Graphical abstract
Keywords: Antimicrobial, Buriti oil, Droplet size, Nanoemulsion, Structured lipids, X-ray
Highlights
-
•
Nanoemulsions of buriti oil (NBO) and nanoemulsions of structured lipids (NBO6h, NBO24h) were prepared by high-pressure homogenization.
-
•
Use of nanoemulsion ofstructured lipid NBO24 h reduced droplet size.
-
•
Nanoemulsions (NBO24 h) had higher oxidative stability.
-
•
Nanoemulsion of structured lipid showed bacteriostatic activity.
Abstract
Buriti oil nanoemulsions were prepared using non-interesterified buriti oil or buriti oil interesterified for 6 or 24 h (NBO, NBO6h, and NBO24 h), respectively. The aim was to investigate the effects of interesterified oils on the physicochemical and biological properties of nanoemulsions. Samples were stored at 4 and 25 °C for 30 days, and their physicochemical properties and biological activities were evaluated. The mean droplet diameter of nanoemulsions ranged from 196 to 270 nm. NBO24 h had the smallest droplet size and was the most stable during the storage period. Furthermore, NBO24 h demonstrating the good oxidative stability, had a high antioxidant capacity, and was less susceptible to droplet aggregation. NBO and NBO24 h had similar biological activity against Gram-negative bacteria (Escherichia coli O157: H7); bacterial growth was inhibited by at least 60% at 3.12 mg mL−1. The nanoemulsions have interesting properties for the production of pharmaceutical, cosmetic, and food formulations with antimicrobial activity.
1. Introduction
The buriti palm (Mauritia flexuosa L.f.) occupies extensive areas in Brazil, especially in the central part of the country and in the southern Amazon lowlands. Buriti oil, extracted from the fruit of the buriti palm, is traditionally used in cooking and for medicinal purposes. The unprocessed oil has considerable levels of minor compounds, such as β-carotene and tocopherols, which help reduce total and low-density lipoprotein cholesterol and lower the risk of coronary artery disease [1,2]. The functional properties of this unconventional vegetable oil have been extensively investigated: buriti oil is rich in oleic acid and other compounds that exert positive health effects, shows antimicrobial activity, and has potential applications in the cosmetic, pharmaceutical, and food industries [3].
Chemical or enzymatic interesterification reactions can be used for the synthesis of structured lipids. Structured lipids are triacylglycerols (TAGs) that have been modified in their fatty acid composition or restructured in their positional distribution of fatty acids in the glycerol backbone [4] to impart desirable physicochemical, nutritional, and functional properties for food applications or other purposes. Structured lipids are also referred to as the “new generation of nutraceutical fats” [5]. Previous work by our research group has shown that enzymatic interesterification can improve physicochemical and biological properties of buriti oil. Enzymatic interesterification is widely used to synthesize oils and fats, as it has potential health and nutrition benefits [6].
Oils and fats find many commercial applications in the form of emulsions. Nanoemulsions are dispersion or delivery systems that are composed of at least two immiscible liquids, one of which is present as nanosized droplets [7,8]. These systems are regarded as valuable alternatives for encapsulating bioactive lipophilic compounds of hydrophobic nature. Oil-in-water (O/W) colloidal dispersions are often used for this purpose. Nanoemulsions show advantages over conventional emulsions because of their smaller droplet size and greater stability against gravitational separation. Previous studies confirmed the advantages of nanoemulsions in the food industry, showing that these systems can be used to improve the bioavailability of complex food matrices and extend product shelf life [9,10]. Nanoemulsions are prepared by either low- or high-energy methods [11]. Examples of the latter include high-pressure homogenization, microfluidization, and ultrasonication [12].
The choice of the emulsifier is of critical importance for producing nanoemulsions because this component facilitates emulsification and promotes physical stability [13]. There are various types of food-grade emulsifiers that are used to produce nanoemulsions based products in the food industry, such as gum arabic and small-molecule surfactants (e.g., Tweens) [14]. Tween 80 is one of the most used synthetic emulsifiers for the preparation of nano and conventional emulsions because of its good emulsifying properties [15,16]. The amphiphilic character of emulsifiers allows the entrapment of hydrophilic or hydrophobic bioactive compounds, improving their dissolution in the emulsion system.
Results obtained by our research group in a previous study provided the fundamental perspective that enzymatic interesterification of Amazonian oils yields structured lipids with interesting characteristics and antimicrobial potential [3]. Thus, the aim of this study was to produce O/W nanoemulsions of non-interesterified and interesterified buriti oil and compare their physicochemical characteristics and biological potential.
2. Materials and methods
2.1. Materials
Buriti oil was purchased from Beraca (São Paulo, Brazil). Commercially purified and immobilized lipase (Lipozyme® TL IM) was purchased from Novozymes (São Paulo, Brazil). Tween 80 (polyoxyethylene sorbitan monooleate) was purchased from Dinâmica (São Paulo, Brazil). Nile red was purchased from Sigma–Aldrich (St. Louis, USA). Escherichia coli O157:H7 ATCC 11,775 was obtained from the Chemical, Biological, and Agricultural Pluridisciplinary Research Center (CPQBA) of the University of Campinas, Campinas, Brazil.
2.2. Methods
2.2.1. Preparation of nanoemulsions
Buriti oil was interesterified for 6 and 24 h, following the method of Speranza et al. [17]. O/W nanoemulsions with 10% oil phase were prepared according to Ozturk et al. [18]. Three different formulations were manufactured by varying the oil phase: nanoemulsions of buriti oil (NBO), nanoemulsions of buriti oil interesterified for 6 h (NBO6h), and nanoemulsions of buriti oil interesterified for 24 h (NBO24h). Coarse emulsions were homogenized in a high-pressure homogenizer (GEA Niro Soavi, Parma, Italy) for 3 cycles at 800 bar. After homogenization, 100 mL of each sample was stored in amber glass bottles and kept in the dark at 4 or 25 °C for 30 days.
2.2.2. Mean droplet size determination
The mean droplet diameter (d32) of nanoemulsions was measured using a static light-scattering system (Mastersizer 2000, Malvern Instruments Ltd., Malvern, UK). Aliquots of nanoemulsions were diluted with distilled water under constant stirring (1750 rpm) until an obscuration of 5% was achieved. Nanoemulsions were analyzed after 1, 15, and 30 days of storage at refrigeration (4 °C) or room temperature (25 °C). Each sample was analyzed in triplicate.
2.2.3. Zeta-potential measurements
The surface charge of nanoemulsions during the storage period (days 1, 15, and 30 at 4 and 25 °C) was determined using Instrument Zetasizer Nano-Z (Malvern Instruments Ltda., Malvern, UK). Measurements were carried out in triplicate.
2.2.4. Confocal laser scanning microscopy
Confocal laser scanning microscopy (CLSM) was carried out using a 63× objective lens (TCS SP5 II C1 Digital Eclipse, Leica, Tokyo, Japan). Samples were stained with Nile red, a fat-soluble fluorescent dye, by mixing 100 mL of sample with 20 μL of 1 mg mL−1 Nile red in propylene glycol. Excitation and emission wavelengths were respectively 488 nm and 515 nm. Images were processed using LAS lite (Zeiss Inc., Toronto, Canada).
2.2.5. Oxidative stability
The thiobarbituric acid reactive species (TBARS) assay is based on the spectrophotometric measurement of the pink pigment produced by the reaction of thiobarbituric acid (TBA) with malondialdehyde (MDA) and other secondary lipid peroxidation products. The assay was performed by adding 100 μL of nanoemulsion and 1 mL of 2-TBA into screw cap tubes, stirring the mixture, and reading the absorbance at 532 nm [19]. TEP (1,1,3,3-tetraethoxypropane) was used to construct a standard curve.
2.2.6. Antioxidant assays
The ferric reducing antioxidant power (FRAP) assay was carried out according to Benzie and Strain [20] with modifications. A solution of TPTZ (2,4,6-tripyridyl-s-triazine) was prepared in 40 mM HCl, and a solution of 20 mM FeCl3·6H2O was prepared in distilled water. Nanoemulsions were diluted to 1:2, 1:3, and 1:5. Absorbance was measured at 595 nm using a UV–vis spectrophotometer. Antioxidant activity was expressed as μmol Trolox equivalents (TE) mL−1 nanoemulsion according to a standard curve. The modified oxygen radical absorbance capacity-fluorescein (ORACFL) assay was carried out following the method of Dávalos et al. [21]. ORAC values were calculated by the difference between the area under the fluorescence decay curve of the sample and that of the blank. A standard curve was constructed using Trolox, and results were expressed as μmol TE mL−1 nanoemulsion.
2.2.7. Polymorphism
The polymorphic form of nanoemulsion crystals was investigated by X-ray diffraction (XRD) according to the AOCS method Cj 2–95 [22]. The measurements were obtained with steps of 0.02° in 2° and acquisition time of 2 s, with scans of 15-40°, at 25 °C using a Philips PW 1710 diffractometer with Bragg–Brentano geometry (θ:2θ) and Cu Kα radiation (λ =1.54178 Å; 40 kV; 30 mÅ). XRD. Polymorphic forms were identified by analyzing the short spacings of each crystal. Relative proportions of the different crystal types were estimated from the relative intensity of the short spacings. Data were analyzed using Origin (Origin Lab Corporation, Northampton, USA).
2.2.8. Antimicrobial activity
The minimum inhibitory concentration (MIC) of NBO and NBO24h against E. coli 0157:H7 ATCC 11,775 was determined using the broth microdilution method NCCLS [23]. Briefly, 50 μL aliquots of each nanoemulsion (NBO and NBO24h) were sequentially diluted (25.0 – 0.39 mg mL−1) in a 96-well plate containing 100 μL of Mueller–Hinton Broth (Difco®) The final concentration of bacterial suspension was adjusted to 1.5 × 106 colony forming units (CFU) mL−1. Plates were incubated at 37 °C for 24 h. Chloramphenicol was used as positive control.
2.3. Statistical analysis
Data were submitted to analysis of variance (ANOVA) followed by Tukey’s test using Minitab v. 16 (Minitab Inc., State College, USA) to evaluate significant differences (p < 0.05) between mean values.
3. Results and discussion
3.1. Size distribution and zeta potential
Overall, nanoemulsions were relatively stable at 4 °C and had little or no variation in droplet size during storage. The results show that temperature had no significant effect on droplet size. Data also suggest that structured nanoemulsions were more resistant to coalescence and gravitational separation, especially NBO24h, as the mean droplet size was smaller than 200 nm and no visible changes in phase separation were observed during storage [24]. Similar droplet diameters were observed in systems containing vitamin E, vitamin D, or lime oil [25]. Physicochemical changes in TAGs caused by the interesterification reaction result in a predominance of unsaturated fatty acids at the sn-1 and sn-3 positions, leading to the formation of tri-unsaturated TAGs that are rich in oleic acid and thereby reduce the melting range of buriti oil. Furthermore, the substitution of palmitic acid for oleic acid at the sn-1 and sn-3 positions of TAG in structured buriti oil [26] might have contributed to the reduction in droplet size. The lower the melting range of a structured lipid, the lower its viscosity, which directly affects droplet size. Oleic acid has a certain degree of mobility provided by the presence of a double bond, and this may alter the crystalline arrangement and the density at which chains can be stacked [27] (Table 1).
Table 1.
Influence of storage temperature on droplet size of nanoemulsions of buriti oil (NBO), nanoemulsions of buriti oil interesterified for 6 h (NBO6h), and nanoemulsions of buriti oil interesterified for 24 h (NBO24h).
| Storage period | Sample | Mean droplet size (nm) |
|
|---|---|---|---|
| 4 °C | 25 °C | ||
| Day 1 | NBO | 271±0.03b | 269±0.02b |
| NBO6h | 208±0.01c | 209±0.01c | |
| NBO24h | 196±0.01d | 196±0.01d | |
| Day 15 | NBO | 275±0.02a | 275±0.02a |
| NBO6h | 206±0.01c | 206±0.01c | |
| NBO24h | 196±0.01d | 196±0.01d | |
| Day 30 | NBO | 268±0.02b | 268±0.02b |
| NBO6h | 206±0.01c | 206±0.01c | |
| NBO24h | 196±0.01d | 195±0.01d | |
a,b,c,d Means within a column followed by different letters differ significantly at p < 0.05.
Another important factor associated with the stability of droplet size during storage is the efficiency of homogenization, which depends on the physicochemical properties of the lipids that form the emulsion, as smaller droplets are formed when the lipid phase is less viscous. When oil droplets are more viscous, they are more difficult to be dissolved by the homogenizer because they may exit the break zone before deforming and rupturing [12]. Emulsifiers play an important role in the preparation of nanoemulsions because they reduce interfacial tension and facilitate the formation of small droplets during homogenization through adsorption of the oil–water interface. However, if the hydrophilic–lipophilic balance is too high, then the interfacial tension of the emulsifier may be too high to form fine oil droplets [28,29].
The high-energy homogenization process combined with the interesterification reaction yielded nanoemulsions that were more stable. Although no studies have investigated the effect of fatty acid position in TAGs on nanoemulsion stability, some authors evaluated the effect of oil type on stability and the behavior of different nanoemulsions during in vitro digestion [30,31]. After droplet formation, the stability of nanoemulsions depends on the oil phase composition, as oils differ in polarity and water solubility [32]. A previous study showed that nanoemulsions containing small droplets (d32 < 200 nm) may find practical application in pharmaceutical, food, and cosmetic industries because of their structure and easy manipulation [28].
The zeta potential is an indirect measure of the surface charge of oil droplets, which provides an indication of stability during storage. The stability of NBO, NBO6h, and NBO24h stored at 4 and 25 °C was analyzed for 30 days (Table 2). Surface charges ranged from −23.5 to −30.8 mV, which indicates that nanoemulsions would be stable against aggregation and coalescence during storage.
Table 2.
Zeta-potential values of nanoemulsions of buriti oil (NBO), nanoemulsions of buriti oil interesterified for 6 h (NBO6h), and nanoemulsions of buriti oil interesterified for 24 h (NBO24 h) after 1, 15, and 30 days of storage at 25 °C and 4 °C.
| Sample | Storage period (days) |
||
|---|---|---|---|
| 1 | 15 | 30 | |
| 25 °C | |||
| NBO | −26.20 ± 0.61ef | −30.86 ± 0.71a | −30.53 ± 0.97ab |
| NBO6h | −25.56 ± 0.74e | −28.23 ± 0.47cde | −29.40 ± 0.10abc |
| NBO24h | −28.50 ± 1.48bcd | −30.13 ± 0.06abc | −26.63 ± 0.06def |
| 4 °C | |||
| NBO | −28.8 ± 0.40acd | −30.17 ± 0.66a | −29.30 ± 0.26cf |
| NBO6h | −26.13 ± 0.49f | −23.50 ± 0.36e | −27.63 ± 0.21cdf |
| NBO24h | −27.43 ± 0.72df | −29.33 ± 1.10cf | −28.43 ± 0.93acd |
a,b,c,d,e,f Means within a row followed by different letters differ significantly at p < 0.05.
We observed significant differences (p < 0.05) between zeta-potential values of NBO, NBO6h, and NBO24h during storage. NBO24h stored at 25 °C showed the highest absolute value of zeta potential (30.6 mV). Statistical analyses revealed significant differences (p < 0.05) between zeta potentials of NBO and NBO6h stored at 4 °C. We also observed that nanoemulsions stored under refrigeration had lower values than those stored at room temperature. Zeta potentials of all samples were negative because of the preference of the oil–water interface to adsorb the hydroxylic ions of water or Tween 80 molecules [12]. Studies have shown that zeta-potential measurement is a useful tool for estimating stability when values below −30 mV are obtained. Nanoemulsion systems of high absolute zeta potentials are usually stable because of the electrostatic repulsion between droplets [15,33,34], which minimizes aggregation [35]. Similar negative zeta potentials were reported in nanoemulsions containing a mixture of copaiba oil and medium-chain TAGs stored at 4 and 25 °C [36].
3.2. Confocal laser scanning microscopy (CLSM) analysis
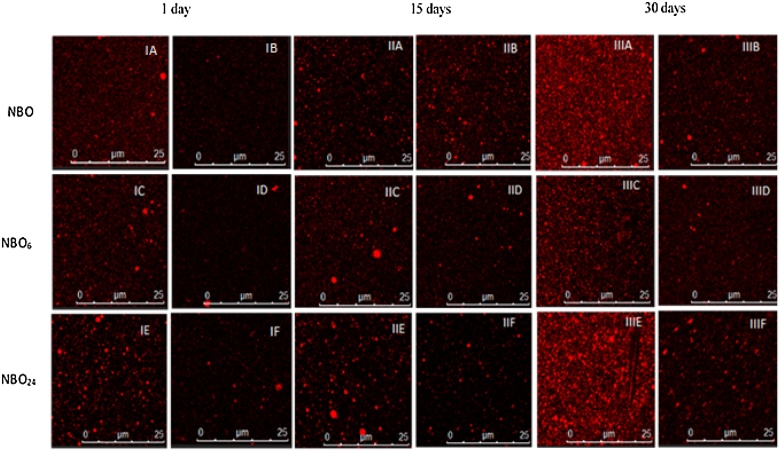
Confocal laser scanning microscopy observations allowed us to gain a better understanding of the microstructure of nanoemulsions and examine nanoscale characteristics that could not be inferred from droplet size results. CLSM images (Fig. 1) revealed that lipids were present as dispersed nanoparticles in suspension. NBO, NBO6h, and NBO24h (Fig. IB, ID, and IF, respectively) stored at room temperature (25 °C) displayed a very similar distribution pattern, with lipid droplets of less than 1 μm. However, it was difficult to distinguish individual droplets because of the resolution limit of the microscope. The number of relatively large droplets increased as the storage time increased, which suggests droplet aggregation and emulsion instability.
Fig. 1.
Confocal laser scanning micrographs of nanoemulsions of buriti oil (NBO) stored at 4 and 25 °C (IA and IB), nanoemulsions of buriti oil interesterified for 6 h (NBO6h) stored at 4 and 25 °C (IC and ID), and nanoemulsions of buriti oil interesterified for 24 h (NBO24 h) stored at 4 and 25 °C (IE and IF).
NBO, NBO6h, and NBO24h (Fig. IA, IC, and IE, respectively) stored at 4 °C showed greater susceptibility to droplet aggregation than nanoemulsions stored at room temperature, according to CLSM images. This phenomenon was more intense after 30 days of storage. Although we did not find a significant increase in droplet size, microstructural observations provided evidence that coalescence took place at refrigeration temperature. In contrast, nanoemulsions stored at 25 °C did not exhibit larger agglomerations with time, especially NBO6h and NBO24h, which were composed of structured lipids. These results can be explained by the fact that structured buriti oil has a higher content of unsaturated TAGs than its unstructured equivalent and thus has lower melting point and viscosity. These physicochemical properties influence droplet formation and stability [26].
Interestingly, nanoemulsions exhibited a specific aggregation behavior that may be related to polymorphic changes during storage. Such changes affect nanoemulsions properties, including crystal shape and particle morphology, as will be discussed in detail later. No studies reporting similar behaviors in structured lipid nanoemulsions were found in the literature. Some studies investigated the behavior of conventional emulsions or nanoemulsions by confocal microscopy using gastric digestion models [9,30].
As nanoemulsions produced with lipids interesterified for 6 or 24 h afforded similar results, NBO6h was excluded from the study and only NBO and NBO24h were subjected to further investigation.
3.3. Lipid peroxidation during storage (TBARS assay)
The oxidative stability of NBO and NBO24h was monitored during 30 days of storage by the TBARS method, used to measure the formation of secondary oxidation products. Results are shown in Table 3. A significant increase in TBARS was observed during storage for both NBO and NBO24h, indicating the occurrence of lipid oxidation. NBO and NBO24h had very similar (p > 0.05) TBARS levels on the first day of storage at room temperature. TBARS levels increased in NBO as the storage time increased, so that at the end of storage at 25 °C, NBO showed significantly higher TBARS than NBO24h. At the end of storage at 4 °C, NBO24h also had significantly lower TBARS than NBO. Droplet size and interfacial area influence lipid oxidation in O/W emulsions, as do various structural parameters and components [37]. Nanoemulsions have a large specific surface area because they are composed of small droplets [38]. Tween 80 is also known to play a role in the oxidation of emulsified lipids through the formation of hydroperoxides [39].
Table 3.
Oxidative stability (TBARS levels in mg MDA mL − 1) of nanoemulsions of buriti oil (NBO) and structured buriti oil (NBO24 h) at days 1, 15, and 30 of storage at 4 or 25 °C.
| Storage temperature | NBO |
NBO24h |
||||
|---|---|---|---|---|---|---|
| day 1 | day 15 | day 30 | day 1 | day 15 | day 30 | |
| 4 °C | 11.90 ± 0.24A | 5.67 ± 0.04A | 16.07 ± 1.43A | 9.87 ± 0.56B | 5.93 ± 0.28A | 10.33 ± 0.55B |
| 25 °C | 10.29 ± 0.62A | 6.12 ± 1.13A | 17.44 ± 0.82A | 10.35 ± 0.81A | 5.34 ± 0.98A | 11.95 ± 0.73B |
A, B Means within a row followed by different letters differ significantly at p < 0.05.
On the basis of the results presented above, we believe that enzymatic interesterification helped prevent lipid peroxidation in buriti oil nanoemulsions. In general, significant amounts of secondary oxidation compounds are formed when fatty acid chains containing three or more double bonds, different from linoleic acid or oleic acid, are involved [40].
Similar results were reported by Nejadmansouri et al. [37], who observed that the oxidation rate of nanoemulsions was greater at refrigeration temperature than at ambient temperature. Wang et al. [41] concluded that interesterification contributed positively to the stability of oils, retarding or preventing oxidation.
This study has important implications for the design and use of nanoemulsions as delivery systems. The present results show that, because of its smaller droplet size, NBO24h is less susceptible to the formation of secondary oxidation products throughout the storage period at both studied temperatures. It is known that the mechanism of lipid oxidation in O/W emulsions is very complex and differs from that which occurs in bulk lipid systems [42]. Because emulsified lipids are highly susceptible to oxidation, combined antioxidant strategies are required to delay oxidation reactions and improve shelf-life. This knowledge served as a basis for developing structured nanoemulsions of buriti oil: it has excellent antioxidant, functional, and biological properties [26,43]. Subsequent experiments were performed using NBO and NBO24h stored at room temperature, as samples were shown to be less stable at 4 °C.
3.4. Antioxidant capacity of nanoemulsions
The FRAP assay, based on the electron transfer reaction between antioxidants and radicals [44], has been widely used to determine the antioxidant capacity of O/W emulsions and nanoemulsions. But because antioxidant capacity varies according to the radicals that participate in the reaction, it must be examined by more than one method. We analyzed the antioxidant activity of structured and non-structured buriti oil nanoemulsions by the FRAP and ORAC assays. Results are presented in Table 4. By the FRAP method, NBO had an antioxidant activity of 419.83 μmol mL−1 and NBO24h of 611.38 μmol mL−1; that is, NBO24h exhibited an antioxidant capacity approximately 31% higher than that of NBO. Our data show that structured lipids in NBO24h were effective in donating electrons to stabilize free radicals, contributing to the antioxidant effect of the system.
Table 4.
Antioxidant capacity of nanoemulsions of buriti oil (NBO) and nanoemulsions of structured buriti oil (NBO24 h) by the ORAC and FRAP assays.
| Sample | Concentration range (mg mL−1) | ORAC (μmol TE* mL−1) | FRAP (μmol TE* mL−1) |
|---|---|---|---|
| NBO | 0.20–0.05 | 3089.7 ± 360.40b | 419.8 ± 7.30b |
| NBO24h | 0.20–0.05 | 3640.6 ± 235.25a | 611.4 ± 47.53a |
TE, Trolox equivalents. a,b Means followed by different letters within a column differ significantly at p < 0.05 by Tukey test.
Minor components of oils, such as tocopherols, carotenoids, phenolics, exert antioxidant effects through various chemical mechanisms, including the elimination of free radicals by electron transfer or hydrogen donation and chelation of transition metals [40]. Thus, the antioxidant activity of nanoemulsions of buriti oil and structured buriti oil is probably also due to the presence of carotenoids, mainly β-carotene [26]. In contrast, the presence of free fatty acids in emulsified oils is known to increase lipid oxidation, which is related to their location in the oil–water interface, and to increase the negative charge of oil droplets, which may attract cationic transition metals such as Fe2+ or Fe3+ to the oil droplet surface [45,46]. Transition metals can originate from various sources, including process water, processing equipment, and oils and fats [37]. When present in the emulsion system, transition metals can catalyze oxidation reactions; in these cases, lipid oxidation cannot be prevented by a radical-scavenging antioxidant alone and a metal chelator is needed [37].
The ORAC assay measures the oxidative degradation of the molecule fluorescein, which when inhibited indicates antioxidant activity [47]. The ORAC value of NBO24h was 3640.56 μmol mL−1 and of NBO was 3089.66 μmol mL−1 (Table 4). NBO24h showed greater antioxidant activity than NBO by both FRAP and ORAC methods, which can be confirmed by further oxidative stability assays. To date, no other study has evaluated ORAC in nanoemulsions of structured lipids.
Previous studies have shown that the levels of phenolics of high antioxidant potential, can vary in buriti oil [48,49]. Thus, buriti oil emulsion systems may differ in composition and concentration of phytochemicals and may even contain compounds that interact in an antagonistic manner [43,50].
3.5. Crystal polymorphism in nanoemulsions
Crystallization processes occur according to the predominant form of crystals and have a great impact on functionality and application [51]. In this study, evidence was collected to show the influence of droplet size on the crystalline structural properties of nanoemulsions, especially in relation to the presence of TAG molecules, which can crystallize into different forms depending on processing methods. Polymorphism analysis of NBO and NBO24h by X-ray diffraction after 30 and 120 days of storage revealed the coexistence of β′ and β polymorphs in samples. The short spacings and polymorphic forms of buriti oil, NBO, and NBO24h are shown in Table 5. The diffractogram of bulk buriti oil stored for 30 days at room temperature showed peaks at 4.4, 4.2, and 3.9 Å, attributed to the polymorphic forms β′ and β. The diffractograms of NBO and NBO24h also showed a combination of the polymorphic forms β′ and β, as evidenced by the peaks at 4.2 and 3.9 Å, corresponding to the polymorph β, and very strong peaks at 4.4 Å, attributed to β′.
Table 5.
Short spacings and polymorphic forms of buriti oil (BO), nanoemulsions of buriti oil (NBO), and nanoemulsions of buriti oil interesterified for 24 h (NBO24 h) determined by X-ray diffraction at −25 °C after 30 and 120 days of storage at room temperature.
| Sample |
Short spacings* |
Polymorphic form | |||||
|---|---|---|---|---|---|---|---|
| 4.4 | 4.2 | 4.0 | 3.9 | 3.8 | |||
| 30 days of storage | |||||||
| BO | 4.38 (vs) | 4.19 (vs) | – | 3.93 (vs) | – | β' + β | |
| NBO | 4.44 (s) | 4.24 (vs) | 3.97 (vs) | – | – | β' + β | |
| NBO24h | 4.44 (vs) | 4.23 (vs) | 4.00 (vs) | – | – | β' + β | |
| 120 days of storage | |||||||
| NBO | 4.42 (s) | 4.23 (s) | – | 3.88 (vs) | 3.77 (w) | β' + β | |
| NBO24h | 4.43 (vs) | 4.24 (vs) | – | 3.88 (vs) | – | β' + β | |
Intensity: vs, very strong; s, strong; and w, weak.
Polymorphic behaviors, such as melting point, may be affected by the chain length of fatty acids in the TAG structure [52]. To avoid interaction with lipid droplets and facilitate crystallization, we performed these experiments using a low concentration of surfactant.
A study on emulsified blends of interesterified fats reported that crystallization (−10 °C) in the β′ form was probably due to changes in the configuration of TAGs, which showed an increase in unsaturated fatty acids in the sn-2 position [53]. In the present study, TAGs in buriti oil and interesterified buriti oil contained a high percentage of tri-unsaturated fatty acids, which cannot crystallize in a metastable form [26]. According to some researchers [51,54], the presence of minor lipid components, monoacylglycerol and diacylglycerol in the oil at concentrations above 5% (data not shown), causes an increase in the energy barrier for nucleation, leading to delayed crystallization and the formation of unstable polymorphs. A better understanding of polymorphic transformations can be gained by analyzing the X-ray diffraction results of nanoemulsions after 120 days of storage. Transition from the polymorphic form α to β occurs more rapidly during storage when the system is emulsified because of the small size of crystals in emulsified systems. The polymorphic form of a lipid was shown to influence the biological activity of hydrophobic drugs in emulsion systems [55], because the molecular packing of lipid crystals can expel the molecules from the crystalline matrix. Although uncharacteristic of emulsified crystalline material, some crystalline nanostructure have previously been found to improve emulsion stability.
3.6. Antimicrobial assay
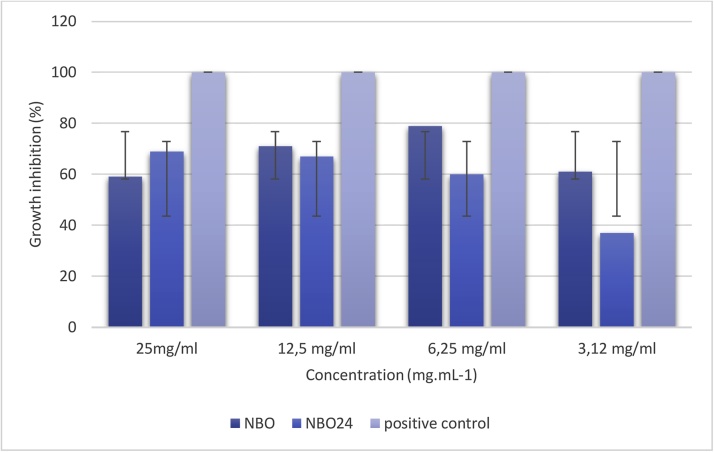
Antimicrobial agents from natural sources are being extensively investigated for their potential application in the food and other industries. In this study, structured and unstructured buriti oil was used to produce nanoemulsions with a mean droplet diameter with approximately 200 nm. It was measured by reading the absorbance in the plate cavities with different concentrations of nanoemulsions and response expressed in (Fig. 2).
Fig. 2.
Antibacterial activity of buriti oil nanoemulsions (NBO), structured buriti oil nanoemulsions (NBO24 h), and positive control (chloramphenicol) against Escherichia coli. Values are presented as mean ± S.D. of three replicates (n = 3).
NBO inhibited bacterial growth by approximately 61% at 3.12 mg mL−1 demonstrating the bacteriostatic effect. Complete inhibition of bacterial growth was not achieved (Fig. 2). The low concentration of unsaturated fatty acids, which have antimicrobial activity, might explain the low antimicrobial effect of the samples [50]. Nevertheless, the results suggest that NBO and NBO24h have physicochemical characteristics that contribute to their biological activity. According to recent studies, droplet size can affect the antimicrobial activity of emulsions. Essential oil nanoemulsions induced faster inactivation of E. coli than conventional emulsions [55]. The antimicrobial activity is due not only to the chemical activity of individual components but also to the structure of the O/W nanoemulsion system. Some studies have correlated droplet size with biological activity [56,57]. However, a conventional emulsion of buriti oil showed bactericidal and bacteriostatic activity against E. coli [3]. The different explanations proposed for the experimental observations were considered, but we were not able to compare our results, as no previous data on the antimicrobial activity of buriti oil nanoemulsions are available in the literature. The study provided relevant contributions to the development of value-added products from natural ingredients, such as vegetable oils. Interesterification positively influenced mean droplet size, and oxidative stability of nanoemulsions, exhibited good physical stability, with little evidence of phase separation or droplet growth during 30 days of storage at 25 °C. Furthermore, interesterification enhanced the antioxidant activity and, to a lesser extent, the antimicrobial action of buriti oil nanoemulsions. Several challenges remain to be overcome in the development of structured nanoemulsions with industrial applications that provide controlled-release of bioactive compounds, good stability thermodynamically and antimicrobial effects.
Declaration of Competing Interest
None.
Acknowledgments
The authors are grateful to Ph.D. Marta Cristina T. Duarte of CPQBA/UNICAMP, SP, Brazil, for her support during the analyses and for providing the microorganisms used in the study. The authors would also like to thank the Brazilian National Council for Scientific and Technological Development (CNPq, grant no. 140883/2016-9) and the São Paulo Research Foundation (FAPESP, grant no. 2014/16530-1) for their financial support.
Contributor Information
K.M.M. Leão, Email: kmagnaleao@gmail.com.
G.A. Macedo, Email: macedoga@gmail.com.
References
- 1.Speranza P., Falcão A.D.O., Macedo J.A., Silva L.H.M. Amazonian Buriti oil : chemical characterization and antioxidant potential. Grasas Y Aceites. 2016;67:1–9. [Google Scholar]
- 2.Silva S.M., Sampaio K.A., Taham T., Rocco S.A., Ceriani R., Meirelles A.J.A. Characterization of oil extracted from buriti fruit (Mauritia flexuosa) grown in the brazilian Amazon region. J. Am. Oil Chem. Soc. 2009;86:611–616. [Google Scholar]
- 3.Speranza P., Badan Ribeiro A.P., Cunha R.L., Macedo J.A., Macedo G.A. Influence of emulsion droplet size on antimicrobial activity of interesterified Amazonian oils. LWT - Food Sci. Technol. 2015;60:207–212. [Google Scholar]
- 4.Kim B.H., Akoh C.C. Recent research trends on the enzymatic synthesis of structured lipids. J. Food Sci. 2015;80:C1713–C1724. doi: 10.1111/1750-3841.12953. [DOI] [PubMed] [Google Scholar]
- 5.Osborn H.T., Akoh C.C. Structured lipids-novel fats with medical, nutraceutical, and food applications. Compr. Rev. Food Sci. Food Saf. 2002;1:110–120. doi: 10.1111/j.1541-4337.2002.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 6.Speranza P., Ribeiro A.P.B., Macedo G.A. Lipase catalyzed interesterification of Amazonian patauá oil and palm stearin for preparation of specific-structured oils. J. Food Sci. Technol. 2015;52:8268–8275. doi: 10.1007/s13197-015-1943-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClements D.J., Li Y. Structured emulsion-based delivery systems: controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010;159:213–228. doi: 10.1016/j.cis.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Tadros T.F. 2002. Emulsion Formation, Stability, and Rheology. [Google Scholar]
- 9.Salvia-Trujillo L., McClements D.J. Influence of nanoemulsion addition on the stability of conventional emulsions. Food Biophys. 2016 [Google Scholar]
- 10.Özogul Y., Durmus M., Ucar Y., Özogul F., Regenstein J.M. Comparative study of nanoemulsions based on commercial oils (sunflower, canola, corn, olive, soybean, and hazelnut oils): effect on microbial, sensory, and chemical qualities of refrigerated farmed sea bass. Innov. Food Sci. Emerg. Technol. 2016;33:422–430. [Google Scholar]
- 11.Mason T.G., Wilking J.N., Meleson K., Chang C.B., Graves S.M. Nanoemulsions: formation, structure, and physical properties. J. Phys. Condens. Matter. 2006 [Google Scholar]
- 12.Troncoso E., Aguilera J.M., McClements D.J. Fabrication, characterization and lipase digestibility of food-grade nanoemulsions. Food Hydrocoll. 2012 [Google Scholar]
- 13.Uluata S., Decker E.A., McClements D.J. Optimization of nanoemulsion fabrication using microfluidization: role of surfactant concentration on formation and stability. Food Biophys. 2016;11:52–59. [Google Scholar]
- 14.Kharat M., Zhang G., McClements D.J. Stability of curcumin in oil-in-water emulsions: impact of emulsifier type and concentration on chemical degradation. Food Res. Int. 2018;111:178–186. doi: 10.1016/j.foodres.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Salvia-Trujillo L., Rojas-Graü A., Soliva-Fortuny R., Martín-Belloso O. Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll. 2015;43 [Google Scholar]
- 16.Arancibia C., Riquelme N., Zúñiga R., Matiacevich S. Comparing the effectiveness of natural and synthetic emulsifiers on oxidative and physical stability of avocado oil-based nanoemulsions. Innov. Food Sci. Emerg. Technol. 2017 [Google Scholar]
- 17.Speranza P., Ribeiro A.P.B., Macedo G.A. Application of lipases to regiospecific interesterification of exotic oils from an Amazonian area. J. Biotechnol. 2016;218:13–20. doi: 10.1016/j.jbiotec.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Ozturk B., Argin S., Ozilgen M., McClements D.J. Nanoemulsion delivery systems for oil-soluble vitamins: influence of carrier oil type on lipid digestion and vitamin D<inf>3</inf> bioaccessibility. Food Chem. 2015 doi: 10.1016/j.foodchem.2015.04.065. [DOI] [PubMed] [Google Scholar]
- 19.Ke P.J., Woyewoda A.D. Microdetermination of thiobarbituric acid values in marine lipids by a direct spectrophotometric method with a monophasic reaction system. Anal. Chim. Acta. 1979 [Google Scholar]
- 20.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 21.Dávalos A., Gómez-Cordovés C., Bartolomé B. Extending applicability of the oxygen radical absorbance capacity (ORAC-Fluorescein) assay. J. Agric. Food Chem. 2004;52:48–54. doi: 10.1021/jf0305231. [DOI] [PubMed] [Google Scholar]
- 22.AOCS . Off. Methods Recomm. Pract. AOCS; 2009. Determination of cis-, trans-, Saturated, Monounsaturated and Polyunsaturated Fatty Acids in Vegetable or Non-Ruminant Animal Oils and Fats by Capillary GLC (Ce 1h-05) [Google Scholar]
- 23.NCCLS Clinical and Laboratory Standards Institute . 2005. Metodologia Dos Testes De Sensibilidade a Agentes Antimicrobianos Por Diluição Para Bactéria De Crescimento Aeróbico : Norma Aprovada - Sexta Edição. [Google Scholar]
- 24.McClements D.J., Rao J. Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011;51:285–330. doi: 10.1080/10408398.2011.559558. [DOI] [PubMed] [Google Scholar]
- 25.Ziani K., Fang Y., Julian D. Vol. 134. 2012. pp. 1106–1112. (Encapsulation of Functional Lipophilic Components in Surfactant-Based Colloidal Delivery Systems : Vitamin E, Vitamin D, and Lemon Oil). [DOI] [PubMed] [Google Scholar]
- 26.Speranza P., Leao K.M.M., Gomes T.S.N., Rodrigues A.P., Macedo J.A., Macedo G.A. Improving the chemical properties of Buriti oil (Mauritia flexuosa L.) by enzymatic interesterification. Grasas Y Aceites. 2018;69:1–8. [Google Scholar]
- 27.Chambi H.N.M., Alvim I.D., Barrera-Arellano D., Grosso C.R.F. Solid lipid microparticles containing water-soluble compounds of different molecular mass: production, characterisation and release profiles. Food Res. Int. 2008;41:229–236. [Google Scholar]
- 28.Bai L., Huan S., Gu J., McClements D.J. Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: saponins, phospholipids, proteins, and polysaccharides. Food Hydrocoll. 2016;61:703–711. [Google Scholar]
- 29.Chang Y., McClements D.J. Optimization of orange oil nanoemulsion formation by isothermal low-energy methods: influence of the oil phase, surfactant, and temperature. J. Agric. Food Chem. 2014;62 doi: 10.1021/jf500160y. [DOI] [PubMed] [Google Scholar]
- 30.Majeed H., Antoniou J., Hategekimana J., Sharif H.R., Haider J., Liu F., Ali B., Rong L., Ma J., Zhong F. Influence of carrier oil type, particle size on invitro lipid digestion and eugenol release in emulsion and nanoemulsions. Food Hydrocoll. 2016 [Google Scholar]
- 31.Cheong A.M., Tan C.P., Nyam K.L. In-vitro gastrointestinal digestion of kenaf seed oil-in-water nanoemulsions. Ind. Crops Prod. 2016 [Google Scholar]
- 32.Davidov-Pardo G., McClements D.J. Nutraceutical delivery systems: resveratrol encapsulation in grape seed oil nanoemulsions formed by spontaneous emulsification. Food Chem. 2015;167:205–212. doi: 10.1016/j.foodchem.2014.06.082. [DOI] [PubMed] [Google Scholar]
- 33.Heurtault B., Saulnier P., Pech B., Proust J.E., Benoit J.P. Physico-chemical stability of colloidal lipid particles. Biomaterials. 2003 doi: 10.1016/s0142-9612(03)00331-4. [DOI] [PubMed] [Google Scholar]
- 34.Pinto M.F., Moura C.C., Nunes C., Segundo M.A., Costa Lima S.A., Reis S. A new topical formulation for psoriasis: development of methotrexate-loaded nanostructured lipid carriers. Int. J. Pharm. 2014;477:519–526. doi: 10.1016/j.ijpharm.2014.10.067. [DOI] [PubMed] [Google Scholar]
- 35.Yukuyama M.N., Ghisleni D.D.M., Pinto T.J.A., Bou-Chacra N.A. Nanoemulsion: process selection and application in cosmetics - A review. Int. J. Cosmet. Sci. 2016;38:13–24. doi: 10.1111/ics.12260. [DOI] [PubMed] [Google Scholar]
- 36.de O. Dias D., Colombo M., Kelmann R.G., Kaiser S., Lucca L.G., Teixeira H.F., Limberger R.P., Veiga V.F., Koester L.S. Optimization of Copaiba oil-based nanoemulsions obtained by different preparation methods. Ind. Crops Prod. 2014 [Google Scholar]
- 37.Nejadmansouri M., Hosseini S.M.H., Niakosari M., Yousefi G.H., Golmakani M.T. Physicochemical properties and oxidative stability of fish oil nanoemulsions as affected by hydrophilic lipophilic balance, surfactant to oil ratio and storage temperature. Colloids Surfaces A Physicochem. Eng. Asp. 2016 [Google Scholar]
- 38.McClements D.J., Rao J. Food-grade nanoemulsions : formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011;51:285–330. doi: 10.1080/10408398.2011.559558. [DOI] [PubMed] [Google Scholar]
- 39.Montes de Oca-Ávalos J.M., Candal R.J., Herrera M.L. Nanoemulsions: stability and physical properties. Curr. Opin. Food Sci. 2017 [Google Scholar]
- 40.Zou L., Akoh C.C. Oxidative stability of structured lipid-based infant formula emulsion: effect of antioxidants. Food Chem. 2015 doi: 10.1016/j.foodchem.2015.01.073. [DOI] [PubMed] [Google Scholar]
- 41.Wang X.Y., Yang D., Gan L.J., Zhang H., Shin J.A., Park S.H., Lee K.T. Degree of oxidation depending on the positional distribution of linolenic acid in perilla oil and interesterified products. Food Sci. Biotechnol. 2014 [Google Scholar]
- 42.Waraho T., Mcclements D.J., Decker E.A. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci. Technol. 2011 [Google Scholar]
- 43.Speranza P., De Oliveira Falcão A., Alves Macedo J., Da Silva L.H.M., Rodrigues A.M.Da C., Alves Macedo G. Amazonian Buriti oil: chemical characterization and antioxidant potential. Grasas Y Aceites. 2016;67:e135. [Google Scholar]
- 44.Huang D., Boxin O.U., Prior R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 45.Waraho T., Cardenia V., Rodriguez-Estrada M.T., Julian Mcclements D., Decker E.A. Prooxidant mechanisms of free fatty acids in stripped soybean oil-in-water emulsions. J. Agric. Food Chem. 2009 doi: 10.1021/jf901270m. [DOI] [PubMed] [Google Scholar]
- 46.Waraho T., McClements D.J., Decker E.A. Impact of free fatty acid concentration and structure on lipid oxidation in oil-in-water emulsions. Food Chem. 2011 doi: 10.1016/j.foodchem.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 47.Fathi M., Varshosaz J., Mohebbi M., Shahidi F. Hesperetin-loaded solid lipid nanoparticles and nanostructure lipid carriers for food fortification: preparation, characterization, and modeling. Food Bioprocess Technol. 2013;6:1464–1475. [Google Scholar]
- 48.Bataglion G.A., da Silva F.M.A., Eberlin M.N., Koolen H.H.F. Simultaneous quantification of phenolics compounds in buriti fruit (Mauritia flexuosa L.f.) by ultra-high performance liquid chromatography coupled to tandem mass spectrometry. Food Res. Int. 2014 [Google Scholar]
- 49.Freitas M.L.F., Chisté R.C., Polachini T.C., Sardella L.A.C.Z., Aranha C.P.M., Ribeiro A.P.B., Nicoletti V.R. Quality characteristics and thermal behavior of buriti (Mauritia flexuosa L.) oil ; Parámetros de calidad y comportamiento térmico del aceite de buriti (Mauritia flexuosa L.) Grasas Y Aceites. 2017;68:9. [Google Scholar]
- 50.Koolen H.H.F., da Silva F.M.A., Gozzo F.C., de Souza A.Q.L., de Souza A.D.L. Antioxidant, antimicrobial activities and characterization of phenolic compounds from buriti (Mauritia flexuosa L. f.) by UPLC-ESI-MS/MS. Food Res. Int. 2013 [Google Scholar]
- 51.Sato K., Bayés-García L., Calvet T., Cuevas-Diarte M.À., Ueno S. External factors affecting polymorphic crystallization of lipids. Eur. J. Lipid Sci. Technol. 2013 [Google Scholar]
- 52.Douaire M., Di Bari V., Norton J.E., Sullo A., Lillford P., Norton I.T. Fat crystallisation at oil-water interfaces. Adv. Colloid Interface Sci. 2014 doi: 10.1016/j.cis.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 53.Da Silva R.C., De Martini Soares F.A.S., Fernandes T.G., Castells A.L.D., Da Silva K.C.G., Gonçalves M.I.A., Ming C.C., Gonçalves L.A.G., Gioielli L.A. Interesterification of lard and soybean oil blends catalyzed by immobilized lipase in a continuous packed bed reactor. JAOCS, J. Am. Oil Chem. Soc. 2011 [Google Scholar]
- 54.Smith K.W., Bhaggan K., Talbot G., Van Malssen K.F. Crystallization of fats: influence of minor components and additives. JAOCS, J. Am. Oil Chem. Soc. 2011;88:1085–1101. [Google Scholar]
- 55.Salvia-Trujillo L., Rojas-Graü M.A., Soliva-Fortuny R., Martín-Belloso O. Impact of microfluidization or ultrasound processing on the antimicrobial activity against Escherichia coli of lemongrass oil-loaded nanoemulsions. Food Control. 2014 [Google Scholar]
- 56.Acosta E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr. Opin. Colloid Interface Sci. 2009;14:3–15. [Google Scholar]
- 57.Salvia-Trujillo L., Rojas-Graü M.A., Soliva-Fortuny R., Martín-Belloso O. Effect of processing parameters on physicochemical characteristics of microfluidized lemongrass essential oil-alginate nanoemulsions. Food Hydrocoll. 2013 [Google Scholar]