hg, and for diastolic blood pressure (DBP) 86.5 mm Hg. In the control group, the respective SBP was 129.3 mm Hg and DBP 81.5 mm Hg. Moreover, Table 2 presents the mean values of arterial blood pressure recorded with the ABPM method in the group with arterial hypertension and in control.
Table 2.
Mean values of arterial blood pressure in office and ABPM measurements.
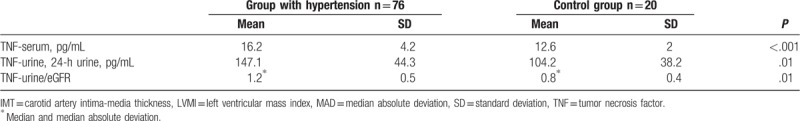
Mean TNF values in blood serum were significantly higher in the group of patients with hypertension. Similar were mean TNF values in 24-hour urine collection. The results are presented in Table 3.
Table 3.
Mean TNF values in serum and 24-h urine collection.
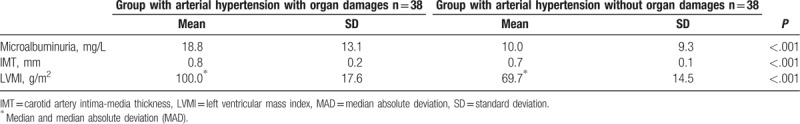
Mean values of albumin excretion and LVMI in the group of patients with arterial hypertension were higher than in the control group. However, those values did not differ statistically significantly. Mean values of IMT in the group of patients with arterial hypertension were statistically significantly higher than in the control group. Data are presented in Table 4.
Table 4.
Mean values of microalbuminuria, IMT, and LVMI in the group of patients with arterial hypertension and in control group.

Among the patients with arterial hypertension, microalbuminuria was diagnosed for 20 patients, IMT >0.9 mm in 13 patients, and LV hypertrophy in 5 patients. On this basis, two, 38-person patient subgroups were distinguished, with arterial hypertension complications and without them.
Mean values of microalbuminuria, IMT, and LVMI for both subgroups are presented in Table 5.
Table 5.
Mean values of microalbuminuria, IMT, and LVMI in the group with arterial hypertension with organ damages and without them.
The mean values of arterial blood pressure obtained by means of office measurement in the group of patients with arterial hypertension with organ damages were for SBP 150.5 mm Hg, and for DBP 88.5 mm Hg. In the group of patients with arterial hypertension without organ damages, the respective SBP was 146.8 mm Hg and DBP 84.2 mm Hg. Moreover, Table 6 presents the mean values of arterial blood pressure recorded with the ABPM method in the group with and without early arterial hypertension complications.
Table 6.
Mean values of arterial blood pressure in office and ABPM measurements in the group of patients with arterial hypertension with and without organ damages.
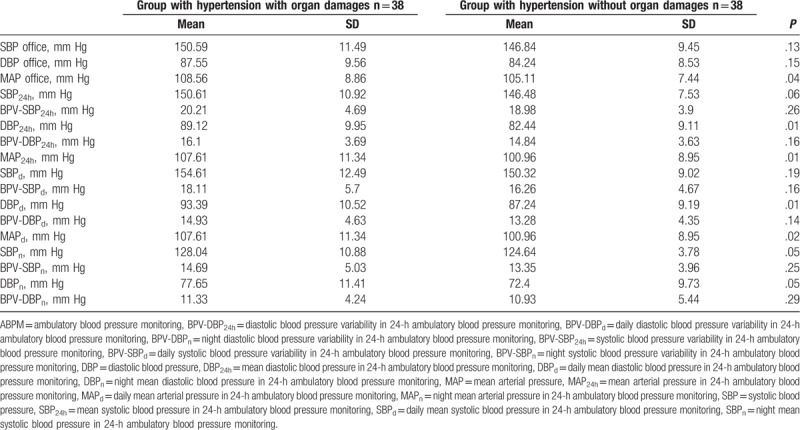
Mean TNF values in the serum and in the 24-hour urine in the group of patients with arterial hypertension and organ damages and without complications did not differ statistically significantly (Table 7).
Table 7.
Mean TNF values in serum and in 24-hour urine in the group of patients with arterial hypertension and organ damages and without complications.

The concentration of the TNFR 1 (which was performed only in 40 patients) in the serum in the group of patients with arterial hypertension with organ damages was 1089.6 ± 186.4 pg/mL, and without complicating conditions was 1126.9 ± 227.6 pg/mL. Those results did not differ statistically significantly (P = .5718).
We observed a positive and statistically significant correlation between TNFR1 concentration in the serum and TNF urine excretion in patients with hypertension (r = 0.369, P < .05).
4. Discussion
Contrary to a series of experimental studies performed on animals, the current research and observations on TNF concentration and its serum soluble receptor in patients with primary arterial hypertension is relatively limited, and its results have been conflicting. Our study has demonstrated that TNF concentration in the serum of patients with primary arterial hypertension is significantly higher than in healthy persons. Similar results were obtained by Bautista et al,[26] who assessed TNF concentration in the blood serum of 196 individuals, of whom 79 examined had been diagnosed with arterial hypertension. It was demonstrated that the higher the blood pressure, the higher was TNF and interleukin 6 concentration in the blood. The CRP concentration was similar between hypertensive and healthy persons.[26] In the Japanese population, Furumoto[27] observed significantly higher TNF concentration in persons with slightly elevated arterial blood pressure (mean 139/80) mm Hg in comparison to persons with arterial blood pressure of mean 118/71 mm Hg. Bogdański et al[28] observed statistically significantly higher TNF concentration in patients with arterial hypertension, particularly in those with obesity. Patients with a history of myocardial infarction or stroke also exhibited higher serum TNF concentration than patients without those complications in the medical history. Huang et al[29] demonstrated significantly increased TNF concentration in a group of 34 patients with arterial hypertension and 60 persons with arterial hypertension and prediabetes condition. On the contrary, in the study of Navarro-Gonzalez et al,[30] the serum TNF concentration in patients with arterial hypertension was only insignificantly higher than in healthy persons. Normal TNF concentration in patients with arterial hypertension was observed by Peeters et al.[31] In the study of Jastrzębski et al,[32] the TNF concentration in patients with primary arterial hypertension without organ damages did not differ from the concentration of this compound in healthy persons.
In the present paper, the mean TNF values in 24-hour urine collection were also higher in the group of patients with arterial hypertension than in the control group and this difference was statistically significant. Elevated TNF concentration in urine, but not in serum, was observed in the study of Lampropoupou et al[33] in patients with diabetes and microalbuminuria. The level of microalbuminuria in this study correlated with the systolic pressure of the examined patients. Navarro-Gonzalez et al[34] described elevated urine TNF concentration as one of the risk factors for microalbuminuria in prehypertensive subjects. The conducted experimental studies in animals proved that TNF administration increases kidney injuries, and blockage of exogenous TNF through its soluble receptors result in reduced activity of tumor necrosis factor and IL-1 concentration and improves renal function.[35,36]
The available literature contains sparse data on significant differences in TNF concentration between patients with arterial hypertension with their already occurring organ damages and patients without such complications.
In the present study, the serum TNF values in the group of patients with arterial hypertension with and without organ damages were comparable.
One of the subclinical complications of arterial hypertension we described is kidney injury, which is expressed by creatinine elevation in serum, reduced creatinine clearance rate, and microalbuminuria. Microalbuminuria occurs as a result of increased permeability of glomerule or reduced renal tubular reabsorption. Increased albumin excretion rate may stem from functional or structural damage of the glomerular ultrafiltration barrier. Microalbuminuria can be determined as a renal symptom of generalized endothelial dysfunction.[22]
In our study, we have not observed the significantly higher concentration of TNF and its soluble receptor in patients with microalbuminuria in comparison with hypertensive patients without microalbuminuria. Cottone et al[37] compared TNF concentration among patients with arterial hypertension with normal renal function and with renal disease (with microalbuminuria or proteinuria) and in control group. Results of the presented study have confirmed that TNF concentration was higher in the group of patients with renal failure compared with hypertensive patients without renal failure and with normotensive persons. However, in the present study, no difference has been demonstrated between persons with normal blood pressure and persons with arterial hypertension without renal failure. Perhaps the obtained results were caused by the chronic inflammatory process or reduced kidney cytokine clearance. In our study, patients with microalbuminuria had normal blood creatinine concentration and normal glomerular filtration rate and perhaps, therefore, TNF concentration was not increased relative to arterial hypertension without organ damages.
Intima-media thickening is another complication of arterial hypertension. It indicates early atherosclerotic changes. Guidelines of the Polish Society of Arterial Hypertension and the European Society of Hypertension and of Cardiology classify IMT increase >0.9 mm and/or presence of atherosclerotic coronary plaques in carotid arteries as an arterial hypertension organ damage. PTNT recommends an assessment of the IMT within the assessment of the risk of cardiovascular events in patients with hypertension.[23,24] The correlation between TNF concentration and the IMT has been studied by a low number of authors. The thickness of this complex and concentration of TNF and its soluble receptors 1 and 2 were assessed by Elkind et al[38] in 279 patients aged 67 ± 8.5 years, of which half had arterial hypertension. However, these authors did not demonstrate a correlation between IMT and TNF concentration. However, the concentration of soluble TNF R1 receptor was significantly elevated in 25% of those individuals with the highest IMT. Also, Morillas et al[39] determined elevated concentration of type 1 and 2 soluble receptor of TNF in hypertensive patients with organ damages, particularly with coexisting IMT thickening, LV hypertrophy, and renal injury. In our study, we have not observed a significant increase of TNF and its soluble receptor in patients with increased IMT. However, the patients we examined were of relatively low age, and the mean IMT was slightly above 0.9 mm.
The increase of LVM, thickening of its walls, and diastolic dysfunction are frequently observed in the course of arterial hypertension. Mass hypertrophy and disorders of the left ventricular geometry are independent risk factors for cardiovascular complications and mortality among patients with arterial hypertension. The LV hypertrophy due to arterial hypertension is caused by a change in the proportion between myocytes, vessels, and the amount of intercellular substance, which constitutes a response to increased end-systolic pressure. The increased amount of collagen I leads to heart wall fibrosis. As a proinflammatory cytokine, TNF may be also produced by cardiomyocytes, which typically occurs under unfavorable hemodynamic conditions with excessive pressure or volume load.[40] It may lead to metabolic disorders in the myocardium, left ventricular dysfunctions, reduced peripheral flow, and left ventricular remodeling. Through stimulation of synthesis and inhibition of protein degradation, TNF leads to myocardial hypertrophy. Moreover, TNF is an important stimulator of angiotensinogen gene transcription.[41] A series of clinical and experimental studies have been published proving that TNF participates in the process of myocardial hypertrophy. Yokoyama et al[42] demonstrated that under the TNF stimulation, increased synthesis of actin and heavy myosin chains occur. Patel et al[43] confirmed the contribution of TNF in the development of heart hypertrophy in hypertrophic myocardiopathy in young persons. In an experimental study in hypertensive rabbits with myocardial hypertrophy, Stamm et al[44] demonstrated increased concentration of TNF in the hypertrophy phase without heart failure. In their study on hypertensive rats, Bergman et al[45] demonstrated increased TNF value already at early stages of hypertension. However, in this study, when no heart failure was developed, these values returned to normal.[46] Jastrzębski et al[32] determined higher TNF concentration in patients with arterial hypertension and organ damages including LV hypertrophy. Also, Navarro-Gonzalez et al[46] determined the correlation between TNF and Cornell product, an electrocardiographic marker of LV hypertrophy. Sriramula et al[47] showed that through angiotensin II, TNF can influence the development of myocardial hypertrophy. In her study, Aksenova[48] demonstrated that proinflammatory cytokines, including TNF, lead to endothelial dysfunction, and their concentration correlates with the presence of left ventricle hypertrophy. On the contrary, the study of Stetson et al [49] demonstrated that only 32% of patients with hypertrophied left ventricle had elevated TNF concentration; however, this correlation was also observed in 14% of patients with normal LV mass. In the present study, we have also been unable to observe a significant increase of TNF and its soluble receptor in patients with myocardial hypertrophy.
We also observed a positive correlation between TNFR1 concentration in the serum and TNF urine excretion in patients with hypertension. We could not find data on this subject in the literature, perhaps because our work was the first to comprehensively evaluate both TNF and TNFR1 in serum and TNF in urine in patients with hypertension.
There were some limitations in our study. First, our study, as an observational study, is limited by lack of randomization. Second, the number of patients was small, and additionally, the concentration of TNFR 1 was performed only in a part of patients, so the results of this study need the further confirmation by studies with larger sample size.
5. Conclusion
Patients with arterial hypertension are characterized by higher TNF concentrations in blood serum and higher TNF excretion in 24-hour urine than healthy persons.
TNF and TNFR1 concentration in blood serum and TNF excretion in 24-hour urine in patients with early organ damages due to arterial hypertension do not differ significantly from those parameters in patients with arterial hypertension without organ damages.
There is a positive correlation between TNFR1 concentration in the serum and TNF urine excretion in patients with hypertension.
Author contributions
Conceptualization: Anna Puszkarska, Jerzy Głuszek.
Data curation: Anna Puszkarska, Arkadiusz Niklas.
Formal analysis: Arkadiusz Niklas, Dawid Lipski, Karolina Niklas.
Investigation: Anna Puszkarska, Arkadiusz Niklas.
Methodology: Anna Puszkarska, Arkadiusz Niklas, Jerzy Głuszek.
Project administration: Anna Puszkarska, Jerzy Głuszek.
Resources: Anna Puszkarska, Jerzy Głuszek.
Supervision: Arkadiusz Niklas, Jerzy Głuszek, Karolina Niklas.
Validation: Arkadiusz Niklas, Dawid Lipski.
Writing – original draft: Anna Puszkarska.
Writing – review & editing: Arkadiusz Niklas, Jerzy Głuszek, Dawid Lipski, Karolina Niklas.
Karolina Niklas orcid: 0000-0002-9770-1019.
Footnotes
Abbreviations: ABPM = ambulatory blood pressure monitoring, BPV-DBP24h = diastolic blood pressure variability in 24-h ambulatory blood pressure monitoring, BPV-DBPd = daily diastolic blood pressure variability in 24-h ambulatory blood pressure monitoring, BPV-DBPn = night diastolic blood pressure variability in 24-h ambulatory blood pressure monitoring, BPV-SBP24h = systolic blood pressure variability in 24-h ambulatory blood pressure monitoring, BPV-SBPd = daily systolic blood pressure variability in 24-h ambulatory blood pressure monitoring, BPV-SBPn = night systolic blood pressure variability in 24-h ambulatory blood pressure monitoring, CRP = C-reactive protein, DBP = diastolic blood pressure, DBP24h = mean diastolic blood pressure in 24-h ambulatory blood pressure monitoring, DBPd = daily mean diastolic blood pressure in 24-h ambulatory blood pressure monitoring, DBPn = night mean diastolic blood pressure in 24-h ambulatory blood pressure monitoring, eGFR = estimated glomerular filtration rate, IMT = carotid artery intima-media thickness, LVMI = left ventricular mass index, MAD = median absolute deviation, MAP = mean arterial pressure, MAP24h = mean arterial pressure in 24-h ambulatory blood pressure monitoring, MAPd = daily mean arterial pressure in 24-h ambulatory blood pressure monitoring, MAPn = night mean arterial pressure in 24-h ambulatory blood pressure monitoring, SBP = systolic blood pressure, SBP24h = mean systolic blood pressure in 24-h ambulatory blood pressure monitoring, SBPd = daily mean systolic blood pressure in 24-h ambulatory blood pressure monitoring, SBPn = night mean systolic blood pressure in 24-h ambulatory blood pressure monitoring, SD = standard deviation, TNF = tumor necrosis factor, TNFR1 = tumor necrosis factor receptor 1, WHO = World Health Organization.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Niklas A, Flotyńska A, Puch-Walczak A, et al. Prevalence, awareness, treatment and control of hypertension in the adult Polish population-Multi-center National Population Health Examination Surveys-WOBASZ studies. Arch Med Sci 2018;14:951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20. [DOI] [PubMed] [Google Scholar]
- [3].Ezzati M, Lopez AD, Rodgers A, et al. Selected major risk factors and global and regional burden of disease. Lancet 2002;360:1347–60. [DOI] [PubMed] [Google Scholar]
- [4].Staessen JA, Wang J, Bianchi G, et al. Essential hypertension. Lancet 2003;361:1629–41. [DOI] [PubMed] [Google Scholar]
- [5].Singh M, Singh AK, Pandey P, et al. Molecular genetics of essential hypertension. Clin Exp Hypertens 2016;38:268–77. [DOI] [PubMed] [Google Scholar]
- [6].Saxena T, Ali AO, Saxena M. Pathophysiology of essential hypertension: an update. Expert Rev Cardiovasc Ther 2018;16:879–87. [DOI] [PubMed] [Google Scholar]
- [7].Sesso HD, Buring JE, Rifai N, et al. C-reactive protein and risk of developing hypertension. JAMA 2002;290:2945–51. [DOI] [PubMed] [Google Scholar]
- [8].Pouvreau C, Dayre A, Butkowski EG, et al. Inflammation and oxidative stress markers in diabetes and hypertension. J Inflamm Res 2018;11:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Park ChS, Kim HY, Park H-J, et al. Association between the JNC 7 classification of the stages of systolic hypertension and inflammatory cardiovascular risk factors. Korean Circ J 2007;37:623–9. [Google Scholar]
- [10].Wallach D. The cybernetics of TNF: old views and newer ones. Semin Cell Dev Biol 2016;50:105–14. [DOI] [PubMed] [Google Scholar]
- [11].Yi F, Shi X, Pei X, et al. Tumor necrosis factor-alpha-308 gene promoter polymorphism associates with survival of cancer patients: a meta-analysis. Medicine (Baltimore) 2018;97:e13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Takaguri A. Elucidation of a new mechanism of onset of insulin resistance: effects of statins and tumor necrosis factor-α on insulin signal transduction. Yakugaku Zasshi 2018;138:1329–34. [DOI] [PubMed] [Google Scholar]
- [13].Han C, Wu W, Ale A, et al. Central leptin and tumor necrosis factor-α (TNFα) in diurnal control of blood pressure and hypertension. J Biol Chem 2016;291:15131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Park YH, Jeong MS, Jang SB. Structural insights of homotypic interaction domains in the ligand-receptor signal transduction of tumor necrosis factor (TNF). BMB Rep 2016;49:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Seta Y, Shan K, Bozkurt B, et al. Basic mechanisms in heart failure: the cytokine hypothesis. J Card Fail 1996;2:243–9. [DOI] [PubMed] [Google Scholar]
- [16].Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004;92:347–55. [DOI] [PubMed] [Google Scholar]
- [17].Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest 2008;118:3537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mehaffey E, Majid DSA. Tumor necrosis factor-α, kidney function, and hypertension. Am J Physiol Renal Physiol 2017;313:F1005–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jackson R, Lawes CM, Bennett DA, et al. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual's absolute cardiovascular risk. Lancet 2005;365:434–41. [DOI] [PubMed] [Google Scholar]
- [20].Kannel WB. Hypertension: reflections on risks and prognostication. Med Clin North Am 2009;93:541–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Głuszek J, Jankowska K. Dementia caused by arterial hypertension. Chor Serca Naczyn 2005;2:125–30. [Google Scholar]
- [22].Futrakul N, Sridama V, Futrakul P. Microalbuminuria: a biomarker of renal microvascular disease. Ren Fail 2009;31:140–3. [DOI] [PubMed] [Google Scholar]
- [23].Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013;31:1281–357. [DOI] [PubMed] [Google Scholar]
- [24].Tykarski A, Narkiewicz K, Gaciong Z, et al. 2015 guidelines for the management of hypertension. Recommendations of the Polish Society of Hypertension–short version. Kardiol Pol 2015;73:6767–7700. [DOI] [PubMed] [Google Scholar]
- [25].Collins HW, Kronenberg MW, Byrd BF., 3rd Reproducibility of left ventricular mass measurements by two-dimensional and M-mode echocardiography. J Am Coll Cardiol 1989;14:672–6. [DOI] [PubMed] [Google Scholar]
- [26].Bautista LE, Vera LM, Arenas IA, et al. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens 2005;19:149–54. [DOI] [PubMed] [Google Scholar]
- [27].Furumoto T. Diminished endothelial function and hypofibrinolysis in high risk patients with hypertension and high plasma levels of inflammatory markers: need for anti-inflammatory strategy. Hypertension 2000;36:684–5. [Google Scholar]
- [28].Bogdański P, Kujawska-Łuczak M, Łacki J, et al. Evaluation of selected interleukins, tumor necrosis factor, insulin and leptin in obese patients with hypertension [Ocena stężenia wybranych interleukin, czynnika martwicy nowotworów, insulinemii i leptynemii u otyłych z nadciśnieniem tętniczym]. Pol Merkur Lekarski 2003;15:347–51. [PubMed] [Google Scholar]
- [29].Huang Z, Chen C, Li S, et al. Serum markers of endothelial dysfunction and inflammation increase in hypertension with prediabetes mellitus. Genet Test Mol Biomarkers 2016;20:322–7. [DOI] [PubMed] [Google Scholar]
- [30].Navarro-Gonzalez J, Mora F, Muros C, et al. Association of tumor necrosis factor-α with early target organ damage in newly diagnosed patients with essential hypertension. J Hypertens 2008;26:2168–75. [DOI] [PubMed] [Google Scholar]
- [31].Peeters AC, Netea MG, Janssen MC, et al. Pro-inflammatory cytokines in patients with essential hypertension. Eur J Clin Invest 2001;31:31–6. [DOI] [PubMed] [Google Scholar]
- [32].Jastrzębski D, Czarnecka M, Rajzer K, Kawecka-Jaszcz K. Increased levels of inflammatory markers in hypertensives with target organ damage. Kardiol Pol 2006;64:802–9. [PubMed] [Google Scholar]
- [33].Lampropoupou IT, Stangou M, Papagianni A, et al. TNF-α and microalbuminuria in patients with type 2 diabetes mellitus. J Diabetes Res 2014;2014:394206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Navarro-González JF, Mora C, Muros M, et al. Relationship between inflammation and microalbuminuria in prehypertension. J Hum Hypertens 2013;27:1191–225. [DOI] [PubMed] [Google Scholar]
- [35].Lan HY, Yang N, Metz C, et al. TNF-alpha upregulates renal MIF expression in rat crescentic glomerulonephritis. Mol Med 1997;3:136–44. [PMC free article] [PubMed] [Google Scholar]
- [36].Le Hir M, Haas C, Marino M, et al. Prevention of crescentic glomerulonephritis induced by antiglomerular membrane antibody in tumor necrosis factor dependent mice. Lab Invest 1998;78:1625–31. [PubMed] [Google Scholar]
- [37].Cottone S, Vadala A, Vella MC, et al. Comparison of tumor necrosis factor and endothelin-1 between essential and renal hypertensive patients. J Hum Hypertens 1998;12:351–4. [DOI] [PubMed] [Google Scholar]
- [38].Elkind MS, Cheng J, Boden-Alba B, et al. Tumor necrosis factor receptor levels are associated with carotid atherosclerosis. Stroke 2002;33:313–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Morillas P, de Andrade H, Castillo J, et al. Inflammation and apoptosis in hypertension. Relevance of the extent of target organ damage. Rev Esp Cardiol (Engl Ed) 2012;65:819–25. [DOI] [PubMed] [Google Scholar]
- [40].Ho KK, Pinsky JL, Kannel WB, et al. The epidemiology of heart failure: the Framingham study. J Am Coll Cardiol 1993;22:6A–13A. [DOI] [PubMed] [Google Scholar]
- [41].Brasier AR, Li J, Wimbish KA. Tumor necrosis factor activates angiotensinogen gene expression by the Rel a transactivator. Hypertension 1996;27:1009–17. [DOI] [PubMed] [Google Scholar]
- [42].Yokoyama T, Nakano M, Bednarczyk JL, et al. Tumor necrosis factor-alpha provokes a hypertrophic growth response in adult cardiac myocytes. Circulation 1997;95:1247–52. [DOI] [PubMed] [Google Scholar]
- [43].Patel R, Lim DS, Reddy D, et al. Variants of trophic factors and expression of cardiac hypertrophy in patients with hypertrophic cardiomyopathy. J Mol Cell Cardiol 2000;32:2369–77. [DOI] [PubMed] [Google Scholar]
- [44].Stamm C, Friehs I, Cowan DB, et al. Inhibition of tumor necrosis factor-alpha improves postischemic recovery of hypertrophied hearts. Circulation 2001;10412 suppl 1:I350–5. [DOI] [PubMed] [Google Scholar]
- [45].Bergman MR, Kao RH, McCune SA, et al. Myocardial tumor necrosis factor-alpha secretion in hypertensive and heart failure-prone rats. Am J Physiol 1999;277:H543–50. [DOI] [PubMed] [Google Scholar]
- [46].Navarro-Gonzalez J, Mora F, Muros C, et al. Association of tumor necrosis factor-α with early target organ damage in newly diagnosed patients with essential hypertension. J Hypertens 2008;26:21682–2175. [DOI] [PubMed] [Google Scholar]
- [47].Sriramula S, Haque M, Majid DS, et al. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension 2008;51:1345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Aksenova TA. The immunologic disorders and dysfunction of endothelium as predictors of development of hypertrophy of left ventricle of heart in patients with hypertension disease. Klin Lab Diagn 2013;8:18–20. [PubMed] [Google Scholar]
- [49].Stetson SJ, Perez-Verdia A, Mazur W, et al. Cardiac hypertrophy after transplantation is associated with persistent expression of tumor necrosis factor-alpha. Circulation 2001;104:676–81. [DOI] [PubMed] [Google Scholar]


