Abstract
Background:
High pretreatment plasma D-dimer levels can predict poor prognosis in various types of gastrointestinal carcinomas. Our meta-analysis explored the correlation between plasma D-dimer levels and prognosis in gastrointestinal malignancies.
Methods:
Two independent reviewers conducted a comprehensive search from PubMed, ScienceDirect, Embase, Web of Science and the Cochrane Library. All articles evaluating the correlation between pretreatment plasma D-dimer levels and prognosis in gastrointestinal malignancies were searched. We chose overall survival (OS) as the primary survival outcome measure and progression-free survival (PFS), disease-free survival (DFS) and cancer-specific survival (CSS) as the secondary survival outcome measures. We extracted hazard ratios (HRs) and 95% confidence intervals (CIs) from the eligible publications.
Results:
We included 30 studies involving 5928 gastrointestinal cancer patients. There was an obvious correlation between high D-dimer levels and poor OS (HR = 2.01, 95% CI = 1.72–2.36, P < .01). High plasma D-dimer levels were correlated with shorter PFS (HR = 1.34, 95% CI = 1.05–1.70, P = .32), DFS (HR = 1.67, 95% CI = 1.12–2.50, P < .01) and CSS rates (HR = 1.93, 95% CI = 1.49–2.49, P = .66).
Conclusions:
Elevated pretreatment plasma D-dimer levels might help predict poor prognosis in patients with gastrointestinal malignancies.
Keywords: clinical value, D-dimer, gastrointestinal carcinoma, meta-analysis, prognosis
1. Introduction
Gastrointestinal cancer, including esophageal cancer, gastric cancer, pancreatic cancer, hepatoma, cholangiocarcinoma and colorectal cancer, is the main type of digestive system neoplasm worldwide.[1] Nearly 30% of carcinoma morbidity and 32% of carcinoma mortality worldwide are attributed to gastrointestinal carcinoma.[2] Gastrointestinal carcinomas pose a dramatic clinical challenge because of their high morbidity and mortality. Patients with gastrointestinal cancers are often already in advanced or terminal stages and show resistance to chemotherapy at the time of diagnosis. In fact, gastrointestinal malignancies can be treated at an early stage.[3] For example, gastroscopy has been indicated to effectively decrease the incidence rate of gastric cancer by approximately 30%.[4] Similarly, colorectal cancer could be prevented by performing regular colonoscopies to find precancerous lesions across the large intestine.[5] The early diagnosis of cancer is crucial because colorectal cancer could be treated if diagnosed early; the American Cancer Society reported dramatic differences in 5-year survival rates between non-metastatic and metastatic colorectal cancer of 90% and 11%, respectively.[6] However, endoscopic techniques are expensive and invasive, thus further limiting the practicality of detecting gastrointestinal cancer by these techniques. Thus, effective, inexpensive and non-invasive biomarkers for patient diagnosis and prognosis need to be discovered.
The inappropriate activation of both coagulation and fibrinolysis is usually discovered in carcinoma patients, especially in patients with metastatic carcinoma.[7–11] Coagulation is the process by which blood changes from liquid to gel and then forms clots. Cancer cells can have significant procoagulant activities, activating the coagulation system and depositing fibrin, therefore causing the phenomenon of coagulation.[12] The formation of a platelet-fibrin-carcinoma cell offers an extracellular microenvironment to promote carcinoma cell proliferation and survival.
D-dimer is the product of fibrin degradation and is composed of 2 cross-linked D fibrin fragments.[13] Some studies have reported that pretreatment plasma D-dimer levels are obviously increased in patients with various carcinomas, including nasopharyngeal carcinoma, lung cancer, breast cancer and cervical cancer.[14–17] The association between elevated plasma D-dimer levels and poor survival outcomes is also observed in gastrointestinal carcinomas, such as esophageal carcinoma, gastric cancer, pancreatic carcinoma, hepatoma, cholangiocarcinoma and colorectal cancer.[18–47] However, no systematic studies have identified the prognostic significance of D-dimer in gastrointestinal carcinomas. Thus, the aim of our systematic review and meta-analysis was to assess the prognostic significance of D-dimer levels in gastrointestinal carcinomas.
2. Materials and methods
2.1. Search strategy
This meta-analysis was strictly conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[48] Two independent reviewers conducted a comprehensive electronic search to find eligible studies in ScienceDirect, PubMed, Embase, Web of Science and the Cochrane Library dating up to July 5, 2018. The key terms of this analysis included “D-dimer” or “D-dimer” and “tumor” or “cancer” or “carcinoma” or “neoplasm” and “prognosis” or “survival” from 2001 to 2018. The search outcomes were restricted to human research published in English.
2.2. Inclusion and exclusion criteria
The inclusion criteria of our analysis were as follows:
-
1.
clinical studies about the association between D-dimer levels and prognosis in gastrointestinal tumors;
-
2.
outcome indicators including overall survival (OS), progression-free survival (PFS), disease-free survival (DFS) or cancer-specific survival (CSS); and
-
3.
data on hazard ratios (HRs) and 95% confidence intervals (CIs) or survival curves.
The exclusion criteria of this analysis were as follows:
-
1.
the same studies published more than once;
-
2.
animal studies;
-
3.
non-English articles;
-
4.
reviews, case reports, conference abstracts, letters and meta-analyses; and
-
5.
unavailable HR and 95% CI or survival curve data.
There independent researchers (Guoyi Rong, Wenxin Fan, and Jian Shen) evaluated all titles and abstracts of the eligible studies to identify duplicated data and irrelevant records. If the included study was not identified by the abstract, the full publication was read. Any disagreements were settled by discussion to reach a consensus.
2.3. Data extraction
All information was extracted from the included studies by 2 independent investigators (Guoyi Rong and Jian Shen). The survival outcomes were extracted, including OS, PFS, DFS, CSS, and HRs with 95% CIs or survival curves with P values. Other data from these studies were also collected: first author, research institute, publication year, country, research design, patient number, patient age, follow-up period, survival analysis models, cancer stage, cancer site, cut-off value, detection method, and therapy.
2.4. Quality assessment
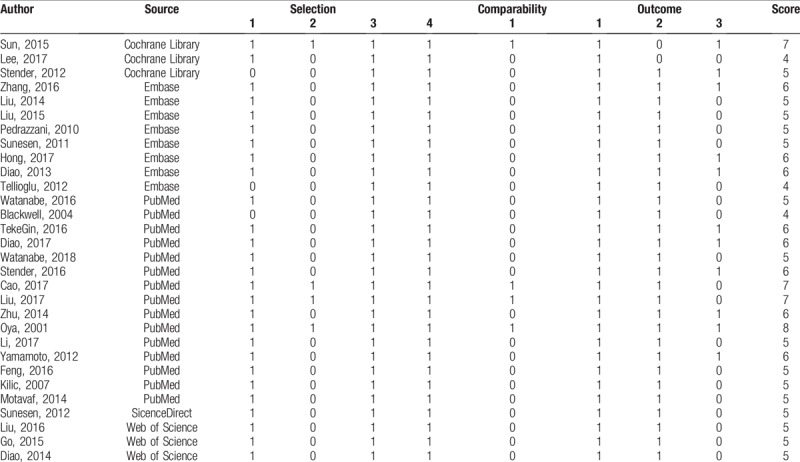
The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the eligible studies by 2 independent investigators (Guoyi Rong and Jian Shen).[49] An assessment of every study was performed in 3 parts, including the selection, comparability and outcomes of the study.
2.5. Statistical analysis
We carried out this meta-analysis by employing R software (version 3.50) (https://www.r-project.org/). We regarded OS as the primary outcome in our research, while PFS, DFS and CSS were regarded as the secondary outcomes. HRs with 95% CIs were directly extracted from every study. If a study provided only survival curves, data would be assessed by employing the Engauge Digitizer software (version 4.1).[50,51] The prognostic significance of plasma D-dimer levels in patients with gastrointestinal tumors was assessed by HRs and 95% CIs. HRs > 1 predicted poor prognosis in patients with high serum D-dimer levels. HRs < 1 frequently implied a favorable prognosis in patients with increased serum D-dimer levels. The statistical heterogeneity of the eligible studies was calculated by Q-test and the I2 statistic. When heterogeneity was nonsignificant (P ≥ .10, I2 value <50%), a fixed effect model was employed; however, if there was significant heterogeneity, a random effects model was employed and a subgroup analysis was conducted to find the sources of heterogeneity. Subgroup analyses were stratified by
-
1.
tumor site;
-
2.
country (Asian, non-Asian);
-
3.
therapy;
-
4.
detection method;
-
5.
D-dimer cut-off value;
-
6.
HR estimation; and
-
7.
the HRs provided from the study.
In addition, we employed sensitivity analyses to identify each study's influence on the pooled effect by sequentially eliminating one study at a time. Simultaneously, a meta-regression was carried out to evaluate whether any relevant variables impacted the pooled effect size for OS, PFS, DFS, and CSS. Publication bias was assessed by Begg funnel plots.
3. Results
3.1. Literature search
The flowchart for the selection of literature is shown in Figure 1. A total of 1152 publications were first confirmed following our search scheme. After excluding duplicate studies, a total of 901 studies were included. Animal research, reviews, case reports, conference abstracts, letters and meta-analyses were removed after screening the titles and abstracts of each article. Then, 43 included studies were evaluated in full text. After further inspection, 13 studies were removed, of which HRs could not be extracted from 3 studies, and 10 were missing relevant outcome indicators. Finally, 30 studies involving 5928 patients were included in our research.
Figure 1.
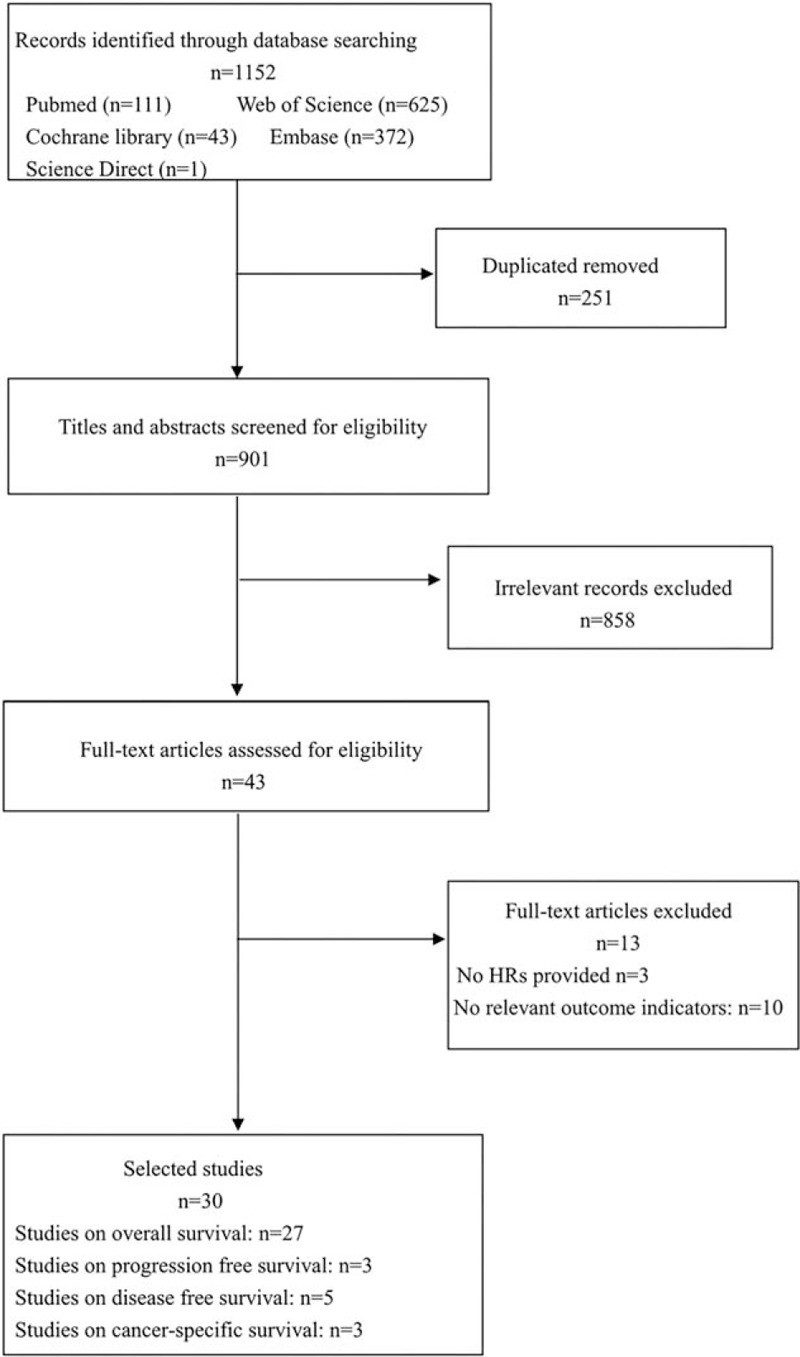
Flowchart of search strategy in the meta-analysis.
3.2. Study characteristics
The main features of the eligible studies are listed in Table 1. All eligible articles were published between 2001 and 2018. Fourteen studies acquired data retrospectively, while the remaining studies applied a prospective research design. The D-dimer levels were tested by immunoturbidimetry, enzyme-linked immunosorbent assay (ELISA), latex agglutination test or enzyme-linked immunofiltration assay (ELIFA). Of all the eligible studies, 5 publications reported on esophageal tumors (n = 5),[21,27,39,41,45] 4 on gastric cancer (n = 4),[22,32,46,47] 4 on pancreatic cancer (n = 4),[18,23,34,35] 1 on hepatoma (n = 1),[36] 1 on cholangiocarcinoma (n = 1),[29] and 15 on colorectal cancer (n = 15).[19,20,24–26,28,30,31,33,37,38,40,42–44] Twenty-seven studies used OS, 3 studies employed PFS, 5 studies employed DFS and 3 studies employed CSS as the survival outcomes. The quality assessment of each included article measured by NOS is shown in Table 2.
Table 1.
Main characteristic of the included studies in the meta-analysis.
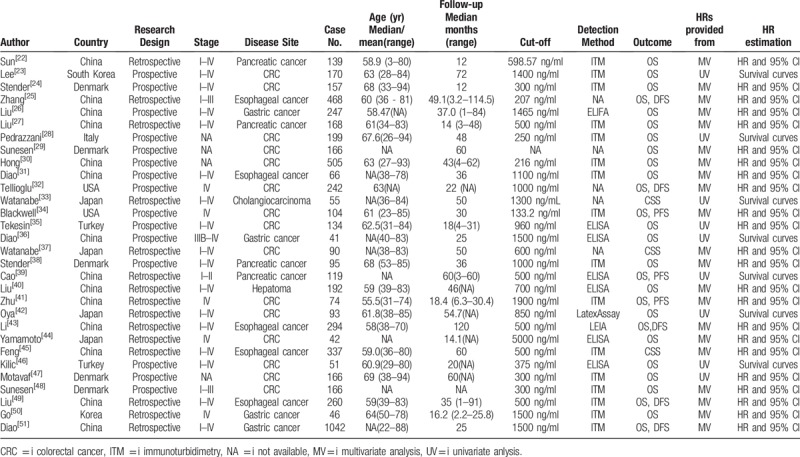
Table 2.
Quality assessment of included articles.
3.3. Primary outcome: overall survival
Twenty-seven studies involving 5446 patients provided appropriate information about OS analysis. The results indicated that elevated pretreatment D-dimer levels were predictive of shorter OS in a random effects model (HR = 2.01, 95% CI = 1.72–2.36) with significant heterogeneity among the studies (I2 = 67%, P < .01) (Fig. 2A).
Figure 2.
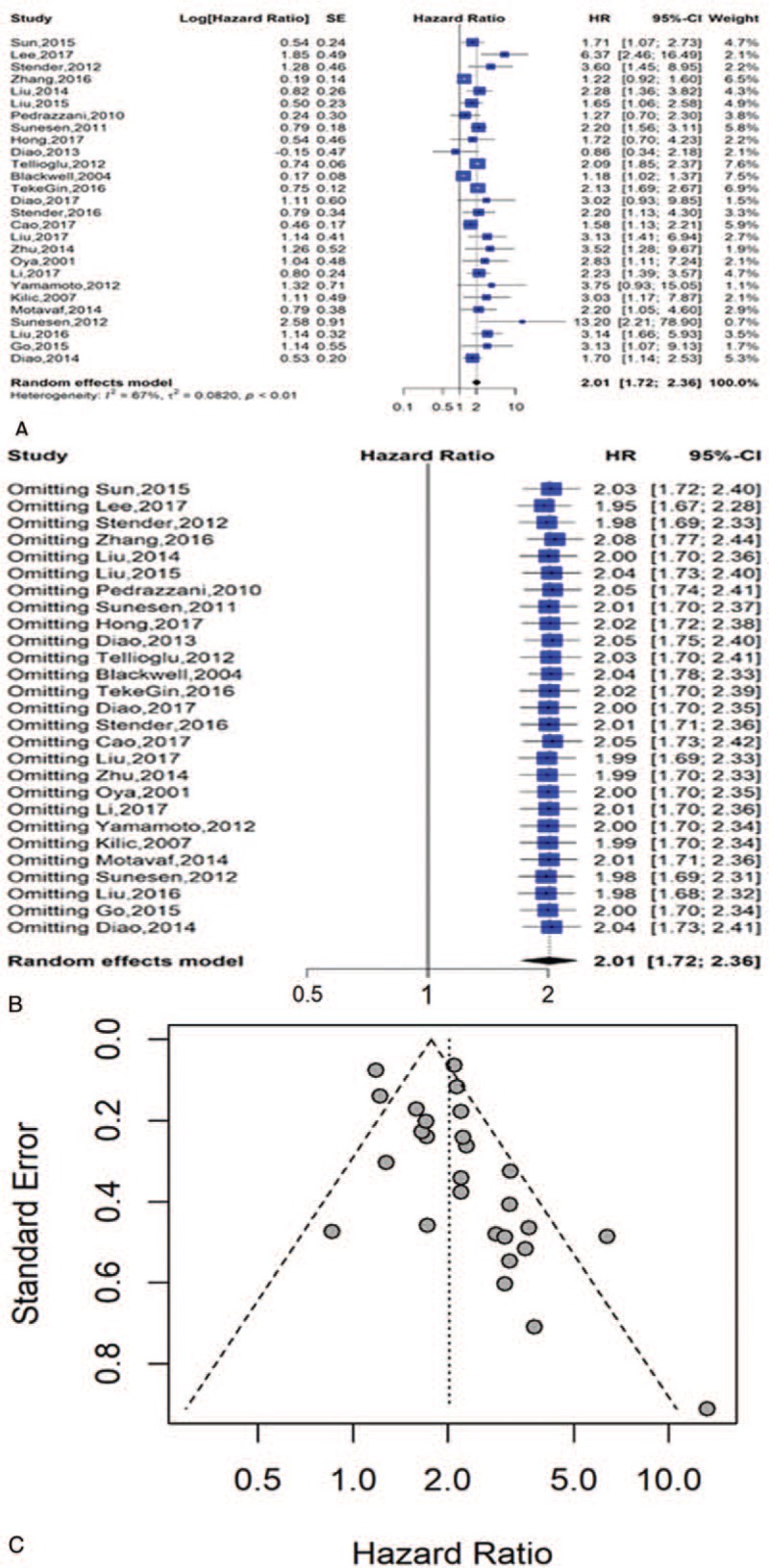
Analysis for overall survival; (A) forest plots of hazard ratios for overall survival; (B) sensitivity analysis of all eligible publications for overall survival; (C) funnel plot of publication bias in the meta-analysis.
The stability of our result was verified through a sensitivity analysis that employed a model in which 1 study was removed at a time. The observed effect size (multivariable adjusted HR) of OS was not dramatically influenced when a certain study was removed in every round (Fig. 2B).
3.4. Subgroup analysis
3.4.1. Tumor site
We performed subgroup analyses on the basis of the cancer site. As shown in Figure 3A, we found that the highest prognostic significance of high D-dimer levels on OS was in hepatoma (HR = 3.13, 95% = 1.41–6.94), followed by colorectal cancer (HR = 2.24, 95% CI = 1.73–2.88), gastric cancer (HR = 2.02, 95% CI = 1.51–2.71), pancreatic cancer (HR = 1.69, 95% CI = 1.35–2.10) and esophageal cancer (HR = 1.69, 95% CI = 1.01–2.82). High heterogeneity was discovered among the studies on colorectal tumors (I2 = 78%, P < .01) and esophageal cancer (I2 = 74%, P < .01).
Figure 3.
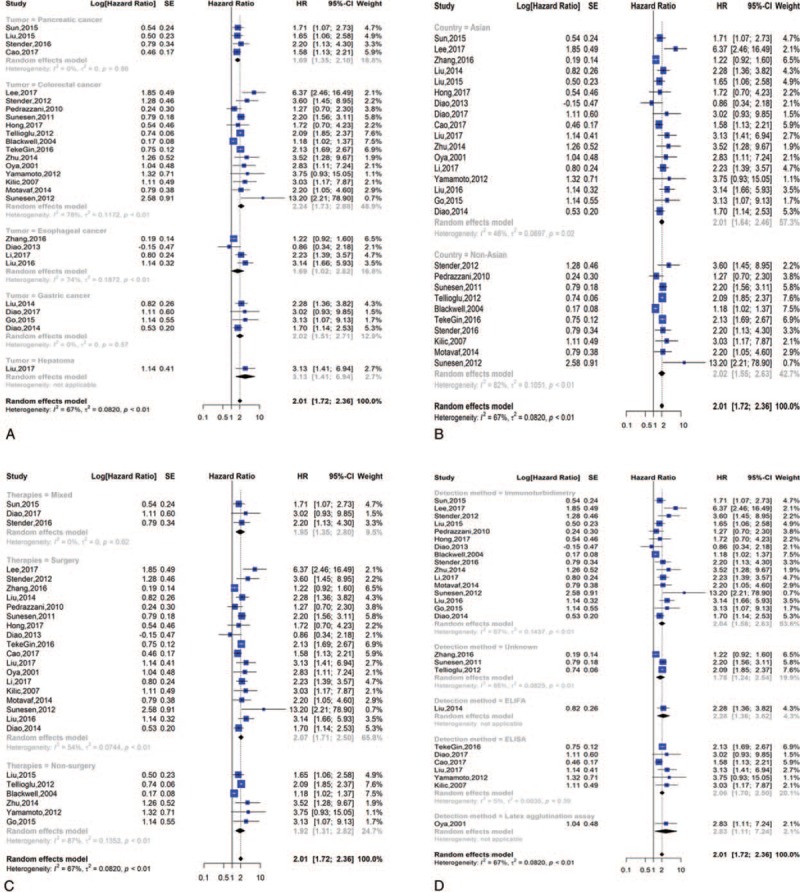
Subgroup analysis of overall survival; (A) forest plot of tumor site; (B) forest plot of countries; (C) forest plot of therapies; (D) forest plot of detection methords.
3.4.2. Asian and non-Asian countries
Ten publications originated from Europe and America (Denmark, Turkey, Italy, and America), and seventeen originated from Asia (China, Japan, South Korea, and North Korea). When we conducted a subgroup analysis of patients’ countries, we found a dramatic correlation between high D-dimer values and poor OS in patients from Asian (HR = 2.01, 95% CI = 1.64–2.46) and non-Asian countries (HR = 2.02, 95% CI = 1.55–2.63) (Fig. 3B).
3.4.3. Therapies
The main therapies in the included studies were surgery, non-surgery, and mixed therapy (chemotherapy and surgery). Since therapies might affect prognosis, we employed subgroup analysis to further study the prognostic significance of D-dimer levels. The HR and 95% CI for OS were 2.07 [1.71, 2.50] in the surgery group, 1.92 [1.31, 2.82] in the non-surgery group, and 1.95 [1.35, 2.80] in the mixed therapy group (Fig. 3C).
3.4.4. Detection methods
The detection methods used in the eligible studies were immunoturbidimetry assay, ELISA, latex agglutination assay, ELIFA and unknown. Because detection methods might affect prognosis, we conducted subgroup analysis to further identify the prognostic significance of D-dimer levels. The HR and 95% CI for OS were 2.10 [1.59, 2.79] for the immunoturbidimetry assay, 1.99 [1.70, 2.34] for ELISA, 2.83 [1.11, 7.24] for the latex agglutination assay, 2.28 [1.36, 3.82] for ELIFA, and 1.78 [1.24, 2.54] for unknown method used (Fig. 3D).
3.4.5. Other groups
We also divided the studies according to a cut-off value (cut-off ≥ 600 ng/ml or < 600 ng/ml), the HR estimation (HR and 95% CI or survival curves) and the HRs provided from multivariate analysis or univariate analysis groups. We discovered that the HR and 95% CI for OS in the cut-off ≥600 group was 1.76 [1.42, 2.19] and in the cut-off <600 group was 2.17 [1.89, 2.50]. In the HR and 95% CI and survival curve groups, the HRs and 95% CI for OS were 1.98 [1.66, 2.36] and 2.30 [1.46, 3.64], respectively. We also discovered that the HR and 95% CI for OS in the multivariate analysis was 1.97 [1.62, 2.39] and for the univariate analysis was 2.12 [1.62, 2.78] (Fig. 4).
Figure 4.
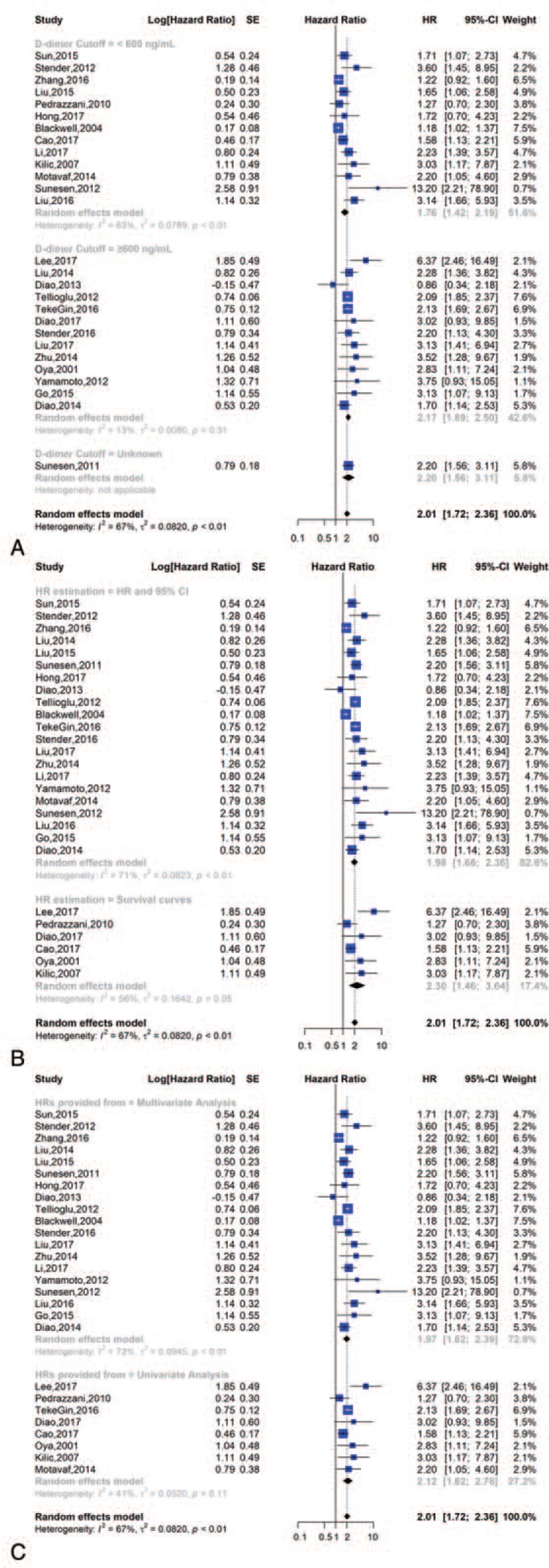
Subgroup analysis of overall survival; (A) forest plot of D-dimer cut-off value; (B) forest plot of HR estimation; (C) forest plot of HRs provided from.
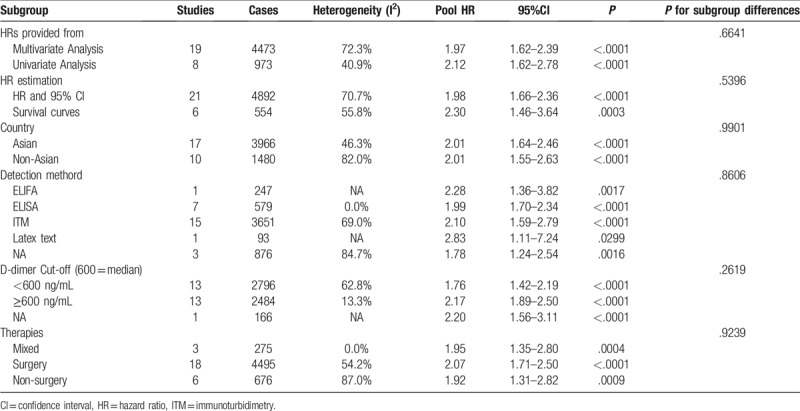
The results of the subgroup analysis for OS are shown in Table 3.
Table 3.
Pooled multivariable-adjusted HRs for OS according to subgroup analyses.
3.4.6. Publication bias
We employed Begg funnel plot to inspect publication bias. The result indicated no publication bias and was statistically significant (Fig. 2C).
3.4.7. Secondary outcome: progression-free survival, disease-free survival and cancer-specific survival
Three studies involving 294 patients provided appropriate data for PFS analysis. The results indicated that elevated pretreatment D-dimer levels predicted shorter PFS in a fixed effects model (HR = 1.34, 95% CI = 1.05–70) with significant heterogeneity among the studies (I2 = 12%, P = .32) (Fig. 5A).
Figure 5.
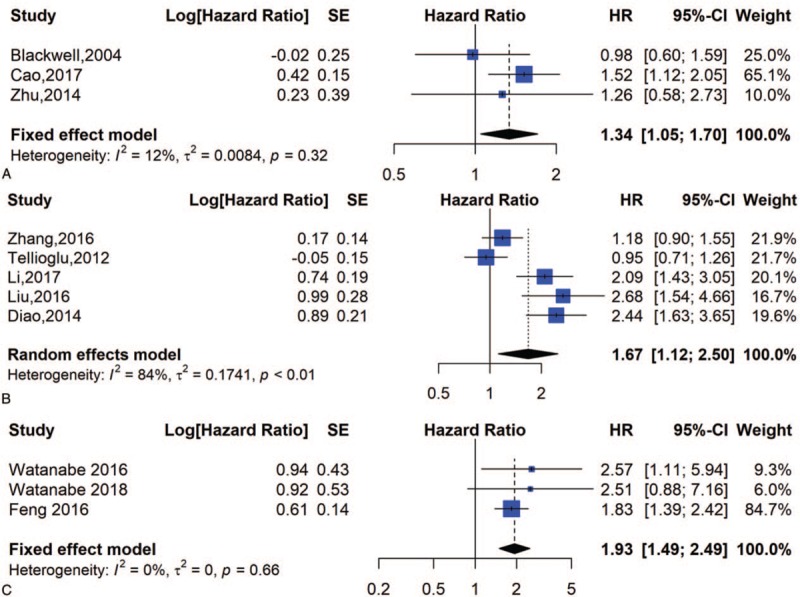
Forest plot of PFS, DFS and CSS; (A) forest plots of hazard ratios for PFS; (B) forest plots of hazard ratios for DFS; (C) forest plots of hazard ratios for CSS.
Five studies involving 2306 patients provided appropriate information for DFS analysis. The results indicated that a high D-dimer level predicted poor DFS in a random effects model (HR = 1.67, 95% CI = 1.12–2.50) with nonsignificant heterogeneity among the studies (I2 = 84%, P < .01) (Fig. 5B).
Five studies involving 482 patients provided appropriate information for CSS analysis. As Figure 5C shows, the HR and 95% CI for CSS was 1.93 [1.49–2.49].
The stability of our result was verified through a sensitivity analysis that employed a model in which 1 study was removed at a time. The observed effect sizes (multivariable adjusted HR) of PFS, DFS, and CSS were not dramatically impacted when 1 study was removed in every round (Fig. 6). PFS and CSS did not require subgroup analysis because there were 3 eligible articles about PFS and CSS.
Figure 6.
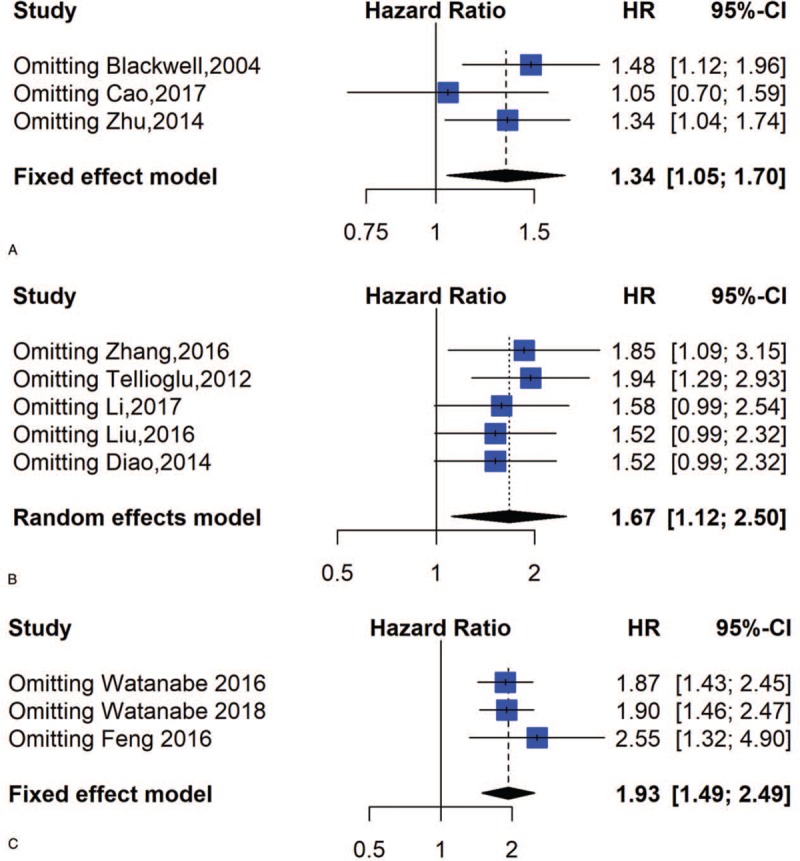
Sensitivity analysis of PFS, DFS and CSS; (A) Sensitivity analysis for PFS; (B) Sensitivity analysis for DFS; (C) Sensitivity analysis for CSS.
3.5. Subgroup analysis
3.5.1. Tumor site
We performed subgroup analyses on the basis of the cancer site. As Figure 7A shows, we found that the highest prognostic significance of high D-dimer levels on DFS was in gastric carcinoma (HR = 2.44, 95% CI = 1.63–3.65), followed by esophageal cancer (HR = 1.81, 95% CI = 1.10–2.98) and colorectal carcinoma (HR = 0.95, 95% CI = 0.71–1.26). High heterogeneity was discovered among the studies on esophageal tumors (I2 = 84%, P < .01).
Figure 7.
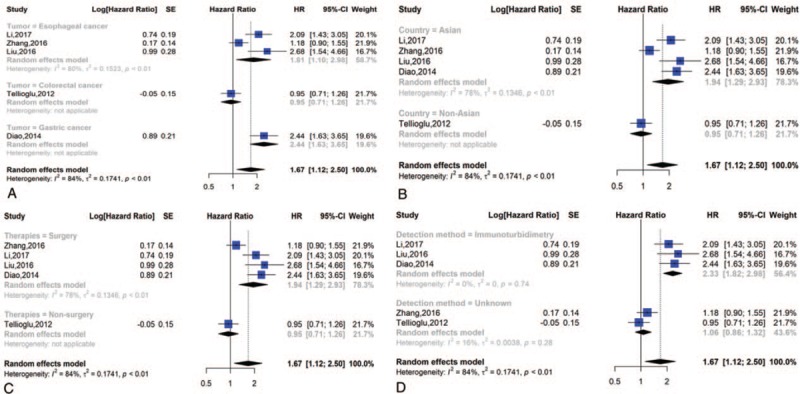
Subgroup analysis of DFS; (A) forest plot of tumor site; (B) forest plot of countries; (C) forest plot of therapies; (D) forest plot of detection methords.
3.5.2. Asian and non-Asian countries
One report originated from America, and 4 originated from Asia. When we conducted a subgroup analysis of patients’ countries, we found a dramatic correlation between high D-dimer values and poor DFS in both Asian (HR = 1.94; 95% CI = 1.29–2.93) and non-Asian countries (HR = 0.95; 95% CI = 0.71–1.26) (Fig. 7B).
3.5.3. Therapies
The main therapies in the included studies were surgery and non-surgery. Since therapies might affect prognosis, we employed subgroup analysis to further identify the prognostic significance of D-dimer levels. The HR and 95% CI for DFS were 1.94 [1.29, 2.93] in the surgery group and 0.95 [0.71, 1.26] in the non-surgery group (Fig. 7C).
3.5.4. Detection method
The main detection methods in the eligible studies were immunoturbidimetry assays and unknown. Because detection methods might affect prognosis, we performed subgroup analysis to further identify the prognostic significance of D-dimer levels. The HR and 95% CI for DFS were 2.33 [1.82, 2.98] for the immunoturbidimetry assay and 1.06 [0.86, 1.32] for the unknown methods used (Fig. 7D).
3.5.5. Other groups
We also divided the studies according to a cut-off value (cut-off ≥600 ng/ml or <600 ng/ml), the HR estimation (HR and 95% CI or survival curves) and the HRs provided by multivariate analysis or univariate analysis. We discovered that the HR and 95% CI for DFS in the cut-off ≥600 group was 1.50 [0.60, 3.80] and in the cut-off <600 group was 1.81 [1.10, 2.98]. In the HR and 95% CI and survival curve groups, the HRs and 95% CIs for DFS were 1.58 [0.99, 2.54] and 2.09 [1.43, 3.05], respectively. We also discovered that the HR and 95% CI for DFS in the multivariate analysis was 1.58 [0.99, 2.54] and in the univariate analysis was 2.09 [1.43, 3.05] (Fig. 8).
Figure 8.
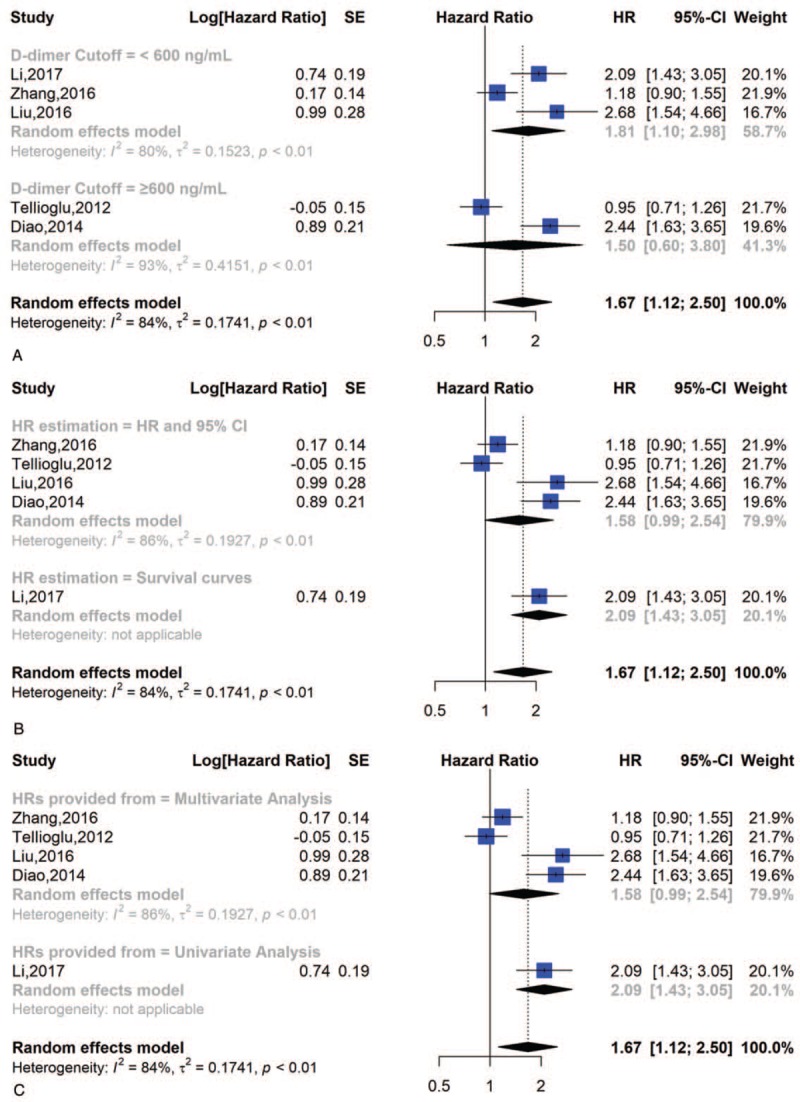
Subgroup analysis of DFS; (A) forest plot of D-dimer cut-off value; (B) forest plot of HR estimation; (C) forest plot of HRs provided from.
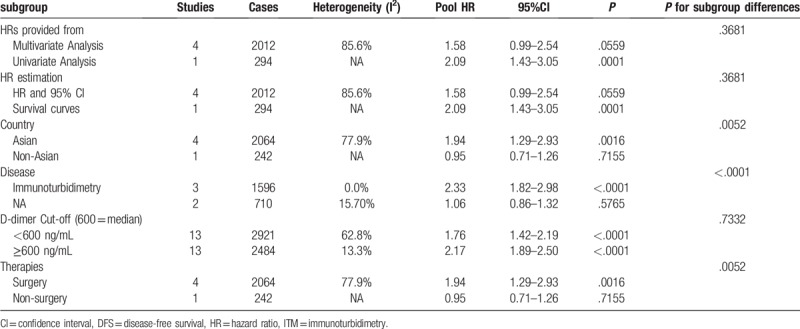
The results of the subgroup analysis for DFS are shown in Table 4.
Table 4.
Pooled multivariable-adjusted HRs for DFS according to subgroup analyses.
3.6. Publication bias
We employed Begg funnel plot to inspect publication bias. The result indicated no publication bias and was statistically significant (Fig. 9).
Figure 9.
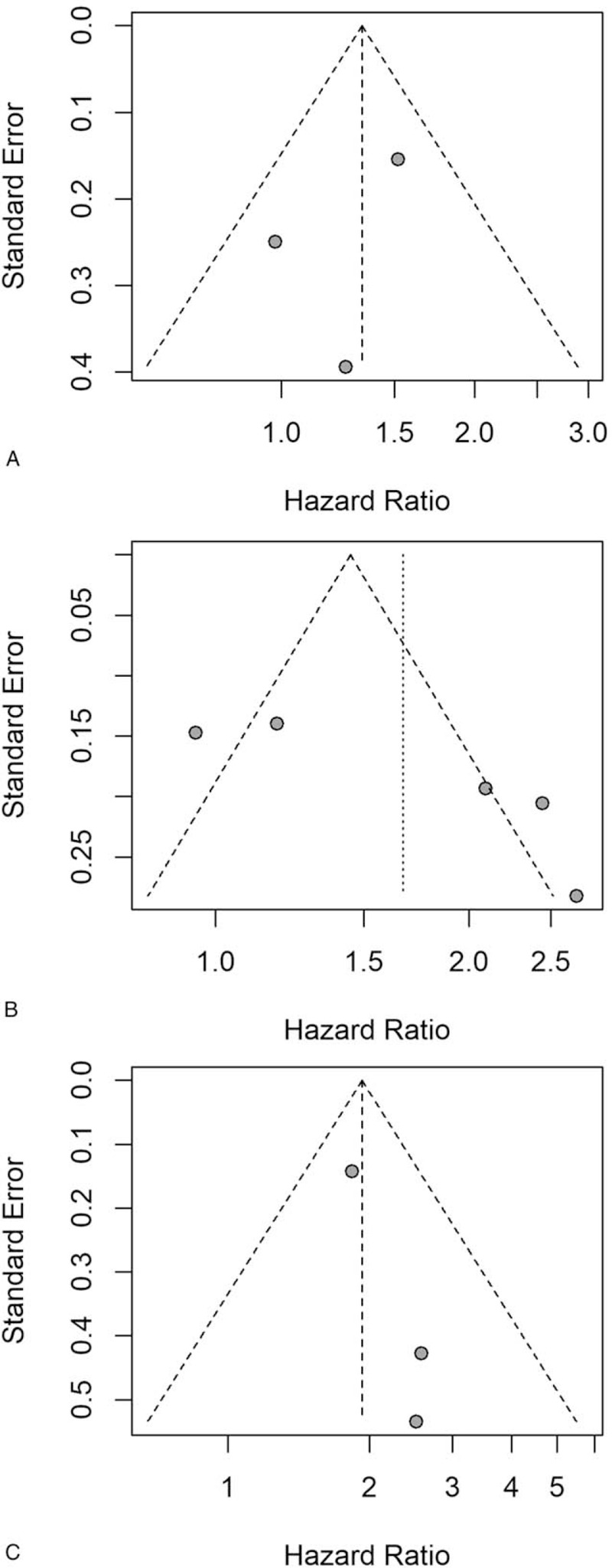
Funnel plots of PFS, DFS and CSS; (A) funnel plots of PFS; (B) funnel plots of DFS; (C) forest plot of CSS.
4. Discussion
In this study, we assessed the correlation between poor survival outcomes and pretreatment plasma D-dimer levels in gastrointestinal cancers. The HR and 95% CI for OS were 2.01 [1.72–2.36]. The correlation between shorter secondary outcomes (PFS, DFS, and CSS) and elevated pretreatment plasma D-dimer levels was in accordance with that of OS with all HRs >1. This finding demonstrates that the D-dimer level is an adverse factor of prognosis for gastrointestinal carcinoma patients. The outcomes regarding the prognostic significance of D-dimer levels (OS, PFS, DFS, and CSS) were robust after sensitivity analysis, indicating that the HRs were not dramatically influenced by any individual study. In the subgroup analysis of OS, the adverse prognostic effects of elevated D-dimer levels were still significant with different tumor sites, different countries, different therapies, various detection methods, different cut-off values, different HR estimations and different HRs provided from the studies. We believe that our research is beneficial in determining the significance of the prognostic value of plasma D-dimer levels in gastrointestinal cancers.
To the best of our knowledge, our research was the first study that employed prognostic publications on all gastrointestinal carcinomas to identify the prognostic significance of pretreatment plasma D-dimer levels. We employed only HR rather than OR or relative risks (RRs) to evaluate the significance of prognosis because the latter 2 parameters are not credible or are difficult to interpret. Moreover, we included only studies that had data on pretreatment D-dimer levels and HRs. For these reasons, our study may be unique from others, and the quality and credibility of our research are guaranteed.
There are some defects in the carcinoma prognostic evaluation system, such that patients with the same TNM stage frequently have disparate prognoses. D-dimer is a kind of easily available, routinely measured molecular biomarker. In addition, some studies have reported that pretreatment plasma D-dimer levels are dramatically increased in patients with various carcinomas.[14–17] Thus, D-dimer levels could be used as a complementary biomarker to increase the accuracy of prognosis estimations.
Although the exact mechanism by which D-dimer influences survival outcomes is still unclear, some publications postulated that D-dimer affects carcinoma patients’ survival outcome by means of the formation of venous thromboembolisms (VTEs). VTEs are a common complication in malignancies.[52] Nevertheless, Ay et al[53] reported that D-dimers and VTEs are independent of each other regarding the poor survival outcomes of carcinoma patients.
The unfavorable survival outcome of carcinoma patients is consistent with metastasis and angiogenesis. Fibrin remodeling is involved in multiple processes of metastasis and has been proven to play a significant role in angiogenesis.[54] D-dimer is a sensitive biomarker of the fibrinolytic process. Some clinical trials involving carcinomas that could activate the coagulation system indicate that high D-dimer levels could be related to advanced tumor stage and unfavorable survival outcomes.[55–57] The effect of the mechanism by which D-dimer affects the progression of malignant tumors needs to be determined in further studies.
Although our research provides a more persuasive conclusion that D-dimer levels can be used as a method for predicting the survival outcome of gastrointestinal cancer patients, certain inevitable limitations should be taken into consideration:
-
1.
some HR estimations could be extracted directly, while other HR estimations were extracted from the survival curve, and these were jointly incorporated to guarantee data integrity. We intensively deliberated the calculations of each publication 3 times through the above methods to avoid using unreasonable outcomes.
-
2.
Some studies provided low-quality data with a short follow-up period.
-
3.
The cut-off value that determined high and low D-dimer levels varied among the eligible studies, which enhanced the difficulty of performing a pooled study.
-
4.
Some studies that reported on the prognostic significance of D-dimer levels were eliminated if they did not report HRs or allow HRs to be calculated.
-
5.
Although no apparent publication bias was discovered in our research, there might have been some potential biases that were not published.
5. Conclusions
In summary, this research suggests that higher pretreatment plasma D-dimer levels could predict adverse survival outcomes among patients with different types of gastrointestinal carcinomas. Additionally, we should conduct further observation and research to determine whether plasma D-dimer levels could be introduced into the carcinoma staging system. Moreover, additional studies need to be conducted to demonstrate the correlation and mechanism between elevated plasma D-dimer levels and gastrointestinal carcinoma progression.
Author contributions
Conceptualization: Guoyi Rong, Wenxin Fan, Jian Shen.
Data curation: Guoyi Rong, Wenxin Fan, Jian Shen.
Formal analysis: Guoyi Rong, Wenxin Fan, Jian Shen.
Funding acquisition: Guoyi Rong, Wenxin Fan, Jian Shen.
Investigation: Guoyi Rong, Wenxin Fan, Jian Shen.
Methodology: Guoyi Rong, Wenxin Fan, Jian Shen.
Project administration: Guoyi Rong, Wenxin Fan, Jian Shen.
Resources: Guoyi Rong, Wenxin Fan, Jian Shen.
Software: Guoyi Rong, Wenxin Fan, Jian Shen.
Supervision: Guoyi Rong, Wenxin Fan, Jian Shen.
Validation: Guoyi Rong, Wenxin Fan, Jian Shen.
Visualization: Guoyi Rong, Wenxin Fan, Jian Shen.
Writing – original draft: Guoyi Rong, Wenxin Fan, Jian Shen.
Writing – review & editing: Guoyi Rong, Wenxin Fan, Jian Shen.
Footnotes
Abbreviations: CI = confidence interval, OS = overall survival, PFS = progression-free survival, DFS = disease-free survival, CSS = cancer-specific survival, DIC = disseminated intravascular coagulation, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, NOS = Newcastle-Ottawa Scale.
GR and WF are both co-first authors. This research was funded by the Chongqing Municipal Health Bureau.
The authors do not have any conflicts of interest.
References
- [1].Garla P, Waitzberg DL, Tesser A. Nutritional therapy in gastrointestinal cancers. Gastroenterol Clin North Am 2018;47:231–42. [DOI] [PubMed] [Google Scholar]
- [2].Mislang AR, Di Donato S, Hubbard J, et al. Nutritional management of older adults with gastrointestinal cancers: an International Society of Geriatric Oncology (SIOG) review paper. J Geriatr Oncol 2018;9:382–92. [DOI] [PubMed] [Google Scholar]
- [3].Wong CC, Li W, Chan B, et al. Epigenomic biomarkers for prognostication and diagnosis of gastrointestinal cancers. Semin Cancer Biol 2018;55:90–105. [DOI] [PubMed] [Google Scholar]
- [4].Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014;23:700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014;348:g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104–17. [DOI] [PubMed] [Google Scholar]
- [7].Wang Y, Wang Z. Predictive value of plasma D-dimer levels in patients with advanced non-small-cell lung cancer. Onco Targets Ther 2015;8:805–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fukumoto K, Taniguchi T, Usami N, et al. Preoperative plasma D-dimer level is an independent prognostic factor in patients with completely resected non-small cell lung cancer. Surg Today 2015;45:63–7. [DOI] [PubMed] [Google Scholar]
- [9].Im JH, Fu W, Wang H, et al. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res 2004;64:8613–9. [DOI] [PubMed] [Google Scholar]
- [10].Luo YL, Chi PD, Zheng X, et al. Preoperative D-dimers as an independent prognostic marker in cervical carcinoma. Tumour Biol 2015;36:8903–11. [DOI] [PubMed] [Google Scholar]
- [11].Ferroni P, Roselli M, Portarena I, et al. Plasma plasminogen activator inhibitor-1 (PAI-1) levels in breast cancer - relationship with clinical outcome. Anticancer Res 2014;34:1153–61. [PubMed] [Google Scholar]
- [12].Aliustaoglu M, Yumuk PF, Gumus M, et al. D-dimer--can it be a marker for malignant gastric lesions? Acta Oncol 2004;43:770–1. [DOI] [PubMed] [Google Scholar]
- [13].Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood 2009;113:2878–87. [DOI] [PubMed] [Google Scholar]
- [14].Chen WH, Tang LQ, Wang FW, et al. Elevated levels of plasma D-dimer predict a worse outcome in patients with nasopharyngeal carcinoma. BMC Cancer 2014;14:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen Y, Yu H, Wu C, et al. Prognostic value of plasma D-dimer levels in patients with small-cell lung cancer. Biomed Pharmacother 2016;81:210–7. [DOI] [PubMed] [Google Scholar]
- [16].Liu YL, Lu Q, Liang JW, et al. High plasma fibrinogen is correlated with poor response to trastuzumab treatment in HER2 positive breast cancer. Medicine (Baltimore) 2015;94:e481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nakamura K, Nakayama K, Ishikawa M, et al. High pre-treatment plasma D-dimer level as a potential prognostic biomarker for cervical carcinoma. Anticancer Res 2016;36:2933–8. [PubMed] [Google Scholar]
- [18].Sun W, Ren H, Gao CT, et al. Clinical and prognostic significance of coagulation assays in pancreatic cancer patients with absence of venous thromboembolism. Am J Clin Oncol 2015;38:550–6. [DOI] [PubMed] [Google Scholar]
- [19].Lee S, Huh SJ, Oh SY, et al. Clinical significance of coagulation factors in operable colorectal cancer. Oncol Lett 2017;13:4669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stender MT, Larsen TB, Sorensen HT, et al. Preoperative plasma D-dimer predicts 1-year survival in colorectal cancer patients with absence of venous thromboembolism (VTE): a prospective clinical cohort study. J Thromb Haemost 2012;10:2027–31. [DOI] [PubMed] [Google Scholar]
- [21].Zhang F, Chen Z, Wang P, et al. Combination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. Tumour Biol 2016;37:9323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu L, Zhang X, Yan B, et al. Elevated plasma D-dimer levels correlate with long term survival of gastric cancer patients. PLoS One 2014;9:e90547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu P, Zhu Y, Liu L. Elevated pretreatment plasma D-dimer levels and platelet counts predict poor prognosis in pancreatic adenocarcinoma. Onco Targets Ther 2015;8:1335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pedrazzani C, Cerullo G, Marrelli D, et al. Is circulating D-dimer level a better prognostic indicator than CEA in resectable colorectal cancer? Our experience on 199 cases. Int J Biol Markers 2010;25:171–6. [DOI] [PubMed] [Google Scholar]
- [25].Sunesen KG, Stender MT, Thorlacius-Ussing O. Preoperative carcinoembryonic antigen levels predict short-term survival and D-dimer levels long-term survival in patients with curatively intended resection for colorectal cancer. Colorectal Dis 2011;13:26. [DOI] [PubMed] [Google Scholar]
- [26].Hong T, Shen D, Chen X, et al. Preoperative plasma fibrinogen, but not D-dimer might represent a prognostic factor in non-metastatic colorectal cancer: a prospective cohort study. Cancer Biomark 2017;19:103–11. [DOI] [PubMed] [Google Scholar]
- [27].Diao D, Zhu K, Wang Z, et al. Prognostic value of the D-dimer test in oesophageal cancer during the perioperative period. J Surg Oncol 2013;108:34–41. [DOI] [PubMed] [Google Scholar]
- [28].Tellioglu G, Agcaoglu O, Siperstein A, et al. Serum D-dimer as a prognostic marker in patients undergoing radiofrequency ablation of colorectal liver metastasis. J Invest Surg 2012;25:295–300. [DOI] [PubMed] [Google Scholar]
- [29].Watanabe A, Araki K, Hirai K, et al. A novel clinical factor, D-dimer platelet multiplication, may predict postoperative recurrence and prognosis for patients with cholangiocarcinoma. Ann Surg Oncol 2016;23Suppl 5:886–91. [DOI] [PubMed] [Google Scholar]
- [30].Blackwell K, Hurwitz H, Lieberman G, et al. Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer 2004;101:77–82. [DOI] [PubMed] [Google Scholar]
- [31].Tekein K, Bayrak S, Esatolu V, et al. D-dimer and carcinoembryonic antigen levels: useful indicators for predicting the tumor stage and postoperative survival. Gastroenterol Res Pract 2016;2016:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Diao D, Cheng Y, Song Y, et al. D-dimer is an essential accompaniment of circulating tumor cells in gastric cancer. BMC Cancer 2017;17:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Watanabe A, Araki K, Harimoto N, et al. D-dimer predicts postoperative recurrence and prognosis in patients with liver metastasis of colorectal cancer. Int J Clin Oncol 2018;23:689–97. [DOI] [PubMed] [Google Scholar]
- [34].Stender MT, Larsen AC, Sall M, et al. D-Dimer predicts prognosis and non-resectability in patients with pancreatic cancer: a prospective cohort study. Blood Coagul Fibrinolysis 2016;27:597–601. [DOI] [PubMed] [Google Scholar]
- [35].Cao J, Fu Z, Gao L, et al. Evaluation of serum D-dimer, fibrinogen, and CA19-9 for postoperative monitoring and survival prediction in resectable pancreatic carcinoma. World J Surg Oncol 2017;15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liu Z, Guo H, Gao F, et al. Fibrinogen and D-dimer levels elevate in advanced hepatocellular carcinoma: high pretreatment fibrinogen levels predict poor outcomes. Hepatol Res 2017;47:1108–17. [DOI] [PubMed] [Google Scholar]
- [37].Zhu L, Liu B, Zhao Y, et al. High levels of D-dimer correlated with disease status and poor prognosis of inoperable metastatic colorectal cancer patients treated with bevacizumab. J Cancer Res Ther 2014;10 Suppl:246–51. [DOI] [PubMed] [Google Scholar]
- [38].Oya M, Akiyama Y, Okuyama T, et al. High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jpn J Clin Oncol 2001;31:388–94. [DOI] [PubMed] [Google Scholar]
- [39].Li J, Zheng Z, Fang M. Impact of pretreatment plasma D-dimer levels and its perioperative change on prognosis in operable esophageal squamous cell carcinoma. Oncotarget 2017;8:79537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yamamoto M, Yoshinaga K, Matsuyama A, et al. Plasma D-dimer level as a mortality predictor in patients with advanced or recurrent colorectal cancer. Oncology 2012;83:10–5. [DOI] [PubMed] [Google Scholar]
- [41].Feng JF, Yang X, Chen S, et al. Prognostic value of plasma D-dimer in patients with resectable esophageal squamous cell carcinoma in China. J Cancer 2016;7:1663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kilic M, Yoldas O, Keskek M, et al. Prognostic value of plasma D-dimer levels in patients with colorectal cancer. Colorectal Dis 2008;10:238–41. [DOI] [PubMed] [Google Scholar]
- [43].Motavaf E, Sunesen KG, Stender MT, et al. Prognostic value of preoperative D-dimer and carcinoembryonic antigen levels in patients undergoing intended curative resection for colorectal cancer: a prospective cohort study. Int J Colorectal Dis 2014;29:1427–32. [DOI] [PubMed] [Google Scholar]
- [44].Sunesen KG, Stender MT, Thorlacius-Ussing O. Preoperative D-dimer levels predict long-term disease-free survival in patients with curatively intended resection for stage III colorectal cancer: a prospective cohort study. Thromb Res 2012;129:S167. [Google Scholar]
- [45].Liu DQ, Li FF, Jia WH. Cumulative scores based on plasma D-dimer and serum albumin levels predict survival in esophageal squamous cell carcinoma patients treated with transthoracic esophagectomy. Chin J Cancer 2016;35:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Go SI, Lee MJ, Lee WS, et al. D-dimer can serve as a prognostic and predictive biomarker for metastatic gastric cancer treated by chemotherapy. Medicine (Baltimore) 2015;94:e951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Diao D, Wang Z, Cheng Y, et al. D-dimer: not just an indicator of venous thrombosis but a predictor of asymptomatic hematogenous metastasis in gastric cancer patients. PLoS One 2014;9:e101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [49].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [50].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [52].Timp JF, Braekkan SK, Versteeg HH, et al. Epidemiology of cancer-associated venous thrombosis. Blood 2013;122:1712–23. [DOI] [PubMed] [Google Scholar]
- [53].Ay C, Dunkler D, Pirker R, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica 2012;97:1158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Shoji M, Hancock WW, Abe K, et al. Activation of coagulation and angiogenesis in cancer: immunohistochemical localization in situ of clotting proteins and vascular endothelial growth factor in human cancer. Am J Pathol 1998;152:399–411. [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang X, Liu ZQ, Zhang W, et al. A retrospective analysis of plasma D-dimer dynamic variation in terminal stage cancer patients: implications for disease progression. Int J Clin Exp Med 2014;7:2395–401. [PMC free article] [PubMed] [Google Scholar]
- [56].Chaari M, Ayadi I, Rousseau A, et al. Impact of breast cancer stage, time from diagnosis and chemotherapy on plasma and cellular biomarkers of hypercoagulability. BMC Cancer 2014;14:991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fan S, Guan Y, Zhao G, et al. Association between plasma fibrinogen and survival in patients with small-cell lung carcinoma. Thorac Cancer 2018;9:146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


