Abstract
Rationale:
Idiopathic megacolon (IMC) is a rare condition in young children. The association of indigestible food and IMC has never been mentioned in literature.
Patient concerns:
An 11-year-old boy reported with a 1-year duration of intermittent constipation and abdominal distension after having eaten a large amount of fried sticky rice in 1 consumption.
Diagnoses:
Chronic low colonic obstruction, IMC and malnutrition.
Interventions:
This patient was managed conservatively for 1 week at first. Then he underwent loop ileostomy since conservative therapy was poorly tolerated. Enteral decompression, gut biopsy, peritoneal lavage, and drainage were performed in the same procedure.
Outcomes:
Rapid weight gain was observed 4 months after operation.
Lessons:
IMC is difficult to diagnose due to the lack of specific clinical manifestations and pathological features. The protocols for management of IMC remains controversial. To achieve a good long-term outcome, early intervention is recommended.
Keywords: hyperphagia, idiopathic megacolon, juvenile, surgery
1. Introduction
Megacolon, the irreversible dilation of a colonic segment, is a structural sign associated with various gastrointestinal disorders.[1] Generally, there are 3 types of megacolon, congenital megacolon (Hirschsprung disease), idiopathic megacolon (IMC), and acquired megacolon (AMC). Hirschsprung disease is characterized by congenital absence of ganglion cells from a distal segment of bowel.[2] IMC is a condition involving persistent dilatation of the colon in the absence of organic disease, also called functional or psychogenic megacolon or megarectum, colonic or rectal inertia, or simply chronic constipation.[2,3] AMC, developing in patients with a demonstrable causative disorder. Identified causes include anorectal obstruction, prescription medications such as antispasmodics, intestinal infections, Chagas disease, necrotizing enterocolits, inflammatory bowel disease, autoimmune or metabolic conditions, and disorders of the endocrine or central nervous system.[2,4,5]
IMC is a rare condition in young children. The association of indigestible food and IMC has never been mentioned in literature. We report a case of IMC after excessive intake of indigestible sticky rice in a single feeding. Our experience indicated pediatric surgeons should be alert to this rare but severe condition.
2. Case report
An 11-year-old boy, previously in good health, was presented to pediatric surgery clinic after a 1-year history of intermittent constipation and abdominal distension. Constipation first occurred 1 year ago after the patient had eaten a large amount of fried sticky rice in 1 consumption. There was no relief of abdominal distension although he received conservative treatments in local hospital, such as Glycerine enema, saline enema and oral Chinese traditional medicine. Abdominal distension was aggravated with bile-like vomiting a month before admission to our hospital. Neither did he use medications known to cause delayed bowel movement nor any family history of gastrointestinal disorders.
On arrival, the patient was emaciated, with a body weight of 28 kg and height of 145 cm. Physical examination showed tense abdominal distension with visible intestinal peristalsis and decreased bowel sounds. Maximum abdomen circumference reached 81 cm. Fecal masses were easily palpable per abdomen as well as per rectum. Anus position was normal and digital rectal examination showed no sign of anal stenosis. Laboratory findings including blood counts, thyroid function and serum electrolytes were all within normal ranges. Plain radiograph and computed tomography of the abdomen revealed dilated colon and distal ileum, full of gas and intestinal contents, measured up to 13 cm in maximum diameter (Fig. 1). He was admitted with the diagnosis of chronic low colonic obstruction, IMC and malnutrition.
Figure 1.
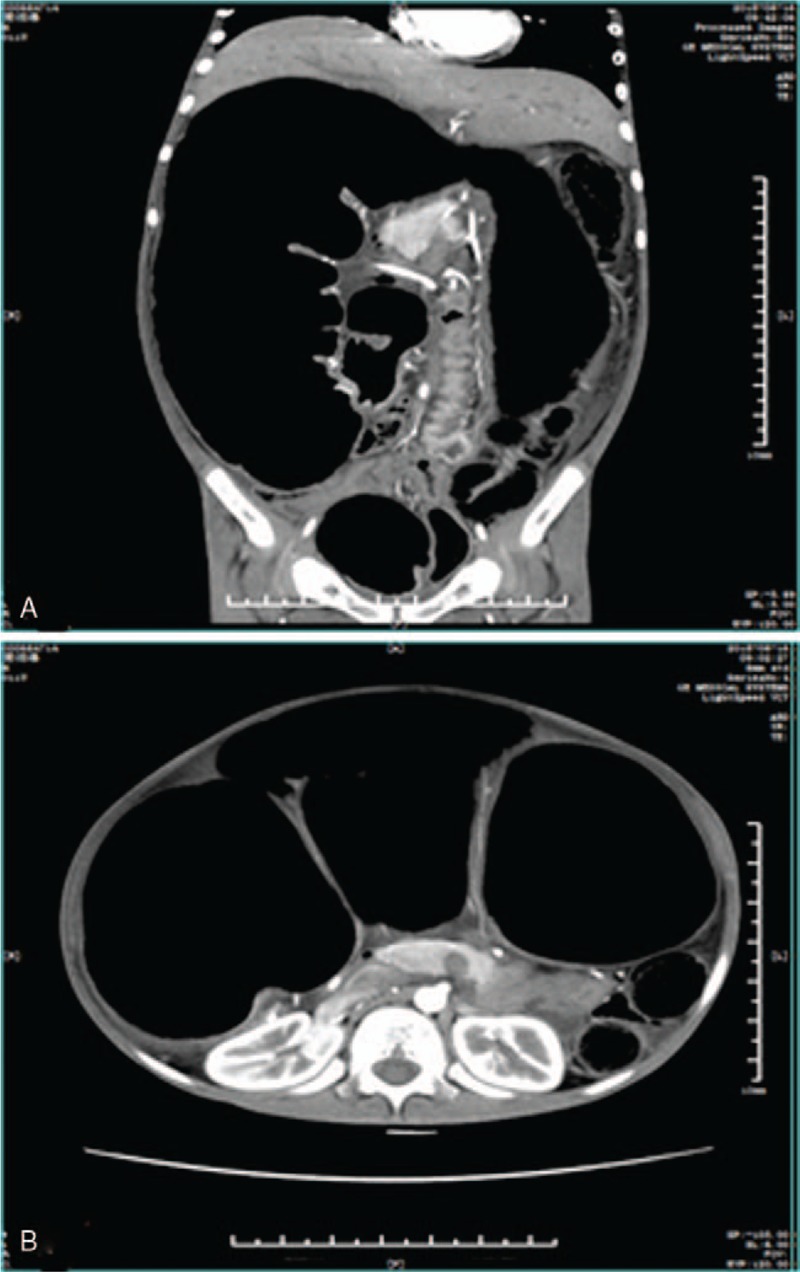
Preoperative abdominal CT scan showing dilated colon and distal ileum.
Fasting, gastrointestinal decompression, anal tube for decompression were given initially due to patient's constipation and intestinal obstruction. His abdominal fullness persisted, saline enema was then prescribed. Total parenteral nutrition was also given to improve patient's nutrition status. Follow-up plain radiograph of abdomen 1 week after admission still revealed prominent dilatation of colon. The patient underwent surgical exploration since conservative therapy was poorly tolerated. On operation, the digestive tract displayed normal anatomy, with no evidence of adhesions, volvulus, intussusception, or torsion but did exhibit massive inflation of the distal loops, more prominent in the distal ileum and proximal descending colon, and measured up to 8 cm in maximum diameter (Figs. 2 and 3). Descending and sigmoid colon were in normal size. Enteral decompression and biopsy were performed through a small incision of splenic flexure. 4000 ml liquid stool was released out of the colon. Meanwhile, loop ileostomy, peritoneal lavage and drainage were performed.
Figure 2.
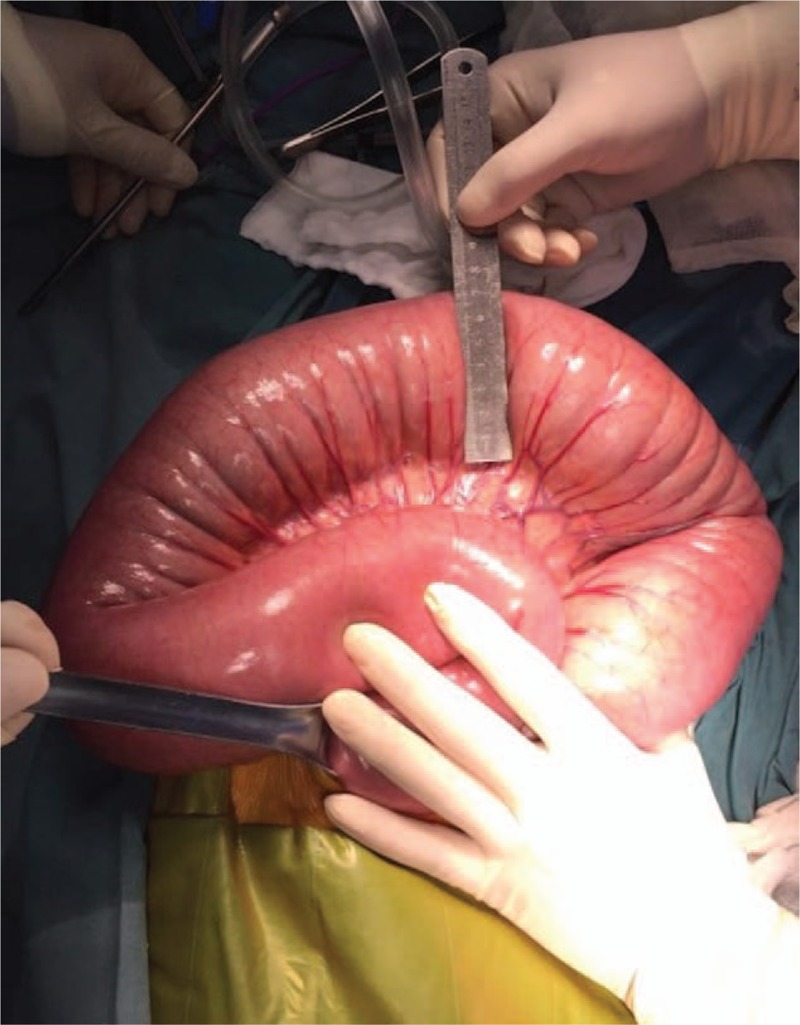
Distal ileum and proximal descending colon showing marked dilation during operation.
Figure 3.
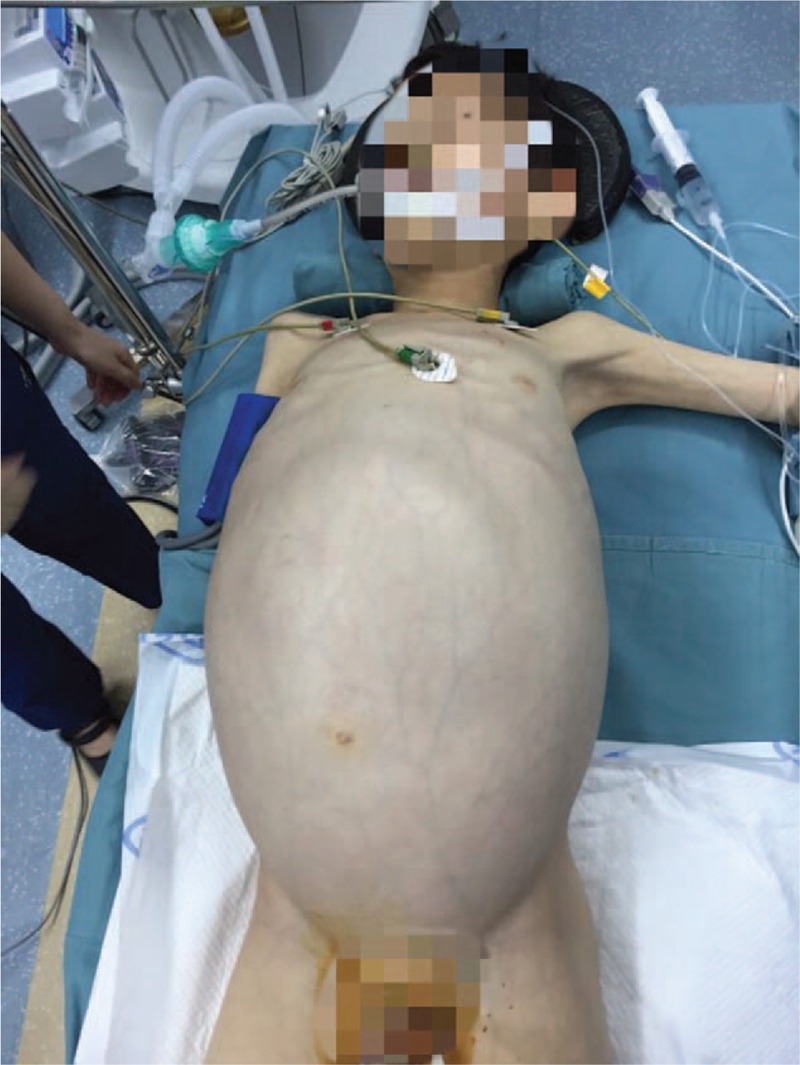
Clinical photography of the 11-year-old boy showing swelling abdomen with visible intestinal peristalsis.
Intraoperative frozen section of the rectum, entire colon, and terminal ileum revealed mild neuron loss parallel decrease of nerve fiber density in the muscular layer and submucosal plexus, with an associated mild chronic inflammatory cells infiltration in lamina propria (Fig. 4). The immunohistochemistry was positive for S-100 protein, SYN, BCL2, CD56, NSE and CGA, leading to the diagnosis of IMC. Postoperative course was smooth. Patient was then discharged without complication after 15 days of hospitalization. He got fast weight gain after 4 months of follow-up visit.
Figure 4.
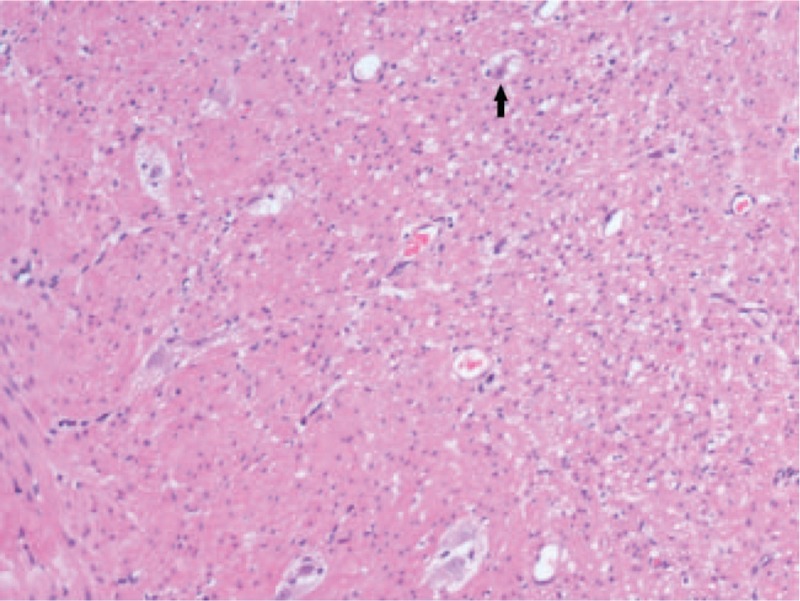
Standard histology (hematoxylin-eosin [HE] staining) of dilated transverse colon sample showing degeneration neuropathy characterized by vacuolar degeneration of ganglion cells (arrows) and a slight reduction of myenteric plexus (×100 magnification).
3. Discussion
For a long time, IMC is a disease of exclusion. The criteria used to diagnose IMC are controversial and no specific criteria exist. Lately, the following criteria for the diagnosis of IMC were proposed by Cuda et al[6]:
(1) the exclusion of organic disease by rectal biopsy or an intact anorectal inhibitory reflex;
(2) symptoms including constipation, distension, abdominal pain, and gas distress;
(3) a sigmoid diameter of around 10 cm on abdominal X-ray or barium enema.
In this case, histologic examination of the rectum, entire colon and terminal ileum showed nerves and ganglion cells within both the submucosal and myenteric plexuses with slight neurodegeneration. The identification of ganglion cells within these plexuses precludes a diagnosis of Hirschsprung disease in cases of IMC, but proposed pathophysiologic abnormalities still permit a decreased number of colonic nerves or ganglion cells[7,8] or a very short aganglionic segment of bowel.[4] Imaging, usually abdominal plain radiograph and barium enema have been used. The sigmoid is often involved radiologically, The Preston et al study, proposes that rectosigmoid diameter at the pelvic brim exceeding 6.5 cm as diagnostic. A mean sigmoid diameter around 10 cm is suggested.[9] In our patient, however, descending and sigmoid colon were in normal size (about 3–4 cm measured in CT scan). Our patient fulfilled the first 2 diagnostic criteria of IMC. But some other studies demonstrated that IMC may exclusively affect the proximal colon for which this definition is not suitable.[10] Radiographic colonic dilatation is defined as a diameter of the most dilated colon segment to larger than 6.5 cm might be more appropriate.
IMC is a rare condition in young children. The natural history of this condition and optimal forms of management are yet to be elucidated. In our patient, there was no past history of constipation, or any infectious, metabolic, mechanical, endocrine-related etiology for constipation since the laboratory tests were all within normal ranges. Other dining companions had no similar symptoms excluded the possibility of food poisoning. We assume that intaking excessive indigestible food may be the inducement of constipation, and subsequent megacolon. Constipation occurred soon after the patient had eaten a large amount of fried sticky rice in 1 meal. As slowly progressive constipation, the proximal bowel cannot compensate, and it dilates and becomes aperistaltic, resulting in megacolon eventually. The pathological basis of IMC remains poorly understood. Review of the literature showed that the non-dilated loops exhibited very similar histopathologic abnormalities to those observed in the dilated loop in most patients with IMC.[11]
Definitive treatment includes medical therapy and surgery. Patients with IMC are managed conservatively in the first instance. Indeed, it is claimed that the majority of patients can be successfully managed nonsurgically.[12] However, surgical therapy is often ultimately required as 50% to 70% of the cases are refractory to medical treatment.[13]
Several case reports showed that IMC may develop devastating complication, such as perforation of the dilated bowel and subsequent peritonitis and sepsis, metabolic and electrolyte abnormalities.[14] Compression of the diaphragm by the megacolon with subsequent respiratory failure,[15] colonic volvulus,[11] compression of the pelvic veins and bladder outlet by the megacolon, with resulting acute shower thromboemboli in the lungs[4,13]also have been reported.
To our knowledge, this is the first report of excessive intake of fried sticky rice complicating megacolon in a previously healthy child. Delay in recognition of this condition may lead to bowel perforation, sepsis, and death. An abdominal radiograph should be considered for patients with history of overeating indigestible food with subsequently persistent abdominal fullness. If IMC is suspected, the patient should receive conservative treatment as early as possible to avoid devastating complications and salvage surgery.
Further research is required on the pathophysiology of the condition, protocols for conservative therapy, and surgery for intractable cases.
Author contributions
Conceptualization: Zhi-bao Lv.
Data curation: Yinghua Liu.
Formal analysis: Yinghua Liu, Xiong Huang.
Writing - Original Draft: Yinghua Liu.
Writing - Review & Editing: Wei-jue Xu, Jiang-bin Liu.
Footnotes
Abbreviations: IMC = idiopathic megacolon, AMC = acquired megacolon, NSE = neuron-specific enolase, SYN = synuclein, CGA = chromogranin A.
The authors have no funding and conflicts of interest to disclose.
Informed written consent was obtained from the patient for publication of this case report and accompanying images.
References
- [1].Jabari S, de Oliveira EC, Brehmer A, et al. Chagasic megacolon: enteric neurons and related structures. Histochem Cell Biol 2014;142:235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ehrenpreis T. Megacolon and megarectum in older children and young adults. Classification and terminology. Proc R Soc Med 1967;60:799–801. [PMC free article] [PubMed] [Google Scholar]
- [3].Gladman MA, Knowles CH. Novel concepts in the diagnosis, pathophysiology and management of idiopathic megabowel. Colorectal Dis 2008;10:531–8. discussion 538-540. [DOI] [PubMed] [Google Scholar]
- [4].Hlavaty L, Sung L. Idiopathic megacolon: report of 2 deaths with review of the literature. Am J Forensic Med Pathol 2017;38:254–7. [DOI] [PubMed] [Google Scholar]
- [5].Govindu RR, Kelley M. Neurogenic megacolon in spinal cord injury. N Engl J Med 2016;375:e45. [DOI] [PubMed] [Google Scholar]
- [6].Cuda T, Gunnarsson R, de Costa A. Symptoms and diagnostic criteria of acquired Megacolon - a systematic literature review. BMC Gastroenterol 2018;18:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ribas Y, Bargallo J, Lamas S, et al. Idiopathic sigmoid megacolon with fecal impaction and giant calcified fecaloma. Am Surg 2013;79:E96–97. [PubMed] [Google Scholar]
- [8].Autschbach F, Gassler N. Idiopathic megacolon. Eur J Gastroenterol Hepatol 2007;19:399–400. [DOI] [PubMed] [Google Scholar]
- [9].Preston DM, Lennard-Jones JE, Thomas BM. Towards a radiologic definition of idiopathic megacolon. Gastrointest Radiol 1985;10:167–9. [DOI] [PubMed] [Google Scholar]
- [10].Min BH, Son HJ, Kim JJ, et al. Idiopathic proximal hemimegacolon: radiologic findings and analyses of clinical and physiological characteristics. Abdom Imaging 2010;35:291–5. [DOI] [PubMed] [Google Scholar]
- [11].Ohkubo H, Masaki T, Matsuhashi N, et al. Histopathologic findings in patients with idiopathic megacolon: a comparison between dilated and non-dilated loops. Neurogastroenterol Motil 2014;26:571–80. [DOI] [PubMed] [Google Scholar]
- [12].CB OS, Anderson JH, McKee RF, et al. Strategy for the surgical management of patients with idiopathic megarectum and megacolon. Br J Surg 2001;88:1392–6. [DOI] [PubMed] [Google Scholar]
- [13].Lane RH, Todd IP. Idiopathic megacolon: a review of 42 cases. Br J Surg 1977;64:307–10. [DOI] [PubMed] [Google Scholar]
- [14].Kabeer S, Dvorkin L, Carrannante J, et al. A life-threatening complication of undiagnosed congenital idiopathic megacolon. BMJ Case Rep 2010;2010: bcr0420102888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chatelain D, Manaouil C, Marc B, et al. Adult Hirschsprung's disease diagnosed during forensic autopsy. J Forensic Sci 2006;51:1160–3. [DOI] [PubMed] [Google Scholar]


