Abstract
To evaluate the value of intraperitoneal hyperthermic perfusion (IPHP) in the treatment of gastric cancer.
Gastric cancer (GC) is a malignancy with poor prognosis, recent years have demonstrated advances in the use of IPHP for the treatment of advanced gastric cancer (AGC), but the outcome is controversial.
Between January 2015 and January 2017, 134 patients with GC were treated with IPHP in our surgery department, 130 of them were advanced GC patients, and other 1439 cases were treated without IPHP for comparison. In this retrospective cohort study, demographic, perioperative data, and follow-up data were analyzed by univariant analysis, Kaplan–Meier and Cox regression survival analysis.
We found the 1-year survival in IPHP group was significantly longer than it in non-IPHP group (85.5% vs 73.8%, P = .027). and IPHP decreased mortality 1.8 times in 2-year course (OR = 0.556, P = .004). The incidence rate of total complications in IPHP group was similar to that in the Non-IPHP group (6.67% vs 7.46%, respectively; P = .718). We classified all patients into four groups, operation alone, operation + chemotherapy, operation + IPHP, and operation + IPHP + chemotherapy. The 1-year survival in the groups was 70.2%, 77.5%, 83.1%, and 93.5%, respectively (P = .001), compared with the group of operation alone, the 2-year mortality risk was decreased 1.76 times (OR = 0.569, P = .030) and 2.59 times (OR = 0.385, P = .022) in operation + IPHP group and operation + IPHP + chemotherapy group.
Our results suggest that IPHP could contribute to improve survival of patients with gastric cancer. And the modality of operation + IPHP + chemotherapy is the optimal treatment modality for gastric cancer.
Keywords: cytoreductive surgery, gastric cancer, hyperthermic intraperitoneal chemotherapy, peritoneal metastases
1. Introduction
Gastric cancer (GC) ranks third morbidity and second morality in all kinds of cancer worldwide.[1] It has reported the median survival of GC is 50, 14 and 3 months for patients who had chemotherapy plus surgery, had chemotherapy alone and best supportive care, respectively.[2] Up to 17% patients with gastric cancer are diagnosed with the presence of peritoneal metastases (PM), these patients have an average survival of 1–3 months.[3] Systemic chemotherapy could improve the survival of patients with gastric metastatic cancer about 7–10 months, however, this benefit could not be reproduced in patients with PM.[4]
Recent years have demonstrated advances in the use of cytoreductive surgery (CRS) in combination with intraperitoneal hyperthermic perfusion (IPHP) or hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment of PM from appendiceal tumors, colorectal cancer, gastric cancer, epithelial ovarian cancer, and rare primary peritoneum based neoplasms.[5–10] Hyperthermia as well as intraperitoneal chemotherapy is effective in killing cancer cells, IPHP/HIPEC achieve a better heat delivery and preservation by a better circulation of the perfusion fluid, by which all peritoneal surfaces are exposed equally throughout the duration of the therapy as well as avoid dangerous temperatures or over-exposure to normal tissues.[11] IPHP/HIPEC has been shown to be an effective tool whenever a complete or an almost complete resection of the peritoneal implants can be performed.[12–14] Together with new therapeutic options such as targeted therapies, IPHP/HIPEC improve the prognosis of these patients, not only by treating clinically manifest carcinomatosis, but also in a prophylactic setting, by eliminating occult peritoneal seeding.[15] In China, an expert consensus on CRS-HIPEC has been reached by the leading surgical and medical oncologists under the framework of the China Anti-Cancer Association.[16]
However, the role of IPHP/HIPEC in the treatment of gastric cancer is controversial, some documents has reported, the outcome with this approach is not encouraging and is influenced by patient selection.[17] In addition, high procedure-related morbidity and mortality associated with CRS-HIPEC have also been reported, and there is a need clearly to outline the appropriate role of CRS-HIPEC in gastric cancer.[18]
In this study, we analyzed IPHP performed in earlier stage after gastric resection, the safety and outcome of this method were evaluated, and we aimed to boost up the efficiency of this approach.
2. Methods
2.1. Patient enrollment
Between January 2015 and January 2017, 4433 consecutive patients with gastric cancer underwent gastrectomy by the First Department of Digestive Surgery of Xijing Hospital, Fourth Military Medical University (Xi’an, China). Part of them were also underwent IPHP treatment. The selective standard for IPHP were as follows: firstly, patient with gastric cancer and aged less than 75 years; secondly, patients had no severe basic disease such as hypertension, heart disease, and chronic obstructive pulmonary disease (COPD), and their liver and renal function were normal, and hemoglobin (Hb) was not less than 80 mg/L; thirdly, patients without Hyperthermia after operation, and body temperature less than 38°C; fourthly, patients without severe abdominal pain and distention. For this retrospective cohort study, all patient data were evaluated by two researchers, and to enhance the comparability, the patient inclusion criteria were set as follows: firstly, adult patient aged from 18 to 75 years; secondly, patients were diagnosed as gastric cancer based on pathologic characteristics and underwent gastrectomy or explorative surgery; thirdly, patients had no severe basic disease such as hypertension, heart disease, liver, and renal function were normal by laboratory test, Hb was not less than 80 mg/L, and at the level of I or II according to American Society Anesthesiology Physical Status Classification System; fourthly, patients had complete data of follow-up.
This study was approved by the Ethics Committee of the Fourth Military Medical University (ethics code: XJYYLL-2015276). All patients received verbal and written information regarding the study and provided informed consent prior to surgery.
2.2. IPHP method
The perfusion tubes were implanted at the end of operation, and IPHPs were performed from the second day after operation, the procedure consists of 3–5 L of saline circulated using an extracorporeal circulation device at an inflow temperature of 43°C and a flow rate of 200–400 mL/minute for 30–120 minute, and perform 2–3 times with a 1–2 days interval.
2.3. Demographic and preoperative data
Demographic data, including sex, age, preoperative data, including TNM clinical and pathological staging classification, routine hematological and biochemical tests, and X-rays were collected to enable subsequent analysis of the comparability of the groups. The patients were divided into two groups: with IPHP, and without IPHP.
2.4. Perioperative observations
The highest postoperative temperature was recorded. The histological subtype and pathological stage were determined using the Union for International Cancer Control and TNM classification for gastric cancer. Postoperative complications, including anastomotic complication, wound infection, wound rupture, lung infection, bleeding, reoperation, duodenal leak, and intestinal obstruction were observed and evaluated. Anastomotic complication assessment was performed using a water-soluble radiological contrast enema at 6–8 days postoperatively. A clinical leak was defined as the appearance of food material in the abdominal drains, or the development of systemic sepsis associated with local peritoneal signs during the postoperative period. Any extravasation of the contrast medium detected on radiography was considered as a radio-logical leak.
2.5. Follow-up data
All patients were followed for two year from the beginning of operation. And at the end of follow-up, the status of patients were recorded, which included survival, death, and lost follow-up.
2.6. Statistical analysis
Statistical analysis was performed using SPSS 17 software (SPSS Inc., Chicago, IL). Differences among groups consisted of measurement data were analyzed by students’ t test, and when unequal variance existed, the adjust-T test was used; differences in expression rate among groups were analyzed by Pearson's Chi-squared (χ2) test. The Fisher's exact test was used to assess the difference of positive rate when the number of total cases was less than 40. P value <.05 was considered statistically significant. Survival analysis was used by Kaplan–Meier and Cox regression. For Cox model, factors previously demonstrated to be prognostically significant or thought to be clinically important, and covariates identified in bivariate analyses as predictors of mortality were considered.
3. Results
3.1. IPHP and baseline characteristics
According to patient inclusion criteria, 2848 cases were excluded from this study. Of them, 205 cases for age <18 years or >75 years; 935 cases for severe basic disease or severe abdominal pain and distention after operations; 204 cases for Hb <80 g/L; and 1494 cases for incomplete follow-up data. And a total of 1573 cases met the inclusion criteria and were analyzed in this cohort study (Fig. 1), of whom 134 cases were treated with IPHP, and other 1439 cases were treated without IPHP for comparison. The comparison of baseline data between the IPHP and Non-IPHP groups was described in Table 1.
Figure 1.
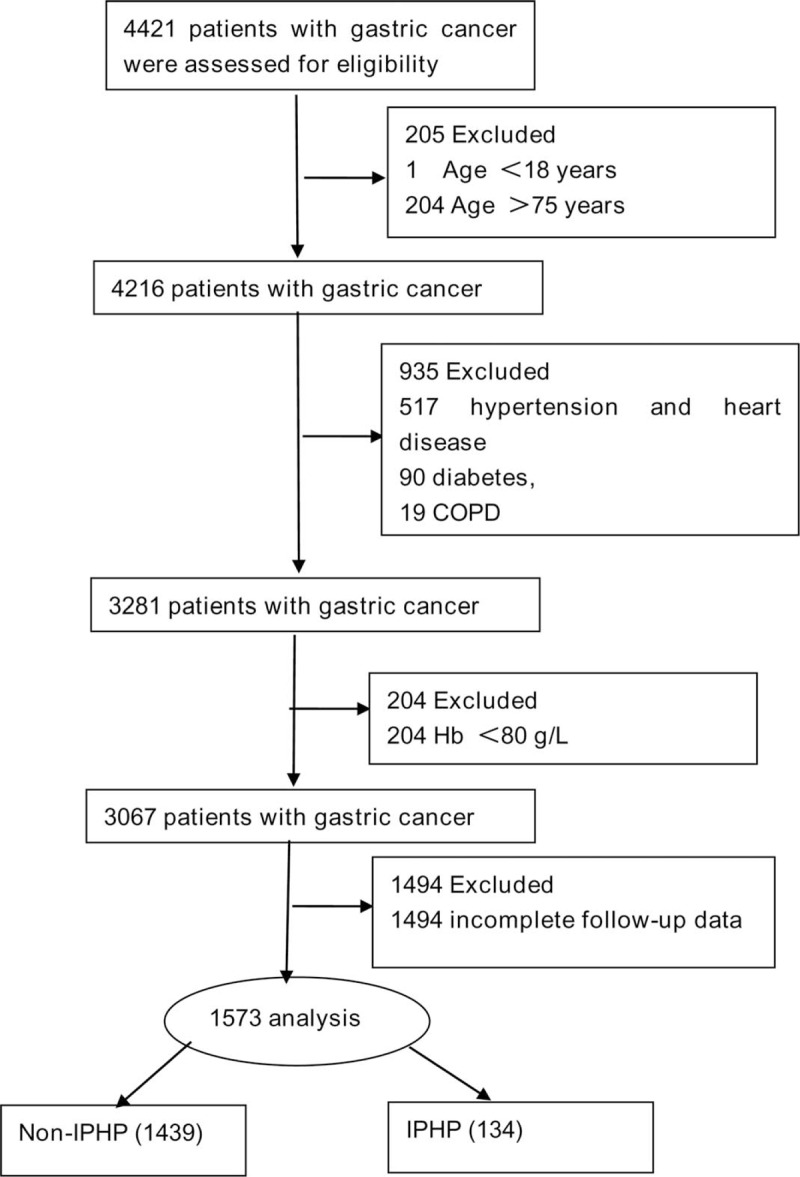
Flow diagraph of patients enrollment. COPD = chronic obstructive pulmonary disease, Hb = hemoglobin, IPHP = intraperitoneal hyperthermic perfusion.
Table 1.
Characteristics of patients with intraperitoneal hyperthermic perfusion and without intraperitoneal hyperthermic perfusion.
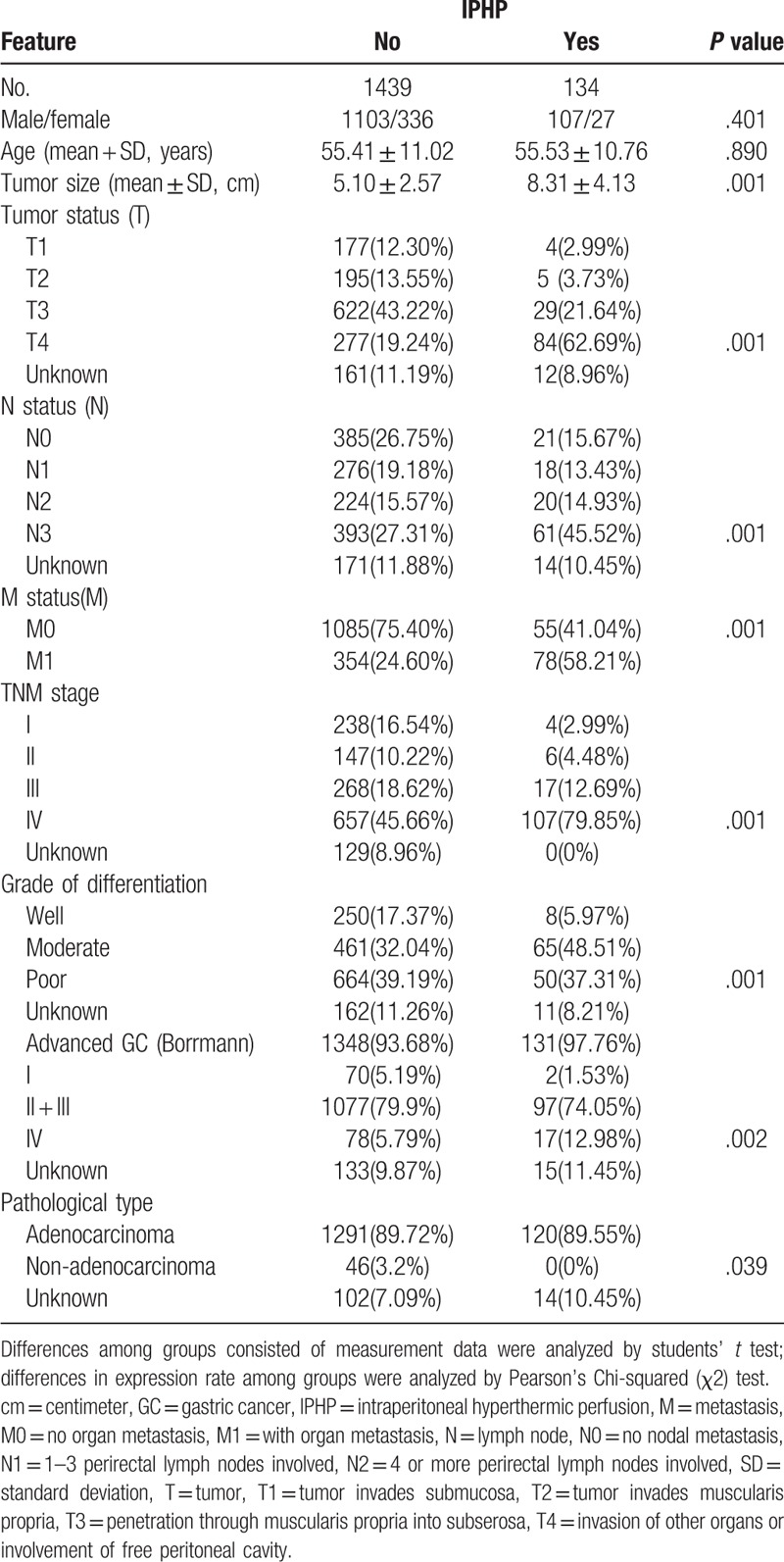
There were no significant differences between the two groups regarding preoperative variables, such as age, sex, but the variants of tumor differentiation, pathological stage, histological subtype, and TNM stage in IPHP group were worse than those in non-IPHP group.
3.2. IPHP could not increase postoperative complications
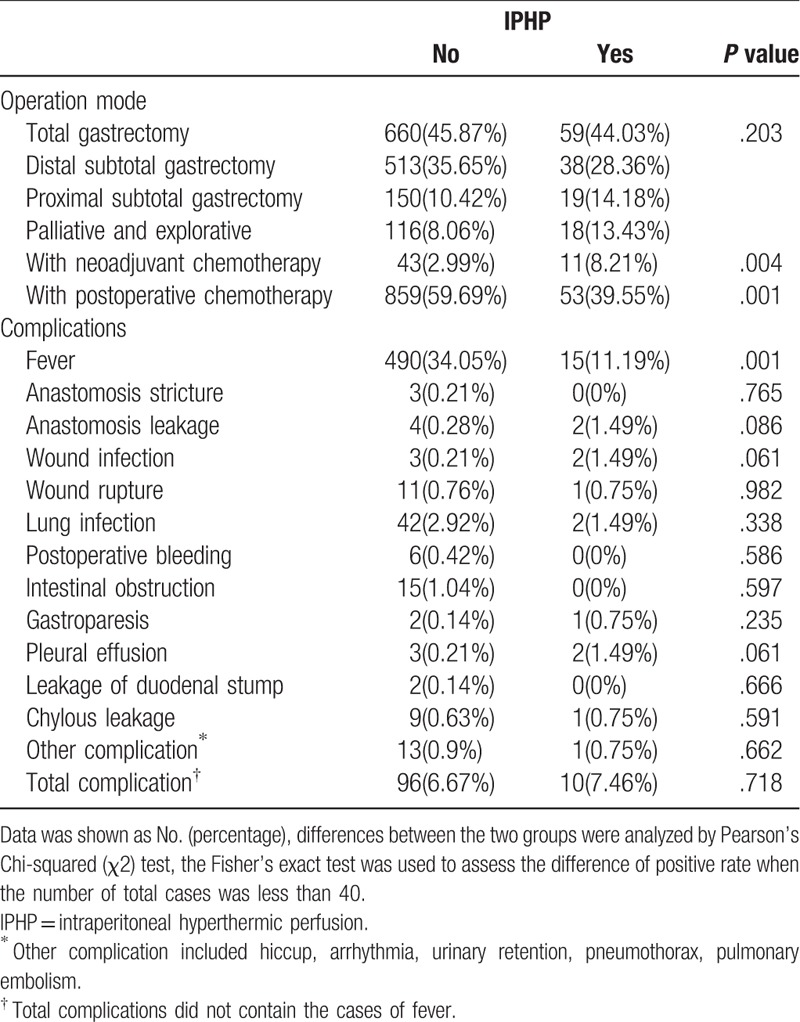
The postoperative complications included anastomosis stricture 0.19%, anastomosis leakage 0.38%, wound infection 0.32%, wound rupture0.76%, lung infection 2.80%, postoperative bleeding 0.38%, intestinal obstruction 0.95%, gastroparesis 0.19%, pleural effusion 0.32%, leakage of duodenal stump 0.13%, chylous leakage 0.64%, other complication 0.89%, and total complication was 6.74% in all the patients.
Fewer patients developed a fever in the IPHP group compared with the non-IPHP group (34.05 vs 11.19%, respectively; P = .003). The incidence rate of total complications in IPHP group was not statistically different from that in the non-IPHP group (6.67% vs 7.46%, respectively; P = .718). In addition, there were no differences regarding lung infection, wound infection, wound rupture, anastomotic leakage, bleeding, duodenal leak, chylous leakage, and intestinal obstruction according to the univariate analysis (Table 2).
Table 2.
Comparison of complication between group with intraperitoneal hyperthermic perfusion and without intraperitoneal hyperthermic perfusion.
3.3. IPHP contribute to improve 1-, 2-year survive
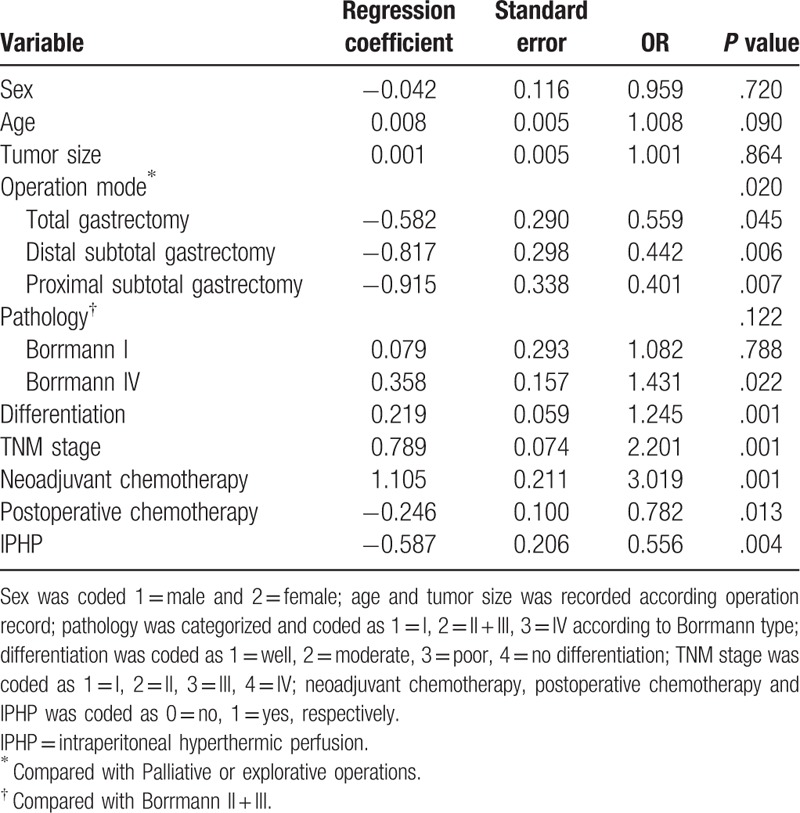
We found the 1-year survival in IPHP group was significantly longer than it in non-IPHP group (85.5% vs 73.8%, P = .027) and 2-year survival in IPHP group was also longer than it in non-IPHP group, but the difference was not significant statically (60.6% vs 73.1%, P = .851) (Fig. 2A–C). to further investigate the role of IPHP in gastric cancer, multivariable including sex, age, tumor size, operation mode, differentiation, TNM stage, neoadjuvant chemotherapy, postoperative chemotherapy, was considered by Cox regression analysis, and operation mode and Borrmann classification were assigned as dummy variables, We found IPHP decreased mortality1.8 times in 2-year course (OR = 0.556, P = .004), and the factors of, operation mode, IV type of Borrmann classification, differentiation, TNM stage, neoadjuvant chemotherapy and postoperative chemotherapy play a role in the survival of patients with gastric cancer(Table 3).
Figure 2.
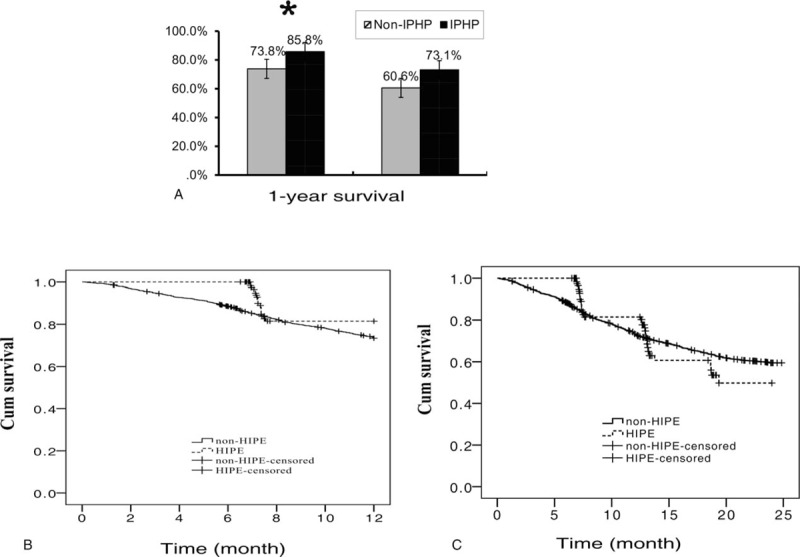
The survival difference between groups with intraperitoneal hyperthermic perfusion and without intraperitoneal hyperthermic perfusion in patients with gastric cancer. (A) The outcome of IPHP in the 1-, 2-survival of patients with gastric cancer. ∗ denoted there was a statistically difference between the two groups, P value <.05. (B) 1-year survival curve of patients with or without IPHP. (C) 2-year survival curve of patients with or without IPHP. IPHP = intraperitoneal hyperthermic perfusion. Solid line = non-IPHP, dotted line = IPHP.
Table 3.
Relationship between cancer specific mortality and perioperative variables-Cox multiple-regression analysis.
3.4. The optimal treatment combined with IPHP
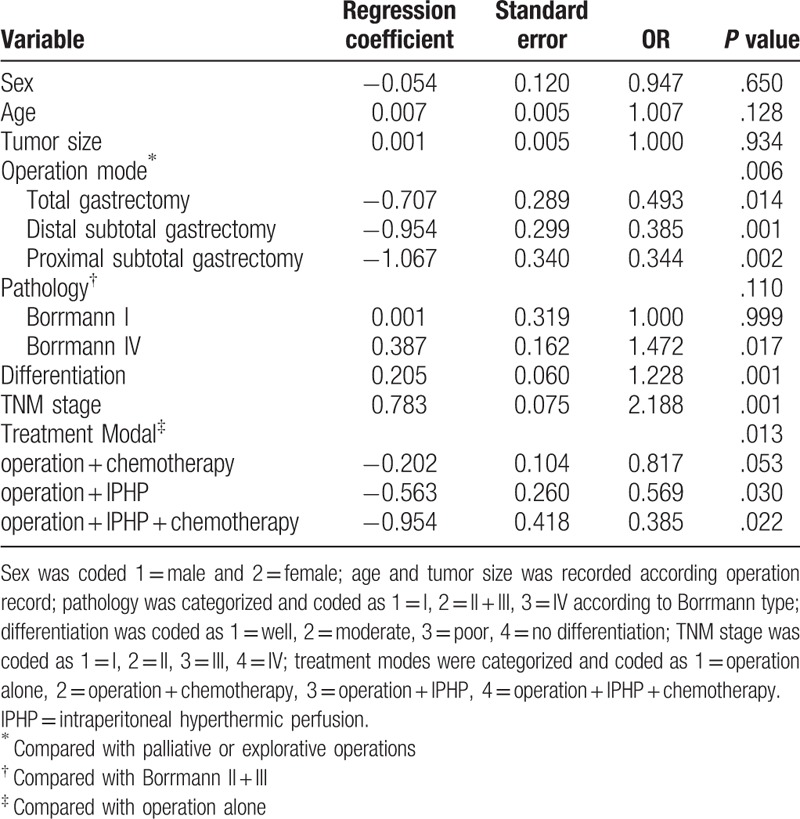
Based on the previous study,[19] treatment modal could influence the therapeutic outcome, we classified all patients into four groups, operation alone, operation + chemotherapy, operation + IPHP, and operation + IPHP + chemotherapy. The 1-year survival in the groups was 70.2%, 77.5%, 83.1%, and 93.5%, respectively (P = .001), and 2-year survival in the groups was 60.6%, 62.2%, 70.1%, and 80.4% (P = .332) (Fig. 3A–C). Compared with the reference group (operation alone), the 2-year mortality risk was decreased 1.76 times (OR = 0.569, P = .030) and 2.59 times (OR = 0.385, P = .022) in operation + IPHP group and operation + IPHP + chemotherapy group, respectively. The result demonstrated that the modal of operation + IPHP + chemotherapy was the optimal treatment modal for gastric cancer (Table 4).
Figure 3.
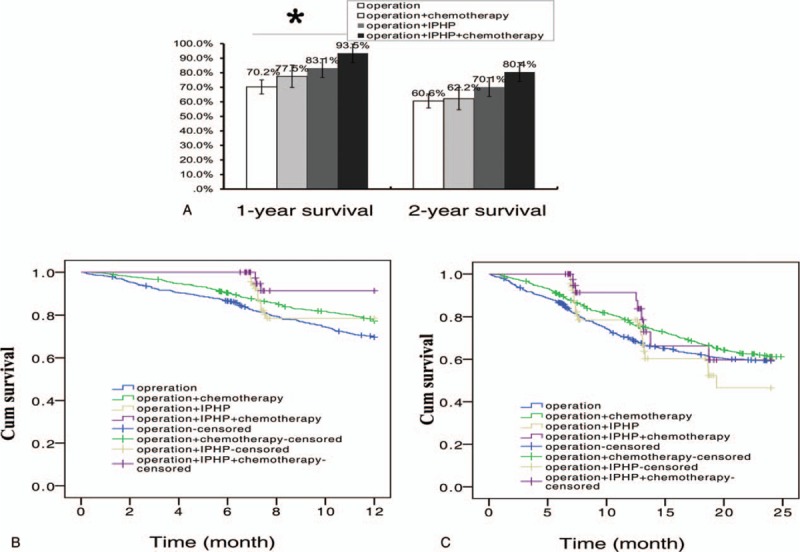
The survival difference among four therapeutic modalities in patients with gastric cancer. (A) The modalities with or without IPHP in the 1-, 2-survival of patients with gastric cancer. ∗ denoted there was a statistically difference among the groups, P value <.05. (B) 1 year survival curve of patients treated with four modalities. (C) 2-year survival curve of patients treated with four modalities. IPHP = intraperitoneal hyperthermic perfusion. Blue line = operation alone, green line = operation + chemotherapy, yellow line = operation + IPHP, purple line = operation + IPHP + chemotherapy.
Table 4.
Treatment mode could influence the mortality of gastric cancer-Cox multiple-regression analysis.
4. Discussion
The aim of the present study was to investigate the value of IPHP for gastric cancer. Compared with the non-IPHP method, IPHP contributed to improve 1-, 2-year survival, moreover, it did not increase the rate of complications.
The first human was subjected to IPHP/HIPEC for locally abdominal malignancy in 1979,[20] till now, IPHP/HIPEC has been used in three situations in gastric cancer. Firstly, it has been used in patients with established PM,[21] secondly, it has been used as a prophylaxis against peritoneal recurrence after curative surgery,[22] thirdly, IPHP/HIPEC contribute to reduce malignant ascites,[23,24] palliate symptom, and improve quality of life in AGC patients .[25] But there is not a standard procedure for IPHP/HIPEC. We replaced IPHP tubes at the end of operation, and performed the perfusion earlier from the second day after operation, our method contributed to reduce residual tumor cells, which had a chance to implantation and reduced the need for frequent paracentesis .[26,27]
In this study, although the baseline data including tumor size, TNM stage and grade of differentiation, in IPHP group were significantly worse than non-IPHP group, we found the 1-year survival in IPHP group was significantly higher than it in non-IPHP group (85.5% vs 73.8%),and we also found IPHP decreased 2-year mortality risk about 1.8 times (OR = 0.556). Our results provided powerful evidence for the therapeutic value of IPHP in gastric cancer. It has reported that CRS and HIPEC could improve the OS to 11 months compared to best supportive care in selected patients.[3] Yuan reported the rate of tumor disappear and decrease in the HIPEC + group was 82.60%, which was statistically significantly superior to that of the IPHPC-group (54.80%).[28] And the median OS after CRS + HIPEC was 13.3, the median 1-, 2-, and 5-year survival rates after CRS + HIPEC were 50.0%, 35.8%, and 13.0%, respectively.[29] Our results was consistent with those reports, and combined with the above study boomed up the application of IPHP/HIPEC. However, the value of HIPEC in gastric cancer is controversial, It has reported CRS plus HIPEC yield fewer benefits in patients with PM from gastric cancer, and the median OS in GC is shorter than in other malignancies such as colorectal cancer, ovarian cancer, and appendicular cancer.[30,31] And clinical usefulness of systemic chemotherapy and HIPEC is judged to be moderate to high for PM of ovarian and colorectal origin but moderate to poor for gastric origin.[32] Contrast to these studies, the above studies mainly targeted the advanced gastric cancer with peritoneal metastasis, while, we aimed each stage of gastric cancer, and our IPHP procedure was not combined with chemotherapeutic agent, which may increase morbidity of adverse event.
Fewer patients developed a fever in the IPHP group compared with the non-IPHP group. And the incidence rate of total complications in IPHP group was not statistically significantly different from that in the non-IPHP group. In addition, there were no differences regarding lung infection, wound infection, wound rupture, anastomotic leakage, bleeding, duodenal leak, chylous leakage, and intestinal obstruction between the two groups according to the univariate analysis, therefore, our results proved that IPHP was safety for gastric cancer treatment. The morbidity of complication in our study was lower than others. Which reported that the morbidity approximate 11%–37.5%, and the mortality is 0%–4.8%.[33–35] Some documents reported there is higher incidence of procedure-related morbidity in the HIPEC group, whereby higher incidence of myelotoxicity and renal insufficiency. CRS-HIPEC causes surgery-related morbidity including abscess, fistula, and anastomotic leak, and chemotherapy-related morbidities such as leucopenia, anemia, thrombopenia, and heart, liver or renal toxicity. In addition, it has reported 5.7% develop delayed major complications correlated with IPHPC, which included pancreatic pseudocyst/pancreatitis, abdominal wall dehiscence, gastric perforation, and ureteral stricture with associated hydronephrosis.[36] The higher morbidity and mortality limit the application of HIPEC. Our lower morbidity of complications were attribute to IPHP procedure, which was not combined with chemotherapeutic agent, moreover, the selection of patients for IPHP was performed strictly, the candidate aged less than 75 years, and no severe basic disease. Repeated evidence demonstrates that incidence of morbidity or mortality is significantly influenced by the institutional experience and selecting candidate patients.[37,38] Overall, application of pathogenetic ways of protection from thermal injury, timely control and correction of homeostasis caused by the toxic effects of chemotherapy and burn peritoneum, may reduce the risk of complications.
IPHP/HIPEC is frequently combined with CRS, and IPHP/HIPEC can performed pre-, intra,- and post- CRS or radical resection of gastric cancers.[26,39] We investigated four treatment modality in our study, and found the 2-year mortality risk was decreased 1.76 times and 2.59 times in operation + IPHP group and operation + IPHP + chemotherapy group respectively. Moreover, we found the modality of operation + IPHP + chemotherapy was the optimal treatment modality for gastric cancer to increase 2-year survival. Our results were consistent with the study which demonstrate the modality of gastrectomy + chemotherapy + HIPEC showed an optimum survival.[19] Using HIPEC and systematic chemotherapy followed by a staged CRS (HIPEC + Chemo + CRS), Wu has reported the tolerance and compliance of the new modality is better than simultaneous CRS and HIPEC, and the mortality and the complications of both modality were similar.[40] But some authors hold a opposite view and reported that the morbidity and mortality rates of CRS and HIPEC, in combination with gastrectomy, were significant and the survival rates of this approach may not extend beyond that of treatment with systemic chemotherapy.[41]
Helicobacter pylori infection is the major cause of gastric cancer. Inducing tolerogenic dendritic cells and inhibiting effector T cell responses, H. pylori can successfully evade the strong innate and adaptive immune responses and colonize in the gastric epithelium. The mechanisms related to H. pylori persistence involve bacterial virulence factors such as cytotoxin-associated gene A, vacuolating cytotoxin A, or gamma-glutamyltranspeptidase.[42]H. pylori could against gastric acidity, shape its helical morphology and flagella, and change genetic diversity to facilitate persistent infection.[43] In this study, whether IPHP play a role in anti-H. pylori treatment and affected the immune evasion of H. pylori was unclear. And besides thermal injury, how did IPHP change inhospitable microniche and play a role in molecular pathogenesis and signal transduction of H. pylori need further investigate.
Our study have limitations, because of poor prognosis of AGC and a relatively small sample size in IPHP group, we could only analyzed 2-year survival, and we could not evaluate the preventive role of IPHP in PM. And for the safety, the patients with serious basic disease such as heart disease, hypertension and COPD, or with hyperpyrexia, severe abdominal pain, and distension after operation were unsuitable for IPHP, and were excluded from this study. The quality of life in patients with IPHP and the tolerance of IPHP need further investigate. Therefore, we couldn’t extrapolate the value of IPHP to all GC patients. And a prospective randomized controlled study about IPHP should be performed eventually and hopefully to confirm the observation in the future.
In conclusion, our results suggest that IPHP is safety and efficacy in GC treatment, and could become one of important therapeutic strategy for GC. And establishment of standard of IPHP is very necessary in current clinical practice. Moreover, the appropriate selection of patients and modality need further investigate.
Acknowledgments
The authors would like to thank Dr. XuGH, Dr. GuoM, and Dr. LianX for their help in the design, data collection, and analysis.
Author contributions
Conceptualization: Hong-Wei Zhang, Guo-Cai Li.
Data curation: Jian-Jun Yang, Li Sun.
Formal analysis: Jian-Jun Yang, Li Sun, Guo-Cai Li.
Investigation: Ji-Yang Zheng, Xue-Wen Yang, Guo-Cai Li.
Methodology: Ji-Yang Zheng.
Project administration: Guo-Cai Li.
Supervision: Hong-Wei Zhang.
Validation: Li Sun, Xue-Wen Yang.
Writing – original draft: Guo-Cai Li.
Footnotes
Abbreviations: AGC = advanced gastric cancer, CRS = cytoreductive surgery, HIPEC = hyperthermic intraperitoneal chemotherapy, IPHP = intraperitoneal hyperthermic perfusion, OS = overall survival, PM = peritoneal metastases.
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- [1].Global Burden of Disease Cancer C, Fitzmaurice C, Allen C, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Morgagni P, Solaini L, Framarini M, et al. Conversion surgery for gastric cancer: a cohort study from a western center. Int J Surg 2018;53:360–5. [DOI] [PubMed] [Google Scholar]
- [3].Brandl A, Pachmayr E, Gül-Klein S, et al. Surgical treatment of peritoneal metastases of gastric cancer. Chirurg 2018;89:669–77. [DOI] [PubMed] [Google Scholar]
- [4].López-Basave HN, Quiroz-Sandoval OA, Padilla-Rosciano AE, et al. Role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Cir Cir 2018;86:277–84. [DOI] [PubMed] [Google Scholar]
- [5].Maeda H, Kobayashi M, Sakamoto J. Evaluation and treatment of malignant ascites secondary to gastric cancer. World J Gastroenterol 2015;21:10936–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].de Mestier L, Lardiere-Deguelte S, Volet J, et al. Recent insights in the therapeutic management of patients with gastric cancer. Dig Liver Dis 2016;48:984–94. [DOI] [PubMed] [Google Scholar]
- [7].Dehal A, Smith JJ, Nash GM. Cytoreductive surgery and intraperitoneal chemotherapy: an evidence-based review-past, present and future. J Gastrointest Oncol 2016;7:143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Feingold PL, Kwong ML, Sabesan A, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for gastric cancer and other less common disease histologies: is it time? J Gastrointest Oncol 2016;7:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Marrelli D, Polom K, de Manzoni G, et al. Multimodal treatment of gastric cancer in the west: where are we going? World J Gastroenterol 2015;21:7954–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mihmanli M, Ilhan E, Idiz UO, et al. Recent developments and innovations in gastric cancer. World J Gastroenterol 2016;22:4307–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Spratt JS, Adcock RA, Sherrill W, et al. Hyperthermic peritoneal perfusion system in canines. Cancer Res 1980;40:253–5. [PubMed] [Google Scholar]
- [12].Di Vita M, Cappellani A, Piccolo G, et al. The role of HIPEC in the treatment of peritoneal carcinomatosis from gastric cancer: between lights and shadows. Anticancer Drugs 2015;26:123–38. [DOI] [PubMed] [Google Scholar]
- [13].Spiliotis J, Halkia E, de Bree E. Treatment of peritoneal surface malignancies with hyperthermic intraperitoneal chemotherapy-current perspectives. Curr Oncol 2016;23:e266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: progress toward a new standard of care. Cancer Treat Rev 2016;48:42–9. [DOI] [PubMed] [Google Scholar]
- [15].Polom K, Marano L, Roviello G, et al. Evolution and emerging future of cytoreducxtive surgery and hyperthermic intraperitoneal chemoperfusion in gastric cancer: from treating the incurable to preventing recurrence. Int J Hyperthermia 2016;32:173–9. [DOI] [PubMed] [Google Scholar]
- [16].Li Y, Zhou YF, Liang H, et al. Chinese expert consensus on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal malignancies. World J Gastroenterol 2016;22:6906–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Loggie BW, Thomas P. Gastrointestinal cancers with peritoneal carcinomatosis: surgery and hyperthermic intraperitoneal chemotherapy. Oncology (Williston Park) 2015;29:515–21. [PubMed] [Google Scholar]
- [18].Peixoto RD, de Sousa TT, Silva P, et al. Complete response for more than 4 years following neoadjuvant FOLFOX and cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for a patient with advanced gastric cancer with extensive peritoneal carcinomatosis. Case Rep Oncol 2018;11:305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Geng X, Liu H, Lin T, et al. Survival benefit of gastrectomy for gastric cancer with peritoneal carcinomatosis: a propensity score-matched analysis. Cancer Med 2016;5:2781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Spratt JS, Adcock RA, Muskovin M, et al. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res 1980;40:256–60. [PubMed] [Google Scholar]
- [21].Desiderio J, Chao J, Melstrom L, et al. The 30-year experience-A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur J Cancer 2017;79:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Murata S, Yamamoto H, Naitoh H, et al. Feasibility and safety of hyperthermic intraperitoneal chemotherapy using 5-fluorouracil combined with cisplatin and mitomycin C in patients undergoing gastrectomy for advanced gastric cancer. J Surg Oncol 2017;116:1159–65. [DOI] [PubMed] [Google Scholar]
- [23].Seshadri RA, Glehen O. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in gastric cancer. World J Gastroenterol 2016;22:1114–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ni X, Wu P, Wu J, et al. Hyperthermic intraperitoneal perfusion chemotherapy and response evaluation in patients with gastric cancer and malignant ascites. Oncol Lett 2017;14:1691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Orgiano L, Pani F, Astara G, et al. The role of “closed abdomen” hyperthermic intraperitoneal chemotherapy (HIPEC) in the palliative treatment of neoplastic ascites from peritoneal carcinomatosis: report of a single-center experience. Support Care Cancer 2016;24:4293–9. [DOI] [PubMed] [Google Scholar]
- [26].Sugarbaker PH. Peritoneal metastases from gastrointestinal cancer. Curr Oncol Rep 2018;20:62. [DOI] [PubMed] [Google Scholar]
- [27].Seshadri RA, Glehen O. The role of hyperthermic intraperitoneal chemotherapy in gastric cancer. Indian J Surg Oncol 2016;7:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yuan M, Wang Z, Hu G, et al. A retrospective analysis of hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Mol Clin Oncol 2016;5:395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ji ZH, Peng KW, Yu Y, et al. Current status and future prospects of clinical trials on CRS + HIPEC for gastric cancer peritoneal metastases. Int J Hyperthermia 2017;33:562–70. [DOI] [PubMed] [Google Scholar]
- [30].Hsieh MC, Lu CY, Chang WW, et al. Experiences with cytoreduction surgery plus hyperthermic intraperitoneal chemotherapy in Taiwan. Medicine 2017;96:e7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Montori G, Coccolini F, Fugazzola P, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in ovarian and gastrointestinal peritoneal carcinomatosis: results from a 7-year experience. J Gastrointest Oncol 2018;9:241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Grass F, Martin D, Montemurro M, et al. Current opinion and knowledge on peritoneal carcinomatosis: a survey among a Swiss Oncology Network. Chemotherapy 2018;63:143–7. [DOI] [PubMed] [Google Scholar]
- [33].Badgwell B, Blum M, Das P, et al. Phase II trial of laparoscopic hyperthermic intraperitoneal chemoperfusion for peritoneal carcinomatosis or positive peritoneal cytology in patients with gastric adenocarcinoma. Ann Surg Oncol 2017;24:3338–44. [DOI] [PubMed] [Google Scholar]
- [34].Ni Z, Li C, Yan C, et al. Efficacy and safety of surgery combined with hyperthermic intraperitoneal chemotherapy in the treatment of advanced gastric cancer: a meta-analysis. Zhonghua Wei Chang Wai Ke Za Zhi 2016;19:1406–13. [PubMed] [Google Scholar]
- [35].Wu HT, Li Y. Reply to: Re: cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with lobaplatin and docetaxel to treat synchronous peritoneal carcinomatosis from gastric cancer: results from a Chinese center, Eur J Surg Oncol (2016). Eur J Surg Oncol 2016;42:1762–6. [DOI] [PubMed] [Google Scholar]
- [36].Bhagwandin SB, Naffouje S, Salti G. Delayed presentation of major complications in patients undergoing cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy following hospital discharge. J Surg Oncol 2015;111:324–7. [DOI] [PubMed] [Google Scholar]
- [37].Khoronenko VE, Shemetova MM, Drozhzhina OV, et al. Anaesthesia and intensive care during intraoperative intraperitoneal hyperthermic chemotherapy in patients with gastric cancer (literature review and own clinical experience). Anesteziol Reanimatol 2015;60:50–4. [PubMed] [Google Scholar]
- [38].Wu Z, Li Z, Ji J. Morbidity and mortality of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in advanced gastric cancer. Transl Gastroenterol Hepatol 2016;1:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yonemura Y, Ishibashi H, Hirano M, et al. Effects of neoadjuvant laparoscopic hyperthermic intraperitoneal chemotherapy and neoadjuvant intraperitoneal/systemic chemotherapy on peritoneal metastases from gastric cancer. Ann Surg Oncol 2017;24:478–85. [DOI] [PubMed] [Google Scholar]
- [40].Wu X, Li Z, Li Z, et al. Hyperthermic intraperitoneal chemotherapy plus simultaneous versus staged cytoreductive surgery for gastric cancer with occult peritoneal metastasis. J Surg Oncol 2015;111:840–7. [DOI] [PubMed] [Google Scholar]
- [41].Badgwell B, Blum M, Das P, et al. Lessons learned from a phase II clinical trial of laparoscopic HIPEC for gastric cancer. Surg Endosc 2018;32:512. [DOI] [PubMed] [Google Scholar]
- [42].Mejías-Luque R, Gerhard M. Immune evasion strategies and persistence of Helicobacter pylori. Curr Top Microbiol Immunol 2017;400:53–71. [DOI] [PubMed] [Google Scholar]
- [43].Abadi ATB. Strategies used by Helicobacter pylori to establish persistent infection. World J Gastroenterol 2017;23:2870–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


